Abstract
Background:
Urushiols are mixtures of olefinic catechols which is isolated from the sap of Korean lacquer tree (Rhus vernicifera Stokes). The aim of this study was to determine the anticancer effects of urushiol in human gastric adenocarcinoma cell lines.
Methods:
The cytotoxicity of urushiols was assessed by MTT assays on the two gastric adenocarcinoma cell lines, MKN-45 (wild type of p53) and MKN-28 (mutant type of p53). We also examined the action mechanisms of urushiol by analyzing its effects on cell cycle progression and apoptosis induction.
Results:
The cytotoxic results from MTT assays indicated that urushiol inhibited human gastric cancer cell growth in a dose-dependent manner, with IC50 values of approximately 15 and 20 μg/ml on MKN-45 and MKN-28 cells, respectively. Urushiol mediated cell death on these two cancer cell lines through different pathways. Urushiol induced apoptosis on MKN-45 cells, concomitant with apoptotic nuclear change, DNA fragmentation, poly (ADP-ribose) polymerase cleavage and apoptotic body formation via extrinsic pathway of apoptosis. However, no apoptotic features were induced by urushiol treatment on MKN-28 cells. Urushiol induced cytostatic cell growth inhibition via upregulation of the cyclin-dependent kinase inhibitors, p21WAF1/CIP1 and p27KIP1 proteins and down-regulation of cyclin-dependent kinase 2 and 4 proteins in a p53-independent manner.
Conclusions:
These data provide evidence that urushiol has the potential to be used as a chemotherapeutic agent in human gastric cancer.
Keywords: Urushiol, Gastric cancer cells, Apoptosis, G1 arrest
INTRODUCTION
Urushiols are mixtures of olefinic catechols with n-C15 or n-C17 alkyl side chain which is isolated from the sap of Korean lacquer tree (Rhus vernicifera Stokes).1–5 The usefulness of urushiol as a potential therapeutic agent has been limited by its hypersensitive reaction.6 Urushiol has been traditionally used as an oriental folk medicine in Korea for various diseases, such as swelling, inflammation, and tumor. The cytotoxic effects of urushiol compounds have been studied in 29 different kinds of human cancer cell lines, including prostate, ovary, brain, colon, kidney, lung and leukemia, and the value of 50% growth inhibition (GI50) has been reported to be approximately 4.0μg/ml, showing cell line-specific cytotoxicity.7 However, only cytotoxic sulforhodamine B assay was conducted and, therefore, the underlying molecular mechanisms of the cytotoxicity of urushiol were not fully elucidated. We recently reported that urushiol induced growth inhibition and apoptosis in MCF-7 human breast cancer cells.8
Gastric cancer is the most prevalent cancer in the world, including Korea and Japan. Despite recent advances in cancer treatments, most newly diagnosed gastric cancer patients are incurable and have a median survival of less than one year.9 It is well known that gastric cancer can be divided into two major categories: the intestinal, expanding, or differentiated type; and the diffuse, infiltrative, or undifferentiated type.10 The differentiated type is characterized by expansive growth and liver metastasis; whereas the undifferentiated type is distinguished by infiltrative growth and peritoneal dissemination.11 MKN-45 and MKN-28 cell lines were derived from human gastric carcinoma.12 The MKN-45 cell line was derived from a metastatic liver tumor of a 62-year-old female with gastric cancer. The parent tumor of MKN-45 cells was a poorly differentiated adenocarcinoma of the medullary type. The MKN-28 cell line was derived from a metastatic tumor in a cervical lymph node of a 70-year-old female with gastric cancer. The parent tumor of MKN-28 cells was a moderately differentiated tubular adenocarcinoma. MKN-45 cell line contains wild type of p53 genes, whereas MKN-28 cell line has mutant type of p53 genes.13 In this study, we examined whether urushiol inhibits cell growth and promotes cell death. Furthermore, we studied potential molecular mechanisms of action by which urushiol inhibit cancer cells if it did.
MATERIALS AND METHODS
1. Cell culture and urushiol solution
MKN-45 and MKN-28, human gastric cancer cell lines, were obtained from Korean Cell Line Bank (Seoul, Korea). All cells were grown in RPMI 1640 (Gibco BRL, Grand Island, NY, USA) with 10% fetal bovine serum in a humidified 5% CO2 at 37°C containing 50 mg/ml gentamicin, and 135 mg/ml glutamine. The medium was changed three times per week. The number of cells was counted with a hematocytometer. Urushiol complex was obtained in a solution form from Lifetree Biotech Co., Ltd. (Suwon, Korea). The aqueous solution was filtered through Whatman No. 2 filter paper, and then concentrated in vacuum to dryness. The yield of urushiol was about 3.5%. The powder was weighted and adjusted to a final concentration with DMSO (Sigma-Aldrich, St. Louis, MO, USA) and stored at −20°C until use. The stock solution of urushiol was diluted with medium to the desired concentration prior to use. The maximal concentration of DMSO did not exceed 0.1% (v/v) in the treatment range (2–10 μg/ml), where there was no influence on the cell growth (data not shown).
2. Assessment of cell proliferation by MTT assay
The proliferation of gastric cancer cells was assessed by using the MTT assay which is based on the conversion of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to MTT-formazan by mitochondrial enzyme.14 In brief, these cells were cultured with 1 ml of medium per well in 24-multiwell plates at 37°C for 24 h under 5% CO2 in air. The cells were then treated with desired concentrations of urushiol. Then, the MTT solution (5 mg/mlPBS) was added to the well with a concentration of 0.25 mg/ml. Following the incubation for 4 h, the mixture of medium and the MTT solution was carefully discarded, after which 1 ml EtOH-DMSO (1 : 1) was added to the well in order to extract the crystallized dye. The amount of blue dye formed was determined by measuring the absorbance at 540 nm. Measurements were performed in triplicates.
3. Observation of nuclear structure
After the cells were treated with or without half-maximal inhibition (IC50) doses of urushiol (15 μg/ml for MKN-28 cells and 20 μg/ml for MKN-45 cells) for 48 h, they were washed twice with PBS containing 1% bovine serum albumin (PBS-B) and fixed with 70% ethanol containing 0.5% Tween 20 at 4°C for 30 min. Fixed cells were washed with PBS-B, and stained with propidium iodide (PI, Sigma-Aldrich) solution (50 μg/ml in PBS) for 30 min at room temperature. The stained cells were washed twice with PBS-B and observed via a fluorescence microscope at ×320 magnification.
4. Agarose gel analysis of DNA fragmentation
Cells were harvested, rinsed twice in cold PBS, and resuspended in lysis buffer [5 mM Tris-HCl (pH 7.5), 5 mM ethylenediaminetetra acetic acid (EDTA), and 0.5% Triton X-100] at 4°C for 30 min. After centrifugating them at 27,000×g for 15 min, the supernatant was treated with RNase, followed by proteinase K digestion, phenol/chloroform/isoamyl alcohol (25 : 24 : 1) extraction and isopropanol precipitation. DNA separated through a 1.5% agarose gel was stained with ethidium bromide (EtBr, Sigma-Aldrich) and visualized by ultraviolet light source.
5. Morphological study of apoptotic cells
After the cells were treated with or without IC50 doses of urushiol (15 μg/ml for MKN-28 cells and 20 μg/ml for MKN-45 cells) for 48 h, and washed with PBS, they were centrifuged (250×g for 10 min) and fixed as a pellet in 2.5% glutaraldehyde-1% osmium tetroxide buffered with PBS (pH 7.2), and processed for ultrathin sectioning by a slightly modified method.15 The samples were dehydrated in a graded ethanol series, embedded in Spury resin, and analyzed with standard procedures under a Hitachi H600-3 electron microscope (Tokyo, Japan).
6. Gel electrophoresis and Western blotting
The cells were harvested, lysed, and protein concentrations were quantified using the Bio-Rad protein assay (Bio-Rad Lab., Hercules, CA, USA) by the procedure described by the manufacturer. For the Western blot analysis, an equal amount of protein was subjected to electrophoresis on SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA) by electroblotting. Blots were probed with the desired antibodies for 1 h, incubated with diluted enzyme-linked secondary antibodies and then visualized by the enhanced chemiluminescence (ECL) according to the recommended procedure (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). The primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and Calbiochem (Cambridge, MA, USA). Peroxidase-labeled secondary antibodies were purchased from Amersham Pharmacia Biotech.
7. Statistics
Data were expressed as the mean±SD of three separate experiments and analyzed by Student’s t-test. The means were considered significantly different at P<0.05.
RESULTS
1. Urushiol showed cytotoxic and growth inhibitory effects on gastric cancer cells
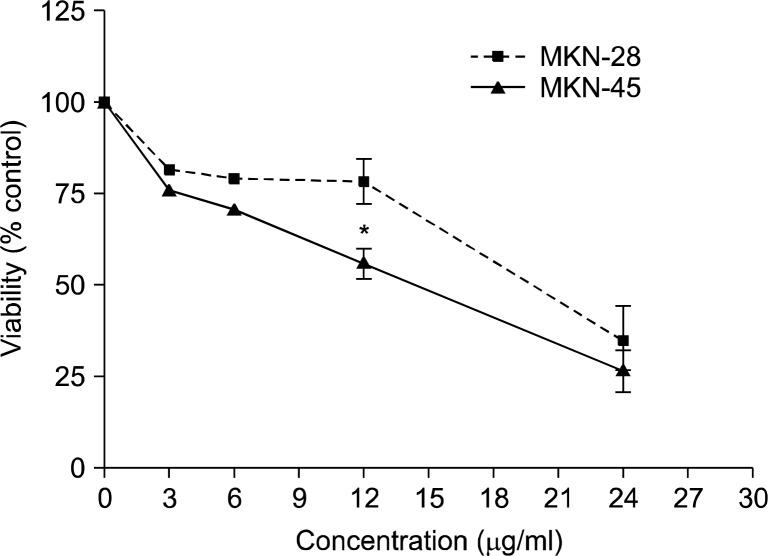
The effect of urushiol on the viability of MKN-28 and MKN-45 cells was assessed via MTT dye assay at the concentrations of 3, 6, 12, and 24 μg/ml for 48 h. Urushiol showed cytotoxic and growth inhibitory effect on both cells in a concentration-dependent manner. The IC50 values of urushiol were approximately 15 and 20 μg/ml on MKN-45 and MKN-28 cells, respectively (Fig. 1). Therefore, urushiol seemed to have stronger anti-proliferative activity on MKN-45 cells than MKN-28 cells.
Fig. 1.
Cytotoxic effect of urushiol on MKN-45 and MKN-28 cells. Cells were treated with different concentration of urushiol for 48 h and the effects of urushiol on cell growth were examined by MTT assay. Results are expressed as percentage of the vehicle treated control±SD of three separate experiments. The significance was determined by Student’s t-test (P*<0.05).
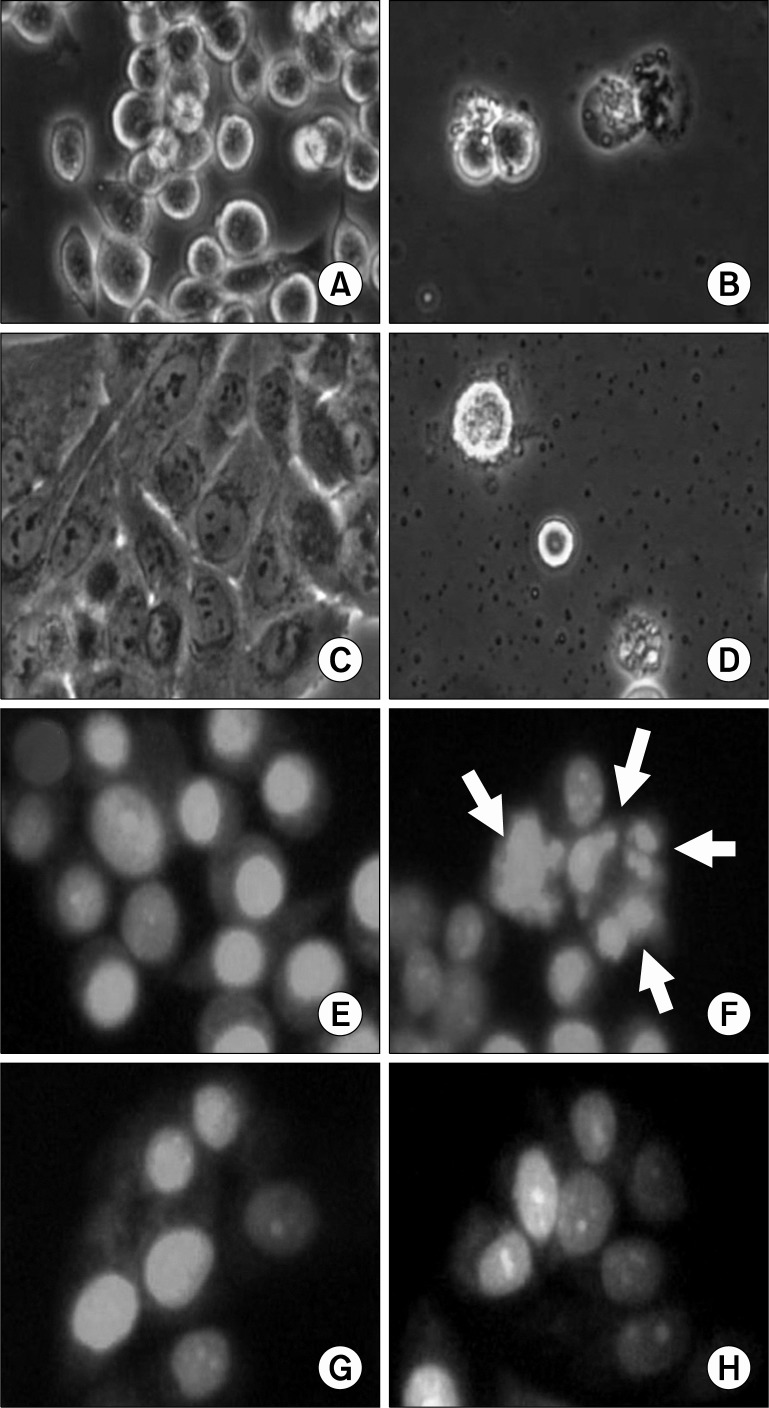
There were significant morphological changes on MKN-45 and MKN-28 cells after the urushiol treatment (Fig. 2). However, the morphologies of two cell lines were significantly different. The sizes of MKN-45 cells were smaller than MKN-28 cells after urushiol treatment. The shape of MKN-45 cell was round, whereas MKN-28 cell was polygonal. Although apparent morphological changes were observed on urushiol-treated MKN-45 (Fig. 2A and B) and MKN-28 cells (Fig. 2C and D), any other differences between two cells were not detectable by light microscopy with no staining. To test whether decreased viability of human gastric cancer cells by urushiol was attributed to apoptosis, the structures of nucleus were studied with PI staining on urushiol-treated cells. In MKN-45 cells, morphological changes were corresponding to apoptosis, including nuclear fragmentation and condensation, observed by the treatment of 15 μg/ml urushiol (Fig. 2E and F). In contrast, any nuclear structural changes were not detectable on 20 μg/ml urushiol-treated MKN-28 cells (Fig. 2G and H).
Fig. 2.
Microphotographs of human gastric cancer cells treated with urushiol. Morphological changes were observed by light microscopy after treatment with urushiol for 48 h. Untreated control of MKN-45 cells (A); 15 μg/ml urushiol-treated MKN-45 cells (B); untreated control of MKN-28 cells (C); 20 μg/ml urushiol-treated MKN-28 cells (D). To detect apoptotic morphology by staining with fluorescent DNA-binding dye, PI, cells were stained with PI after treatment with IC50 doses of urushiol. Untreated control of MKN-45 cells (E); 15 μg/ml urushiol-treated MKN-45 cells (F); untreated control of MKN-28 cells (G); 20 μg/ml urushiol-treated MKN-28 cells (H). Apoptotic cells with small, solid spheres of condensed DNA were shown in the panel F (arrows).
2. Urushiol induced apoptosis in MKN-45 cells
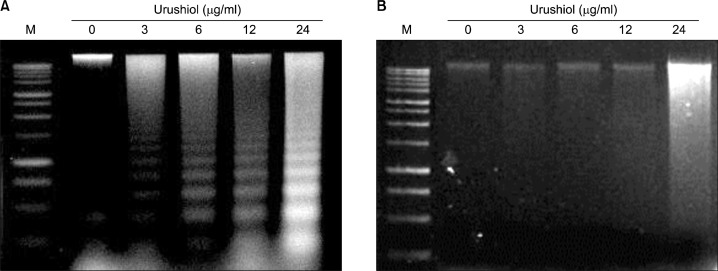
To identify a hallmark of apoptotic cell death, assay for the genomic DNA fragmentation was performed. Urushiol treatment invoked DNA ladder formation, indicating that urushiol induced apoptosis in MKN-45 cell. The formation of DNA ladder was increased concentration-dependently (Fig. 3A). However, urushiol did not mediate DNA ladder formation on MKN-28 cells (Fig. 3B).
Fig. 3.
DNA fragmentation induced by urushiol on MKN-45 cell lines. Effect of urushiol on induction of DNA fragmentation in MKN-45 and MKN-28 cells was observed. Cells were incubated with indicated concentration of urushiol for 48 h. Urushiol induced DNA fragmentation in MKN-45 cells in a dose-dependent manner (A). However, urushiol did not induced DNA fragmentation in MKN-28 cells (B).
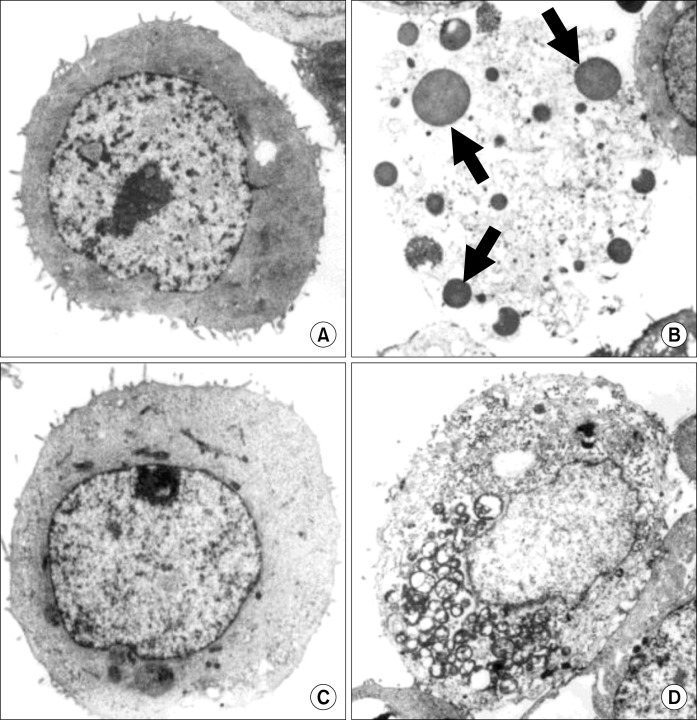
To confirm apoptotic cell death of MKN-45 by urushiol treatment, the cellular morphology was investigated via electron microscopy. The degeneration of apoptotic bodies observed on MKN-45 with the treatment of urushiol (Fig. 4A and B), whereas MKN-28 cells maintained intact cellular structure (Fig. 4C and D).
Fig. 4.
Electron microphotographs of MKN-45 and MKN-28 cells after treatment with urushiol. After treatment with urushiol morphological changes of MKN-45 and MKN-28 cells were observed by electron microscopy. Untreated control of MKN-45 cells (A); 15 μg/ml urushiol-treated MKN-45 cells (B); untreated control of MKN-28 cells (C); 20 μg/ml urushiol-treated MKN-28 cells (D). Multiple nuclear fragments (arrows) were observed on urushiol-treated MKN-45 cells (B).
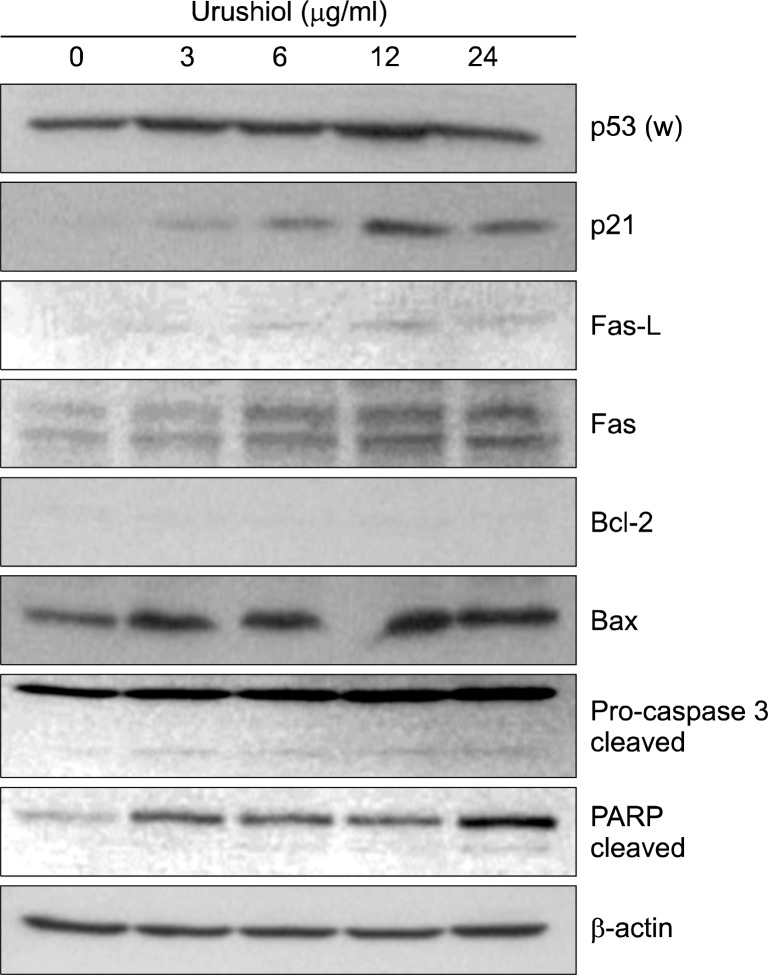
The p53 tumor suppressor gene is a cell cycle regulator and is able to induce cell cycle arrest to allow DNA repair or apoptosis.16 The product of p53 gene mediatesthe regulation of expression of apoptosis-related proteins and transactivates p21WAF1/CIP1, CDK inhibitor.17 The treatment with urushiol mediated up-regulation of p53 and p21WAF1/CIP1 expression on MKN-45 cell (Fig. 5). It was reported that Fas receptor and its ligand were involved in activation of apoptosis.18 Fas (48 kDa) and Fas-L (40 kDa) proteins were up-regulated on MKN-45 cells treated with urushiol (Fig. 5). Recently, it was reported that Bcl-2 family is a major regulator of apoptosis and Bcl-2/Bax ratio appears to be an important determinant in the regulation of apoptosis.19 To investigate the involvement of Bcl-2 family, the expression level of Bcl-2 and Bax was measured on MKN-45 and MKN-28 cells after treatment with urushiol. The expression level of Bcl-2 was very low and unchanged by urushiol on MKN-45 cells, whereas expressions of Bax were up-regulated by urushiol (Fig. 5). However, the expression of Bcl-2 was detectable without changing of expression by urushiol on MKN-28, whereas Bax expression was very low (data not shown).
Fig. 5.
Dose-dependent effect of urushiol on the levels of apoptosis-related proteins in MKN-45 cells. MKN-45 cells were treated with the indicated concentration of urushiol for 48 h. Total cell lysates were prepared and the indicated protein levels were determined by Western blotting. β-actin was used as an internal loading control. A representative blot is shown from three independent experiments. p53 (w), wild type p53.
Caspase-3 has known as executioner of apoptosis that mediated DNA fragmentation and cleavage of many cellular and nuclear proteins.20 To study the involvement of caspase-3, the activation of caspase-3 and PARP cleavage were observed on MKN-45 cells treated with urushiol. After treatment of urushiol the level of proenzyme form (32 kDa) of caspase-3 was increased and active form (19 kDa) of enzyme was also detected on MKN-45 cells (Fig. 5). poly(ADP-ribose) polymerase (PARP), one of well-known substrates of caspase-3, was cleaved on MKN-45 cell by urushiol treatment (Fig. 5).
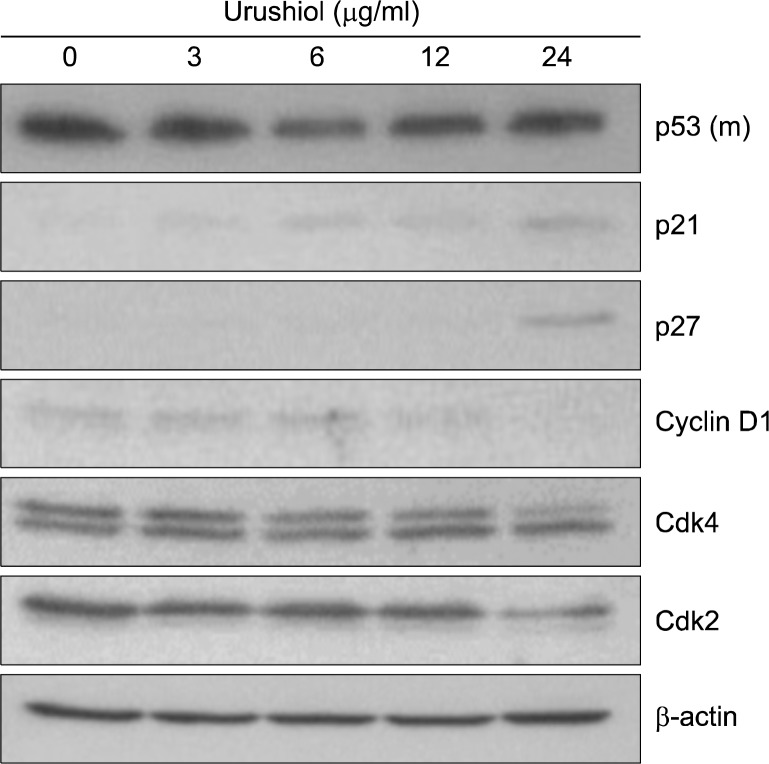
3. Urushiol mediated G1 arrest on MKN-28 cells
Although urushiol mediated apoptosis-associated changes on MKN-45 cell, it seemed that urushiol did not induce apoptosis on MKN-28 cells. DNA ladder formation and nuclear condensation were not observed after treatment with urushiol on MKN-28 cell (Fig. 3B, 4D). This implies that other mechanisms of growth inhibitory effect of urushiol on MKN-28 might be involved. To investigate the effects of urushiol on cell cycle, we studied expression of p21WAF1/CIP1 protein, a well-known inhibitor of cyclin dependent kinase. The expression level of p21WAF1/CIP1 protein was found to be increased by urushiol treatment on MKN-28 cell through a p53-independent manner (Fig. 6). Another inhibitor of cyclin dependent kinase, p27KIP1, was also up-regulated by urushiol on MKN-28 cell (Fig. 6). However, cell cycle regulatory proteins at G1 phase, cyclin D1, was decreased by urushiol treatment on MKN-28 cell (Fig. 6). Additionally, cyclin dependent kinase 4 and cyclin dependent kinase 2 were slightly decreased on MKN-28 cell by urushiol treatment (Fig. 6).
Fig. 6.
Dose-dependent effect of urushiol on the levels of cell cycle regulatory proteins in MKN-28 cells. MKN-28 cells were treated with the indicated concentration of urushiol for 48 h. Total cell lysates were prepared and the indicated protein levels were determined by Western blotting. β-actin was used as an internal loading control. A representative blot is shown from three independent experiments. p53 (m), mutant type p53.
DISCUSSION
In the present study we observed urushiol inhibited the proliferation of human gastric cancer cell lines (MKN-45 and MKN-28). This is the first study to report cytotoxic effects of urushiol on human gastric cancer cells. Urushiol showed cytotoxic effects on the proliferation of human gastric cancer cell lines. Of note, although urushiol exerted cytotoxic effects on both MKN-45 and MKN-28 cell lines, the involved mechanisms of cell death seems to be different.
It has been reported that enhanced apoptosis is responsible for the effects of chemotherapy and for the regression of tumor.21 Moreover, the relationship between apoptosis and the chemosensitivity of cancer cells has been analyzed extensively. From recent studies, it was suggested that the wild-type p53 protein could channel DNA-damaged cells toward apoptosis, while the product of the bcl-2 gene might protect cells from apoptosis.22 Apoptosis was more frequently observed in cells with a wild-type gene for p53 in which the Bcl-2 protein was not expressed than in cells with a mutated gene for p53 or cells containing a detectable level of the Bcl-2 protein after treatment with cisplatin.13 In line with aforementioned study, urushiol mediated up-regulation of p53 on MKN-45 cells (Fig. 5). Moreover, expression of Bax was increased on MKN-45 cells by urushiol treatment without detectable expression of Bcl-2 (Fig. 5). It was well known concept that Bcl-2/Bax ratio appears to be an important determinant in the regulation of apoptosis.19 Decreased expression ratio of Bcl-2/Bax proteins in MKN-45 cells seems to play a role being more sensitive to the cytotoxic effect of urushiol when compared to MKN-28 (Fig. 5).
The binding of agonistic anti-Fas antibody of Fas ligand to Fas receptor result in transduction of a cytolytic signal to the cell, followed by apoptosis.18 This was well examined in a variety of leukemia/lymphoma cell lines in vitro.23 It was also reported that anti-Fas antibody mediated apoptosis on MKN-45 cells.24 Increased levels of Fas and Fas ligand proteins in urushiol-treated MKN-45 cells could possibly lead to induction of apoptosis (Fig. 5).
The activation of caspase-3 and the cleavage of PARP have used as early markers of apoptosis.20 After treatment of urushiol, activation of caspase-3 and concomitant PARP cleavage were observed in MKN-45 cells (Fig. 5). Fragmentation of cellular DNA at the internucleosomal linker regions has been observed in cells undergoing apoptosis induced by a variety of agents. This cleavage produces ladders of DNA fragments that are the sizes of integer multiples of a nucleosome length (180–200 bp).25 Urushiol induced DNA ladder formation on MKN-45 cells with nuclear condensation (Fig. 3A, 4B). However, it was not observed that the DNA fragmentation (Fig. 3B) and nuclear condensation (Fig. 4D) in MKN-28 cell by urushiol treatment. To investigate the morphological changes induced by urushiol treatment, the total cellular morphology was examined under electron microscopy. Urushiol induced significant morphological changes indicating apoptosis. The chromatin was condensed and became segregated into sharply delineated masses. Moreover, the cytoplasmic condensation and segregation also observed on urushiol-treated MKN-45 cells (Fig. 2F, 4A).
The results presented here demonstrated that the treatment with urushiol on MKN-45 cells induced apoptosis, and this was closely linked with the Fas/Fas ligand signal, p53 induction, decreased Bcl-2/Bax ratio, caspase-3 activation, and DNA ladder formation. The mechanisms of action of urushiol have not been fully known to date. It is possible that urushiol directly binds to Fas receptor, and induces apoptosis through caspase activation. In addition, increased Bax protein mediates caspase activation by urushiol, followed by up-regulation of p53 protein. In contrast to MKN-45 cells showed several apoptosis-associated signals by urushiol treatment, urushiol seemed to mediate cell cycle arrest at G1 phase on MKN-28 cells. CDK inhibitor proteins p21WAF1/CIP1 and p27KIP1 were up-regulated on MKN-28 cells by urushiol treatment (Fig. 6). Moreover, cyclin D1, CDK4, and CDK2, which are associated with the cell-cycle transition from G1 to S phase, were decreased on MKN-28 by urushiol treatment (Fig. 6).
In conclusion, these data suggest that urushiol induced apoptosis in MKN-45 cells and mediated cell cycle arrest in MKN-28 cells at G1 phase through different pathway. Understanding such mechanisms of cell death and cell cycle arrest may allow us to enhance apoptotic responses in human gastric cancer cells for tumor regression. Thus, urushiol could be used as a candidate for developing chemotherapeutic agent in human gastric cancer cells.
Acknowledgments
This work was supported by a 2-Year Research Grant of Pusan National University.
REFERENCES
- 1.Symes WF, Dawson CH. Poison ivy “urushiol”. J Am Chem Soc. 1954;76:2959–63. [Google Scholar]
- 2.Markiewitz KH, Dawson CR. On the isolation of the allergenically active components of the toxic principle of poison ivy. J Org Chem. 1965;30:1610–3. doi: 10.1021/jo01016a067. [DOI] [PubMed] [Google Scholar]
- 3.Corbett MD, Billets S. Characterization of poison oak urushiol. J Pharm Sci. 1975;64:1715–8. doi: 10.1002/jps.2600641032. [DOI] [PubMed] [Google Scholar]
- 4.Elsohly MA, Turner CE. GLC analysis of poison ivy and poison oak urushiol components in vegetable oil preparations. J Pharm Sci. 1980;69:587–9. doi: 10.1002/jps.2600690530. [DOI] [PubMed] [Google Scholar]
- 5.Niimura N, Miyakoshi T. Structural study of oriental lacquer films during the hardening process. Talanta. 2006;70:146–52. doi: 10.1016/j.talanta.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Llanchezhian R, Joseph CR, Rabinarayan A. Urushiol-induced contact dermatitis caused during Shodhana (purificatory measures) of Bhallataka (Semecarpus anacardium Linn.) fruit. Ayu. 2012;33:270–3. doi: 10.4103/0974-8520.105250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong DH, Han SB, Lee CW, Park SH, Jeon YJ, Kim MJ, et al. Cytotoxicity of urushiols isolated from sap of Korean lacquer tree (Rhus vernicifera Stokes) Arch Pharm Res. 1999;22:638–41. doi: 10.1007/BF02975339. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Jeong SH, Hossain MA, Kim MY, Kim DH, Kim JA, et al. Urushiol induces growth inhibition and apoptosis in MCF-7 human breast cancer cells. Cancer Pre Res. 2009;14:309–14. [Google Scholar]
- 9.Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437–47. doi: 10.6004/jnccn.2010.0033. [DOI] [PubMed] [Google Scholar]
- 10.Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32:329–47. [PubMed] [Google Scholar]
- 11.Adachi Y, Mori M, Maehara Y, Sugimachi K. Poorly differentiated medullary carcinoma of the stomach. Cancer. 1992;70:1462–6. doi: 10.1002/1097-0142(19920915)70:6<1462::aid-cncr2820700603>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Motoyama T, Hojo H, Watanabe H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn. 1986;36:65–83. doi: 10.1111/j.1440-1827.1986.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 13.Ikeguchi M, Tatebe S, Kaibara N, Ito H. Changes in levels of expression of p53 and the product of the bcl-2 in lines of gastric cancer cells during cisplatin-induced apoptosis. EurSurg Res. 1997;29:396–402. doi: 10.1159/000129549. [DOI] [PubMed] [Google Scholar]
- 14.Tada H, Shiho O, Kuroshima K, Koyoma M, Tsukamoto K. An improved colorimetric assay for interleukin-2. J Immunol Methods. 1986;93:157–65. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Park SE, Hossain MA, Kim MY, Kim MN, Chung HY, et al. 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether induces growth inhibition and apoptosis in MCF-7 human breast cancer cells. Arch Pharm Res. 2007;30:1132–7. doi: 10.1007/BF02980248. [DOI] [PubMed] [Google Scholar]
- 16.Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–6. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 17.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 18.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 19.Wyllie AH. The genetic regulation of apoptosis. Curr Opin Genet Dev. 1995;5:97–104. doi: 10.1016/s0959-437x(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 20.Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–9. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 21.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Zhan Q, Fan S, Bae I, Guillouf C, Liebermann DA, O’Connor PM, et al. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 1994;9:3743–51. [PubMed] [Google Scholar]
- 23.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, et al. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–8. [PubMed] [Google Scholar]
- 24.Hayashi H, Tatebe S, Osaki M, Goto A, Suzuki Y, Ito H. Expression of Fas antigen and its mediation of apoptosis in human gastric cancer cell lines. Jpn J Cancer Res. 1997;88:49–55. doi: 10.1111/j.1349-7006.1997.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiarugi V, Magnelli L, Cinelli M, Basi G. Apoptosis and the cell cycle. Cell Mol Biol Res. 1994;40:603–12. [PubMed] [Google Scholar]