Abstract
Background:
Rg3, a major ginsenoside derived from heat-processed ginseng, has been reported to have anti-inflammatory and anti-proliferative activities. In our previous studies, Rg3 inhibited phorbol ester-induced cyclooxygenase-2 expression and NF-κB activation in cultured human mammary epithelial (MCF-10A) cells and in mouse skin in vivo. In this study, we investigated Rg3-induced apoptosis in human breast cancer (MDA-MB-231) cells and underlying molecular mechanisms.
Methods:
After Rg3 treatment, apoptotic cell death of MDA-MB-231 cell was investigated by the MTT reduction assay and measurement of the mitochondrial membrane depolarization. Flow cytometry was used for cell cycle analysis and detection of apoptotic cells as well as measurement of reactive oxygen species. Expression of apoptotic-related proteins was determined by immunoblot analysis.
Results:
MDA-MB-231 cells treated with Rg3 (30μM) exhibited the increased proportion of hypodiploid or apoptotic cells. Rg3 treatment resulted in an increase in the ratio of proapoptotic Bax to antiapoptotic Bcl-2, depolarization of the mitochondria membrane potential and the release of cytochrome c from mitochondria. Rg3 also induced the proteolytic cleavage of caspase-3 and poly (ADP-ribose) polymerase, which was attenuated by a caspase-3 inhibitor, z-VAD-fmk.
Conclusions:
Based on these findings, it is likely that Rg3 induces apoptosis in MDA-MB-231 cells via classical mitochondria-dependent caspase activation. These data suggest that Rg3 might be a potential candidate as a breast cancer chemopreventive agent.
Keywords: Ginsenoside, Rg3, Apoptosis, Breast cancer, MDA-MB-231 cells
INTRODUCTION
A large number of phytochemicals present in fruits and vegetables or in medicinal plants have been found to retain potential cancer chemopreventive activities.1–4 Ginseng has long been used as a herbal drug in traditional oriental medicine and is now used extensively as a general health supplement. According to an epidemiological study conducted in Korea, chronic intake of ginseng has been associated with a decreased incidence of cancers, such as esophageal, gastric, colorectal, and pulmonary tumors.5–7 Components that have been isolated and characterized from ginseng include ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids.8 Among these, ginsenosides are major pharmacologically active ingredients that have been shown to have anti-oxidation, anti-inflammation, and anti-carcinogenic activities.9,10 It has been reported that heat-treatment of ginseng potentiates its biological activity, and Rg3 is one of the major ingredients in heat-processed ginseng.11,12
Generally, the growth rate of pre-neoplastic or malignant cells outpaces that of normal cells because of malfunctioning or deregulation of their cell-growth and cell-death machineries.13 The normal cell function and tissue homeostasis are maintained by a balance between proliferation and apoptosis. Cancer is a typical disorder in which clones of malignant cells escape such balance and proliferate inappropriately without compensatory apoptosis.14 The success of cancer therapies greatly relies on the extent to which they preferentially induce tumor cell death while allowing survival of normal cells. In this context, induction of apoptosis is recognized as a rational approach to eliminate genetically damaged or pre-neoplastic cells before any malignancy manifests.
Our previous studies have demonstrated that the methanol extract of the heat-processed Panax ginseng C.A. Meyer is capable of inhibiting lipid peroxidation in rat brain homogenates and also scavenging superoxide anion generated by xanthine/xanthine oxidase or 12-O-tetrade-canoylphorbol-13-acetate (TPA) in cultured human pro-myelocytic leukemia (HL-60) cells.15 Ginsenoside Rg3, one of the major ingredients of heat-processed ginseng, also inhibited TPA-induced COX-2 expression and NF-κB activation in both mouse skin9 and cultured human mammary epithelial (MCF-10A) cells.16 In the present study, we have investigated the effects of Rg3 on growth and proliferation of MDA-MB-231 cells.
MATERIALS AND METHODS
1. Materials
Rg3 was prepared and purified as described previously.11 N-Acetyl-L-cysteine (NAC), trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY, USA). Antibodies against caspase-3, poly(ADP)ribosylpolymerase (PARP), cleaved PARP, Bcl-2, and Bax were purchased from Cell Signaling Technology (Beverly, MA, USA). Secondary antibodies for Western blotting were obtained from Zymed Laboratories Inc. (San Francisco, CA, USA). 2,7-Dichlorofluorescein diacetate (DCF-DA) and propidium iodide were purchased from Invitrogen (Carlsbad, CA, USA). Bicinchoninic acid (BCA) protein assay reagent was a product of Pierce Biotechnology (Rockfold, IL, USA). Polyvinylidene difluoride membranes were supplied from Gelman Laboratory (Ann Arbor, MI, USA).
2. Cell culture
MDA-MB-231 cells were grown at 37°C in DMEM supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere of 5% CO2 and 95% air. Cells were plated at an appropriate density according to the scale of each experiment, and the medium was changed at 2-day intervals.
3. Measurement of cell viability
Cell viability was determined by employing the conventional MTT reduction assay. After incubation with Rg3, cells were treated additionally with MTT solution (0.5 mg/ml final concentration) for 2 h at 37°C. Viable cells convert the internalized MTT to insoluble blue formazan crystals by reduction catalyzed by mitochondrial dehydrogenase (e.g., succinate dehydrogenase). The dark blue formazan crystals formed within mitochondria of surviving cells were dissolved by addition of DMSO, and the absorbance of blue color was measured using a microplate reader set at 570 nm (Molecular Devices, Sunnylvale, CA, USA). Cell viability was expressed as the percentage of MTT reduction obtained in the Rg3-treated cells, assuming the absorbance of control cells was 100%.
4. Determination of apoptotic cells (cell cycle analysis)
MDA-MB-231 cells (2×105 cells/well) were seeded onto 6 well plates and treated with the test drugs for the indicated times. Harvested cells were fixed overnight in 70% ethanol at 4°C, then collected by centrifugation and resuspended in phosphate-buffered saline (PBS) containing 25 μg/ml RNase and 0.5% Triton-X100 and incubated for 1 h at 37°C. Finally, cells were stained with 50 μg/ml propidium iodide for 15 min at 4°C in the dark. The relative DNA content of these cells were analyzed by a flow cytometer (Beckman Coulter, EPICS XL-MCL) based on red fluorescence. Ten thousand cells were counted and the histogram of the cell cycle distribution was analyzed by WinMDI 2.8 software. The percentage of cells with hypodiploid DNA content (sub-G1) represented fraction undergoing apoptotic DNA fragmentation.
5. Detection of apoptosis by annexin V/PI staining
MDA-MB-231 cells treated with Rg3 for 24 h and then stained with annexin V conjugated to FITC (fluorescein isothiocyanate) and propidium iodide with an annexin V-FITC apoptosis detection kit (BD Biosciences) according to the manufacturer’s protocol. Briefly, cells were suspended in 100 μl of annexin V binding buffer and incubated with 1μl of annexin V and 10 μl of propidium iodide for 15 min in the dark. Annexin V binding buffer (400 μl) was then added and cells were counted by flow cytometry. The percentage of cells undergoing apoptosis was assessed via dual-colour analysis.
6. Measurement of mitochondrial transmembrane potential (ΔΨm)
To measure the Δψm, we used the lipophilic cationic probe TMRE. After treatment with Rg3 for 24 h, cells (1×105 cells / 800 μl in four-well chamber slides) were rinsed with phosphate-buffered saline (PBS), and TMRE (150 nM) was loaded. After 30 minutes’ incubation at 37°C, cells were examined under a confocal microscope with excitation at 544 nm and emission at 590 nm (Leica Microsystems Heidelberg GmbH, Heidelberg, Germany). TMRE rapidly equilibrated between cellular compartments due to differential membrane potential, and a decrease in fluorescence indicated reduced Δψm.
7. Measurement of intracellular reactive oxygen species (ROS) accumulation
To monitor the intracellular accumulation of ROS, the fluorescent probe DCF-DA was used. Following treatment, cells were rinsed with Kreb’s ringer solution and 10 μM DCF-DA was loaded. After 15 min incubation at 37°C, cells were examined under a confocal microscope with the fluorescence excitation at 488 nm and emission at 530 nm (Leica Microsystems Heidelberg GmbH, Heidelberg, Ger-many). Moreover, fluorescence of oxidized DCF was measured at an excitation wavelength of 480 nm and an emission wavelength of 525 nm using a flow cytometer.
8. Western blot analysis
MDA-MB-231 cells were lysed in RIPA lysis buffer [150 mM NaCl, 0.5% TritonX100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 20 mM EDTA, 1 mM dithiothreitol (DTT), 1 mM Na3VO4, and protease inhibitor] for 30 min at 0°C followed by centrifugation at 12,000×g for 15 min. The protein concentration of the supernatant was measured by using the BCA reagent (Pierce, Rockfold, IL, USA). Protein (40 μg) was separated by running through 8∼15% SDS-PAGE gel and transferred to the PVDF membrane (Gelman Laboratory, Ann Arbor, MI, USA). The blots were blocked with 5% non-fat dry milk-PBST buffer [PBS containing 0.1% Tween-20] for 1 h at room temperature. The membranes were incubated for 2 h at room temperature with 1 : 1000 dilution of the antibodies of Bcl-2, Bax, caspase-3, and PARP (Cell Signaling Technology, Beverly, Massachusetts, USA). Equal lane loading was ensured by using anti-actin (Sigma Chemical Co., St. Louis, MO, USA). The blots were rinsed three times with PBST buffer for 10 min each. Washed blots were treated with 1 : 5000 dilution of the horseradish peroxidase conjugated-secondary antibody for 1 h and washed again three times with PBST buffer. The transferred proteins were visualized with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK).
RESULTS
1. Pro-apoptotic effect of Rg3 in MDA-MB-231 cells
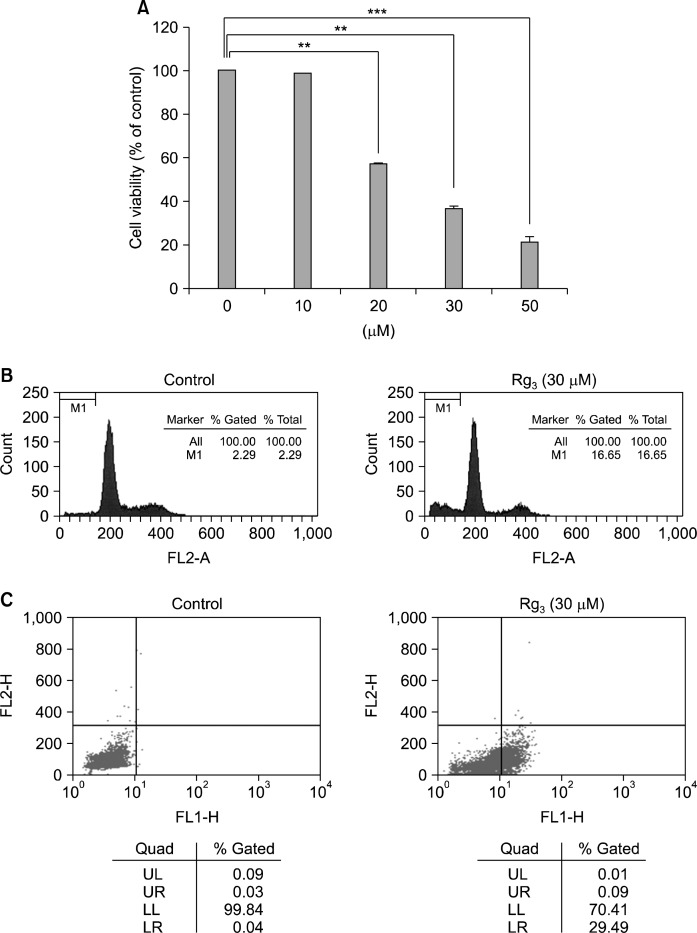
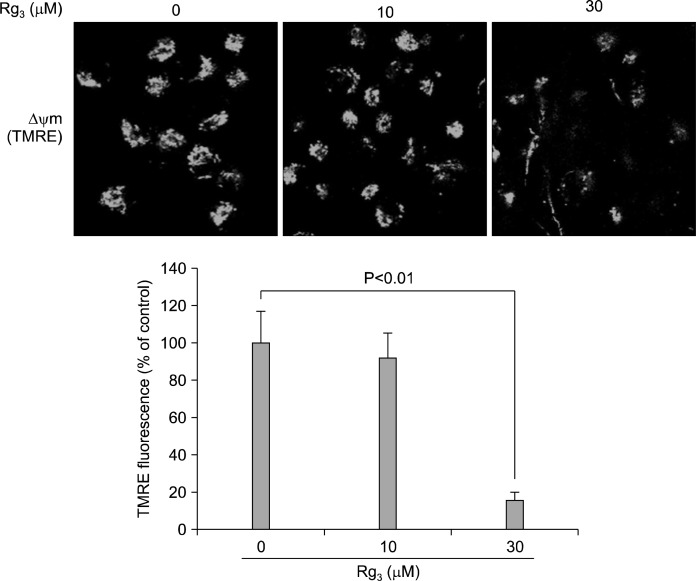
When MDA-MB-231 cells were treated with Rg3 for 24 h, the cell viability declined in a concentration-dependent manner (Fig. 1A). For quantitative assessment of the induction of apoptosis by Rg3, we measured the proportion of cells in the sub-G1 phase by flow cytometric analysis. As shown in Fig. 1B, treatment of the cells with 30 μM Rg3 increased the sub-G1 DNA content. To further assess apoptosis, Annexin V-FITC dye was utilized to detect the translocation of phosphatidylserine from the inner (cytoplasmic) leaflet of the plasma membrane to the cell surface. Rg3 (30 μM) treatment induced 29.49% of apoptotic cells at 24 h (Fig. 1C). Mitochondrial dysfunction is recognized as a critical event in apoptosis. Mitochondria undergo major changes in membrane integrity before classical signs of cell death become manifest. Such abnormalities occur in both the inner and the outer mitochondrial membranes, leading to the dissipation of the transmembrane potential and permeability changes which eventually release soluble intermembrane proteins, such as cytochrome c, through the outer membrane.17 When MDA-MB-231 cells were treated with Rg3 for 24 h, the mitochondrial transmembrane potential was rapidly reduced, as shown by the decrease in red fluorescence using a voltage-sensitive dye, TMRE (Fig. 2).
Fig. 1.
Rg3 induces apoptotic cell death in MDA-MB-231 cells. (A) Cells were treated with Rg3 for 24 h, and cell viability was determined by the MTT reduction assay. The results are presented as mean±SD (n=3). **P<0.01; ***P <0.001. Proportions of cells in the sub-G1 phase (B) and undergoing apoptosis (C), following Rg3 treatment for 24 h, were determined by flow cytometry.
Fig. 2.
Measurement of the mitochondrial transmembrane potential (Δψm) using a voltage-sensitive dye, TMRE. When MDA-MB-231 cells were treated with Rg3 (10 and 30 μM) for 24 h, the mitochondrial transmembrane potential was reduced, as revealed by the decrease in red fluorescence. Values represent mean±SD (n=3).
2. Effects of Rg3 on expression of apoptosis regulatory molecules
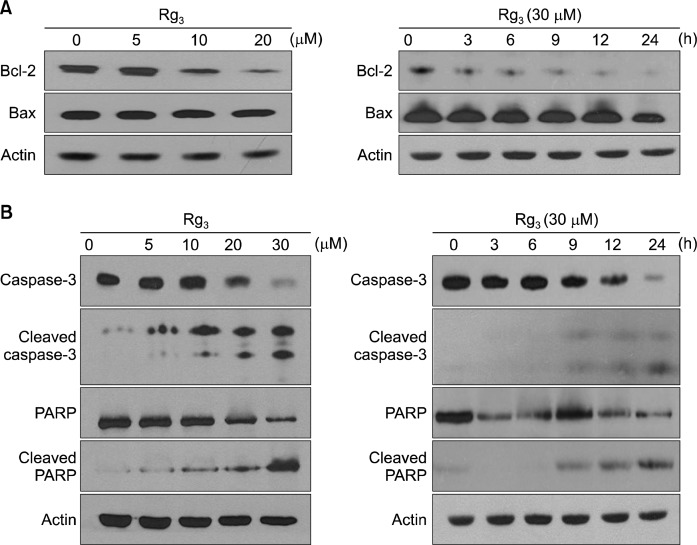
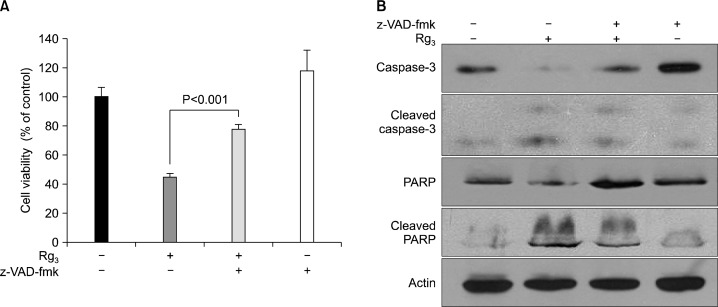
To elucidate the mechanisms underlying the pro-apoptotic effects of Rg3, the expression of several major apoptosis regulators was measured by Western blot analysis. The ratio of pro-apoptotic Bax and the anti-apoptotic Bcl-2 is considered as a molecular rheostat determining cell survival/death.18 As shown in Fig. 3A, Rg3 treatment led to a concomitant decrease in the level of Bcl-2, whereas the expression of Bax remained unchanged. Caspases are important mediators of apoptosis and contribute to the overall apoptotic morphology by cleavage of various cellular substrates. PARP, a known caspase substrate, is a 116-kDa nuclear protein that is specifically cleaved by active caspase-3 into an 85-kDa apoptotic fragment.19 When MDA-MB-231 cells were treated with Rg3 for 24 h, the cleavage of both caspase-3 and PARP increased (Fig. 3B). However, when co-treated with z-VAD-fmk, the caspase-3 inhibitor, cytotoxicity (Fig. 4A) and cleavage of caspase-3 and PARP induced by Rg3 (Fig. 4B) were abrogated.
Fig. 3.
Effects of Rg3 on the expression of the apoptotic markers. MDA-MB-231 cells were treated with Rg3 (5, 10, 20, 30 μM) for 24 h (left panel). MDA-MB-231 cells were treated with 30 μM of Rg3 for the time indicated (right panel). The levels of Bcl-2 and Bax (A) as well as caspase-3, cleaved caspase-3, PARP, and cleaved PARP (B) were determined by Western blot analysis.
Fig. 4.
The protective effects of z-VAD-fmk, the caspase-3 inhibitor, on the Rg3-induced apoptosis. MDA-MB-231 cells were exposed to Rg3 (30 μM) alone or in combination with z-VAD-fmk (25 μM) for 24 h, and cell viability (A) and the expression levels of apoptosis markers (B) were assessed by the MTT reduction assay and Western blot, respectively.
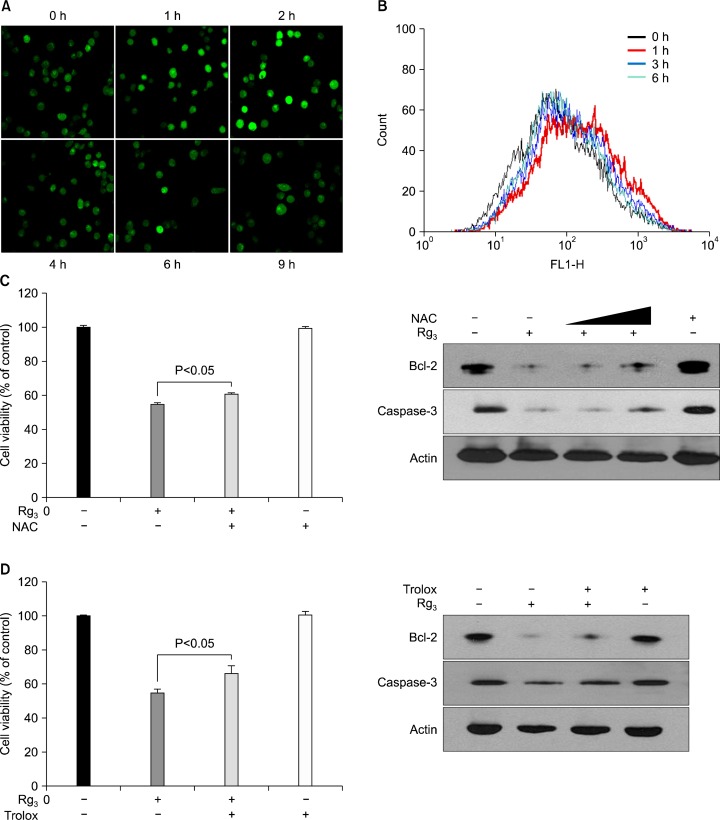
3. Role of reactive oxygen species (ROS) in Rg3-induced apoptosis
Dysregulation of cellular redox status can be a potent mechanism of cell death. Several studies have implicated ROS generation as a plausible mechanism for induction of apoptosis by various anticancer agents.20 Therefore, we tested the possible involvement of ROS in Rg3-induced apoptosis. Fluorescent microscopy and fluorescence-activated cell sorting (FACS) increased generation of ROS in MDA-MB-231 cells following treatment with Rg3 for 2 to 3 h (Fig. 5A and B). To determine whether Rg3-induced ROS accumulation could mediate induction of apoptosis, MDA-MB-231 cells were treated with NAC and trolox which are prototype ROS scavengers. Inhibition of Bcl-2 expression and cleavage of caspase-3 as well as cell viability by Rg3 treatment were only partially blocked when NAC or trolox was added to the medium (Fig. 5C and D).
Fig. 5.
Rg3-indeced ROS accumulation in MDA-MB-231 cells. The intracellular ROS level in Rg3-treated MDA-MB-231 cells was determined based in the DCF-DA fluorescence. Cells were exposed to 20 μM Rg3 for indicated times. (A) Images of the cellular fluorescence were acquired by using a confocal laser-scanning microscope. (B) ROS production was determined by flow cytometric analysis. MDA-MB-231 cells were exposed to Rg3 with NAC (1 mM) (C) or trolox (0.1 mM) (D) for 24 h, and cell viability and expression of apoptosis markers were determined.
DISCUSSION
Ginseng is a valuable agricultural commodity grown for use in many traditional medicinal therapies. More contemporary utilization of ginseng includes formulations prepared for herbal supplements or in functional foods; certain ginsenosides also have therapeutic potential and may be utilized for drug development.21 Ginsenosides possess a common triterpene structure in the backbone and have a structural diversity. So far, about 35 kinds of ginsenosides have been isolated from fresh, white or red ginseng, among which 22 kinds of ginsenosides are the protopanaxadiol type and 12 of them are the protopanaxatriol type, and only one ginsenoside Ro is an oleanane type.8 Although the extracts from fresh or processed P. ginseng have been extensively tested for their pharmacological effects, the precise biological functions and underlying action mechanisms of pure molecules derived from ginseng are still largely unknown.22 It has been reported that heat-treatment of ginseng enhances its biological activity.11,12 Rg3, one of the main ingredients of heat-processed ginseng, has two types of isomers, depending on the stereochemistry at the C-20 atom.
In the present study, we investigated the proapoptotic effects of Rg3, one of the major ingredients of heat-processed ginseng, in MDA-MB-231 cells. Caspases are recognized as important mediators of apoptosis. Once the caspases become active, they exert the apoptotic effects in part by autoactivation, thereby cleaving substrates, such as another caspase or PARP, that are critical for cell survival.23,24 For Rg3-induced apoptosis of MDA-MB-231 cells, it is likely that caspase-3 is activated, which results in the degradation of the DNA repair enzyme PARP. The ratio of pro-apoptotic Bax and the anti-apoptotic Bcl-2 is considered a molecular rheostat determining cell survival/death.25 In our study, the ratio of pro-apoptotic Bax and the anti-apoptotic Bcl-2 was decreased in MDA-MB-231 cells treated with Rg3.
Moderate amounts of ROS can act as mediators of intracellular signaling cascades. However, excessive production of ROS can cause oxidative stress, loss of cell function, and ultimately apoptosis or necrosis. Our results indicate that treatment of MDA-MB-231 cells with Rg3 led to a transient increase of intracellular ROS, which was followed by a reduced ratio of Bcl-2/Bax expression, disruption of the mitochondrial transmembrane potential leading to the activation of the caspase 9/3 cascade. We intended to see whether ROS generation is an upstream event of Rg3-induced mitochondrial membrane damage and apoptosis in our model. Our studies revealed that co-treatment of NAC or trolox resulted in only marginal protection against Rg3-induced apoptosis in MDA-MB-231 cells. These findings imply that ROS-induced oxidative stress does not appear to be a major mechanism underlying Rg3-induced apoptosis of MDA-MB-231 cells.
In conclusion, Rg3 induced apoptosis in human breast cancer (MDA-MB-231) cells, which is mediated by the activation of the mitochondrial death pathway.
Acknowledgments
This work was supported by the grant from Korea Tobacco & Ginseng Corporation through the Korean Society of Ginseng Science.
REFERENCES
- 1.Kim MJ, Kim DH, Na HK, Oh TY, Shin CY, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS) cells. J Environ Pathol Toxicol Oncol. 2005;24:261–9. doi: 10.1615/jenvironpatholtoxicoloncol.v24.i4.30. [DOI] [PubMed] [Google Scholar]
- 2.Manson MM. Cancer prevention-the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9:11–8. doi: 10.1016/s1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 3.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 4.Wolf CR. Chemoprevention: increased potential to bear fruit. Proc Natl Acad Sci U S A. 2001;98:2941–43. doi: 10.1073/pnas.071042698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin HR, Kim JY, Yun TK, Morgan G, Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000;11:565–76. doi: 10.1023/a:1008980200583. [DOI] [PubMed] [Google Scholar]
- 6.Yun TK. Panax ginseng-a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 7.Yun TK, Choi SY, Yun HY. Epidemiological study on cancer prevention by ginseng: are all kinds of cancers preventable by ginseng? J Korean Med Sci. 2001;16(Suppl):S19–27. doi: 10.3346/jkms.2001.16.S.S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms S. Cancer prevention and therapeutics: panax ginseng. Altern Med Rev. 2004;9:259–74. [PubMed] [Google Scholar]
- 9.Keum YS, Han SS, Chun KS, Park KK, Park JH, Lee SK, et al. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat Res. 2003;523–524:75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 10.Nah SY, Park HJ, McCleskey EW. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc Natl Acad Sci U S A. 1995;92:8739–43. doi: 10.1073/pnas.92.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, et al. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–4. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 12.Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, et al. Red American ginseng: ginsenoside constituents and anti-proliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–74. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111:923–5. doi: 10.1016/s0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21:857–64. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 15.Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, et al. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–8. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 16.Surh YJ, Na HK, Lee JY, Keum YS. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl):S38–41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 18.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 19.Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998;273:15540–5. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, Nakazato T, Yamato K, Miyakawa Y, Yamada T, Hozumi N, et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071–8. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- 21.Popovich DG, Kitts DD. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry. 2004;65:337–44. doi: 10.1016/j.phytochem.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Min JK, Kim JH, Cho YL, Maeng YS, Lee SJ, Pyun BJ, et al. 20(S)-Ginsenoside Rg3 prevents endothelial cell apoptosis via inhibition of a mitochondrial caspase pathway. Biochem Biophys Res Commun. 2006;349:987–94. doi: 10.1016/j.bbrc.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 23.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 24.Thornberry NA. Caspases: a decade of death research. Cell Death Differ. 1999;6:1023–7. doi: 10.1038/sj.cdd.4400607. [DOI] [PubMed] [Google Scholar]
- 25.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–32. [PubMed] [Google Scholar]