Abstract
There have been several epidemiologic studies supporting the protective role of pregnancy, although the mechanism is not clear. High level of progesterone, which is crucial in maintaining pregnancy, has been supposed to be one of the causative factors. Progesterone is produced at the corpus luteum in the early pregnancy and the placenta in the late pregnancy period. In several experimental studies, progesterone was reported to induce apoptosis of ovarian cancer cells through intrinsic and extrinsic pathways. In addition, progesterone has been shown to exert its anticancer effect through genomic and non-genomic action. The objective of this review is to discuss the protective mechanism of pregnancy against ovarian cancer focusing on the steroid hormone, progesterone.
Keywords: Steroids, Hormone, Progesterone, Pregnancy, Ovarian neoplasm
INTRODUCTION
Hormone-dependent cancers, such as prostate, breast, uterine, and ovarian cancers, maintain the properties of their progenitor cells.1 In breast cancer, steroid hormones influence the generation of tumor. Estradiol and its metabolites contribute to development of breast cancer. Moreover, hormone replacement therapy with synthetic progesterone and estrogen alleviate the risk of breast cancer.2 On the other hand, hormonal effect is more complicated in ovarian cancer. Infertility is the risk factor of ovarian cancer. However, pregnancy, breast feeding, and oral contraceptive (OC) reduce the risk of ovarian cancer.3
Ovarian cancer is the seventh common gynecologic malignancies the U.S. The age-adjusted incidence rate of ovarian cancer was around 10 to 14 cases per 100,000. The overall 5-year survival rate of ovarian cancer reached 49.7% according to the FIGO 26th Annual Report.4 Almost 90% of ovarian malignancies are originated from the ovarian epithelium, the surface of the ovary.5
Previous researchers investigated that the associations between ovarian cancer and sex steroid hormones have come up to the following conclusions. First, estrogen takes part in malignant transformation of ovarian surface epithelial cells. In addition, estrogen stimulates ovarian tumor growth. Supplementation of the 17β-estradiol increased 4 fold of tumor growth in ovariectomized mice model. Furthermore, 17β-estradiol stimulated the migration potential of ovarian cancer cells.6 Second, progesterone may have a protective effect against ovarian cancer.7 Various theories have been suggested to support the relationship between progesterone and ovarian cancer. However, these processes are not clearly organized yet. The aim of this review is to clarify protective effect of progesterone and discuss the role of pregnancy against ovarian cancer.
STEROID HORMONE
1. Steroidogenesis
Cholesterol-derived steroid hormones are divided into glucocorticoids, mineralocorticoids, and sex steroids. Sex steroids are classified into three types of hormones, such as estrogens, progesterones, and androgens.8 Ovarian follicular steroidogenesis from steroid occurs in theca and granulosa cells under two gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH stimulates the growth of the ovarian preantral follicle. When LH binds to its receptor on the theca cell, cholesterol is converted to androgen, androstenedione, and testosterone by P450scc, P450c17, and 3β-hydroxysteroid dehydrogenase. In granulosa cells, androstenedione and testosterone are aromatized to estrone and estradiol, respectively. Progesterone is derived from pregnenolone through 3β-hydroxysteroid dehydrogenase. The active metabolites of progesterone are 17-hydroxyprogesterone, pregnanediol, and pregnanetriol.9
In non-pregnant women, progesterone is produced mostly from the ovarian corpus luteum and it is in small quantities from the adrenal glands. The peripheral convertsion rate is about 1.5% for androstenedione and 0.15% for testosterone. The production rate and concentration of progesterone is different according to menstrual phase. The production rate is less than 1 mg/day in preovulatory phase and 20 to 30 mg/day in luteal phase. The concentration of progesterone is less than 1 ng/ml in preovulatory phase and 3 to 15 ng/ml in luteal phase. However, progesterone can be converted peripherally only in pregnant women. Normal levels of progesterone range according to the period of pregnancy: 9 to 47 ng/ml in first trimester, 17 to 147 ng/ml in second trimester, 50 to 200 ng/ml in third trimester, respectively.10,11
2. Functions of steroid hormones
Estrogen receptor (ER) has two subtypes which are encoded by different genes: ERα and ERβ. Between the two isoforms, DNA binding domain is similar except the C-terminal ligand-binding domain (LBD) and the N-terminal transactivation domain (AF-1). ERα and ERβ showed different expressions in bone, placenta, prostate, and breast tissues. There are abundant expressions of ERα in the ovarian theca and interstitial cells, and ERβ in the ovarian granulosa cells.12–14 Whereas ERα enhances the growth of hormone-dependent cancer, ERβ is known to suppress tumorigenesis of breast, colon, and prostate cancer.5
Progesterone receptor (PR) also has two types of DNA-binding forms: PR-A and PR-B. Two isoforms are encoded from the different mRNA populations of single-copy progesterone receptor gene. Alternative translational initiation event with co-activators and co-repressors on the same gene results in two subtypes of PR. PR-A and PR-B have different target gene-specific transcriptional manners, especially in promoter context. PR-B protein has an additional domain, a third transactivation function (AF3). The coactivators which bind to AF3 differentiates the function of PR-B from PR-A.15 PR-B more actively inhibits transcription of ER than PR-A by competitive binding with critical transcription activators.12,13 Normal ovulation is sufficiently dependent on PR-A.16 The loss of heterogeneity of PR is involved in 75% of ovarian cancer.17
Steroid hormones simply diffuse across the cell membrane to bind the intracellular receptor protein. Steroid hormones act by binding to steroid hormone receptors which regulate gene expressions.18 The genomic biological activity of steroid hormones is dependent on the time and amount of exposure.19 The stability of steroid hormone receptor is controlled by steroid-dependent genomic actions. Tissue-specific genomic action is performed at the transcription level. In MCF-7 breast cancer cells, estrogen down regulated ERα mRNA via shortening half-life from 4 hours to 40 minutes.20 On the other hand, estrogen up regulated ERα mRNA via prolonging half-life from 9 hours to 24 hours in sheep endometrium.21 Preovulatory LH surge decreased the expression of ERβ mRNA in rat ovarian follicle.22 The activity of steroid receptor is also regulated at the protein level. Estradiol induced the degradation of ER protein in mammalian cells.23
In addition, steroid hormones have non-genomic actions with ubiquitous regulatory cascades. Steroids increases intracellular Ca2+ concentration with the G-protein coupled receptor and the ion channels on cell membrane.24,25 The function of steroid hormone is changed by phosphorylation of steroid receptors. The common sites for phosphorylation are the proteins of serine and tyrosine on ligand-binding domain of ER.26 Phosphorylation of Tyr537 and Ser167 helps to dimerize ER DNA binding.27 Mitogen-activated protein kinase, i.e. MAP kinase, phosphorylates the Ser294 site of PR, to down-regulate PR by accelerating degradation.28
STEROID HORMONE DURING PREGNANCY
1. Estrogen
The 19-carbon steroids make a major role to form the estrogen. However, the 21-carbon steroids don’t produce the 19-carbon steroids during pregnancy. There is low activity of steroids converting enzymes such as, 17α-hydroxylase and 17/20 desmolase (P450cc17) in human placenta. Estrogen is synthesized from the circulating precursors in maternal blood stream during pregnancy. Placental aromatase (P450arom) is in charge of producing estrogen during pregnancy.29
2. Production of progesterone
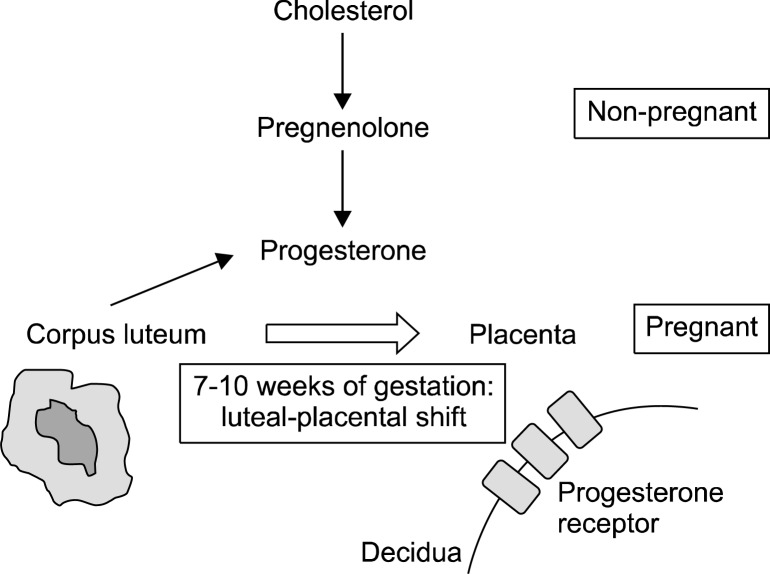
Progesterone is usually synthesized from the circulating cholesterol and pregnenolone. Although maternal circulating progesterone level is approximately 10 ng/ml in early pregnancy, it reaches from 100 to 200 ng/ml in term pregnancy.30 Until about the 10th weeks of gestation, progesterone is produced in the corpus luteum. The luteal-placental shift which is the transition of progesterone producing organ from the corpus luteal cyst to the placenta occurs between the 7th week and 10th week of pregnancy (Fig. 1).31
Fig. 1.
Production of progesterone during pregnancy. Production site of progesterone shifts from corpus luteum to placental decidua during 7–10 weeks of gestation. High level of progesterone is produced during pregnancy, especially late term.
Cortisol is derived from LDL-cholesterol in the fetal liver.32 Low-density lipoprotein (LDL) is the utilized form of cholesterol for the progesterone synthesis.33 Estrogen makes the fetal liver to produce circulating LDL-cholesterol.34 Estrogen stimulates the activity of placental P450scc enzyme which contributes to convert cholesterol to pregnenolone, the precursor for progesterone.35 Human deciduas and fetal membranes synthesize progesterone. Progesterone serves as the substrate for the synthesis of glucocorticoids and mineralocorticoids in the fetal adrenal gland.
Pattern of steroid hormone production of near-term pregnant women is different from that of non-pregnant women. In non-pregnant women, the production rates of progesterone, estriol, and 17β-estradiol is 1.0–40 mg/day, 0.02–0.1 mg/day, and 0.1–0.6 mg/day, respectively. Pregnant women have more amounts of steroids because of the faster production rate. Progesterone, estriol, and 17 β-estradiol are produced at 250–600 mg/day, 50–150 mg/day, and 15–20 mg/day in pregnancy. Whereas progesterone production rate is 250 mg/day in normal singleton pregnancy, it increases to about 600 mg/day in multi-fetal gestation.36 In normal intrauterine pregnancy, early-gestational serum progesterone level is higher than 25 ng/ml, while it is usually lower in ectopic pregnancy.37
3. Role of steroid hormone during pregnancy
Estrogen is closely related to fetal well-being. Declining estrogen level reflects fetal demise and anencephaly. Endogenous progesterone is the key steroid hormone maintaining pregnancy and keeping uterine quiescence. Progesterone level is usually maintained for several weeks after fetal demise. Progesterone withdrawal is the start of parturition. Even exogenous progestin supply after removal of the corpus luteum did not prevent miscarriage in many cases. Progesterone support has been suggested to be the emerging method to prevent preterm birth.38
OVARIAN CANCER AND PREGNANCY
1. Hypotheses for ovarian carcinogenesis
There are several classical hypotheses for ovarian carcinogenesis. Previous studies proposed five hypotheses to explain the etiology of ovarian cancer.39 The first theory is that certain sequelae after ovulation will be related to ovarian carcinogenesis. Potential carcinogenetic process includes repeated trauma and repair process to the ovarian epithelium. The more ovulation occurs, the more aberrant repair processes are induced.40 The damaged fallopian tubal epithelium following ovulation generates high grade serous ovarian cancer according to this theory.41 The exfoliation theory through incessant ovulation suggested that the risk of ovarian cancer depends on total number of ovulatory cycles in life.42 Therefore, ovulation reducing factors are known to be protective against ovarian cancer. In a case-control study, live birth reduced the ovarian cancer risk by 57%, 70%, and 82% in women with parity of 1–2, 3–5, and >5, respectively.43 Furthermore, a previous relevant study also showed strong protective effect with increasing parity. In this study, adjusted RR for ovarian cancer risk compared to women with only one child was 0.69 (95% CI, 0.52–0.90) for those with two children and 0.30 (95% CI, 0.21–0.42) for those with three or more. It is suggested that lower exposure to ovulation is the important factor to decrease the risk of ovarian cancer.44
The second idea is that the circulating levels of pituitary gonadotropins may increase the risk of ovarian malignancy. Pituitary gonadotropin stimulates ovarian stroma to produce estrogen or estrogen precursors. These estrogen products interfere inclusion cyst to disappear in ovarian surface epithelium.45 High gonadotropin help the ovarian stroma entrap the ovarian surface epithelium of the inclusion cyst, which results in carcinogenesis.46 Thus, pregnancy and OC which suppress the secretion of gonadotropins have protective effect against ovarian cancer.47 However, exogenous gonadotropin as a treatment of infertility was reported not to relate with ovarian tumorigenesis. In Danish population-based cohort study, use of gonadotropin did not increase the risk of ovarian cancer.48
The third hypothesis is that androgen stimulates ovarian surface epithelium to proliferate abnormally.49 The association between androgen and ovarian cancer was proved by a number of epidemiologic evidences. For example, a previous cohort study has reported that mean serum level of androstenedione and dehydroepiandrosterone (DHEA) in women with ovarian cancer was higher than that in normal women (4.5 nmol/L versus 3.3 nmol, P=0.03; 15.9 nmol/L versus 9.7 nmol/L, P=0.02, respectively).50
The fourth theory is that the inflammation of the pelvic cavity is involved in ovarian carcinogenesis. Talc use increased the incidence of ovarian cancer.51–54 Inflammation such as endometriosis and pelvic inflammatory disease stimulates ovarian carcinogenesis.47 In a relevant study, higher levels of inflammatory markers such as IL-2, IL-4, IL-6, IL-12, and IL-13 were associated with the increased risk of ovarian cancer (ORs, 1.57, 1.50, 1.63, 1.60, and 1.42; 95% CIs, 0.98–2.52, 0.95–2.38, 1.03–2.58, 1.02–2.51, and 0.90–2.26).55 Moreover, IL-1A and ALOX5 are also known as the rising factors of inherited inflammation related with ovarian carcinogenesis.56
The last theory is in regard to the ovarian stromal hyperactivity. Granulosa and theca cells produce ovarian steroid. Most of them undergo apoptosis after hormone production. Some steroid-producing cells remain in the ovarian stroma, and they still retain the ability to produce steroid.39 Sustained steroid production stimulates ovarian stroma to perform the ovarian tumors. Use of OCs reduces the ovarian hyperactivity by reducing cumulative quantity of ovulation.
2. Protective effect of pregnancy against ovarian cancer
Over 3 years-use of depot medroxyprogesterone acetate significantly reduced the ovarian cancer risk by 83%.57 Progesterone did not increase the number of ovarian cancer cells even though presenting with growth factors.58
Increasing parity is associated with decreased risk of ovarian cancer.59 One birth history had protective effect of 93% against ovarian cancer development compared to nulliparity, because hormonal change during pregnancy induces the apoptosis of ovarian surface epithelial cells.60 In a case-control study, infertility patients had 1.6 times of higher risk of ovarian cancer than fertility group.61 Increased risk of ovarian cancer in infertile population was confirmed by the US Nurses’ Health Study with 107,900 participants.62
Duration and completeness of pregnancy is also related with ovarian cancer risk. If active material is produced throughout pregnancy or during late-pregnancy, the gestational age will be proportional to ovarian cancer protection.63 In fact, number of incomplete pregnancies failed to show significant effect on ovarian cancer prevention in many studies.64 Even one more full-term pregnancy had 29% higher protective effect than incomplete pregnancy among primigravid women.65 Fewer cycles of ovulation was reported to decrease the risk of ovarian cancer.66 However, it is still unknown how many ovulation cycles are significant to inhibit ovarian tumorigenesis.
Maternal age is important to discuss the protective effect against ovarian cancer. The relevant studies insist that age at pregnancy is more important than number of completed pregnancies.67 There are many various results about age effect on ovarian cancer. Although age at first pregnancy after 35 years represented higher risk of ovarian cancer,68 age group under 19 years was more risky to develop ovarian cancer than that above 25 years.69 Furthermore, risk of ovarian cancer decreased with age at delivery in primiparous women. In multiparous women, age at last pregnancy was more important to decrease the risk of ovarian cancer. Longer interval between last delivery and diagnosis of ovarian cancer was likely to increase the risk of ovarian cancer, and decrease the protective effect of pregnancy.70 Thus, pregnancy was suggested to clear the malignant transformed cells from ovary.69 Some investigators had the opposite opinion to the birth recency for ovarian cancer. They insisted that pregnancy had long-lasting protective effect on ovarian carcinogenesis.71 Additional studies reported that period from first delivery to last delivery did not affect ovarian cancer risk.72
PROGESTERONE EFFECT DURING PREGNANCY AGAINST OVARIAN CANCER
1. Reduced exposure to ovulation
Ovulation is widely known as a predisposing factor in epithelial ovarian cancer. Some situations, such as late menarche, higher parity, longer duration of pregnancy, breastfeeding, and OC, that interrupt or postpone ovulation significantly reduce the risk of epithelial ovarian cancer. Because ovulation induces epithelial change, interruption of ovulation lowers the possibility of epithelial damage in ovary and fallopian tube.
Thus, frequent exposure to ovulation is a significant factor for protection against ovarian cancer. The time gap between ovulation age and protected time had positive correlation with ovarian cancer risk in matched case-control study including 150 patients with ovarian cancer. Ovulation age was defined as the period from menarche to the diagnosis of ovarian cancer or menopause in normal women. Protected time was decided as total duration of OC use and pregnancy in this study.73 Higher number of lifetime total ovulation number increased the risk of ovarian cancer in an Australian case-control study. The ovulation number per year increased the risk of ovarian cancer by 6 percent (95% CI: 4–8%).74 In a Mexican case-control study, ovarian cancer risk decreased 4 fold in women with ovulation-free period above 8.7 years than control group.75
2. Integrity of ovarian surface epithelium
Progesterone induces transcriptional change of lipid for biosynthesis and transport. These changes make high levels of intracellular cholesterol and fatty acid. Cell membranes are mainly composed of cholesterol and fatty acid. Thus, these lipid materials disturb the fluidity to maintain the integrity of ovarian surface epithelium.76 Progesterone inhibits ovarian tumorigenesis with disturbance of epithelial integrity.77
3. High levels of progesterone induces apoptosis of ovarian cancer cells
Progesterone had the protective effect against ovarian cancer. Apoptosis pathway which is induced p53 up-regulation is the main mechanism of progesterone-induced growth inhibition of ovarian cancer cells.78 Steroid receptors interact with deranged p53 and Ki-67 which were expressed in ovarian surface epithelium. High ERα expression lowered the level of apoptosis of ovarian cancer cells.79 On the other hand, ovulation is related with p53 gene mutation.80 Overexpression of p53 was involved in total number of ovulatory cycles in a lifetime. Ovulatory cycles make proliferation-associated DNA damage, especially in p53-positive ovarian cancer cells.81 When progesterone was added to ovarian cancer cells, p53 mRNA was transiently increased. Furthermore, progesterone encouraged p53 expression and induced apoptosis of ovarian cancer cell line.82 Progestin treatment was reported to cause apoptosis of ovarian cancer cells.83 In vivo study, progesterone also inhibited ovarian tumorigenesis in immune-deficient mice.84 Combination therapy with progestin and medroxyprogesterone acetate achieved to kill over 85% ovarian cancer cell lines.85
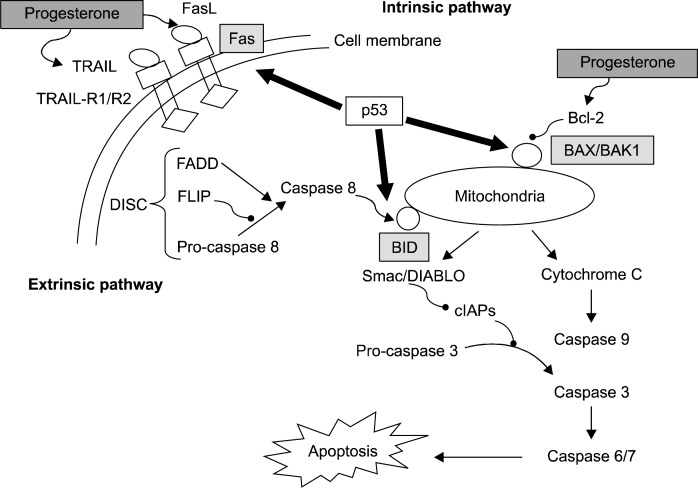
Progesterone induces receptor-mediated extrinsic apoptosis by activating caspase 8.86,87 Fas/FasL singnalling pathway mediates progesterone-induced apoptosis.86 Expression of FasL mRNA and protein were higher in ovarian cancer cell lines than in normal ovarian surface epithelium. Treatment with anti-FasL antibody and progesterone had synergistic effect to die ovarian cancer cells.85,86 In addition, Treatment with progesterone enhances apoptosis of ovarian cancer cells by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). On the contrary, progesterone was involved in mitochondria-related intrinsic apoptosis via Bcl-2 family. Bcl-2, as an anti-apoptotic protein, is down-regulated by progesterone.78,88,89 Furthermore, p53 execute the BID molecule to converge extrinsic and intrinsic pathway of apoptosis.90 Fig. 2 shows progesterone-related apoptotic pathway.
Fig. 2.
Progesterone functions via intrinsic (mitochondria-centered) and extrinsic (cell membrane-mediated) pathway of apoptosis. P53 is the main molecule regulating progesterone-related apoptosis.
Ovarian cancer cell lines are dependent on the concentration of progestin. Low concentrations of progesterone stimulated ovarian cancer cell proliferation with dose-dependent manner while high concentrations of progesterone inhibited it. Only 10−3 M of progesterone was more effective to reduce the density of ovarian cancer cells than 1 mg/ml of 5-FU. The effect of 1 mg/ml of 5-FU was same as that of 10−4 to 10−3 of mifepristone, a kind of progesterone receptor antagonist.91 Normal and malignant human ovarian surface epithelial cells proliferate following progesterone concentration with inverted U-shape dose-response curves. The proliferation rate of progesterone at control and high concentration was slow to be compared with low concentration group. Furthermore, the levels of progesterone which had anti-proliferative effects are acquired during pregnancy. In other words, induction of apoptosis occurs under high-progesterone as the equivalent level during pregnancy.
4. Progesterone as antioxidants
Ovulation induces oxidative damages to the ovarian epithelium, and this process contributes to ovarian carcinogenesis.92 Progesterone treatment inhibited reactive oxygen species (ROS) of ovarian cancer cell lines in time-dependent manner. ROS triggers up-regulation of p53 activation and down-regulation of anti-apoptotic BCL-2 gene expression.93 Progesterone carries out transcriptional regulation of myeloperoxidase and NADPH expression.94 NADPH, the main component of oxidation-reduction system, takes part in progesterone-related cytoprotection.95 Ovariectomy-induced recognitive dysfunction was recovered after long-term high dose progesterone therapy via its antioxidant effects in mice model.96
5. Progesterone level during pregnancy
Pregnancy is the only physiological condition to raise progesterone level above normal secretory phase. There is 10-fold increase in progesterone at term pregnancy compared with non-pregnant condition. Progesterone level during multifetal gestation was higher than that during singleton pregnancy. Women who had the history of twin birth showed low risk of ovarian cancer.70
CONCLUSION
Sex-steroid hormones, such as estrogen and progesterone, play important role in a female hormone-dependent epithelial ovarian cancer. Pregnancy is accepted as a protective factor against ovarian cancer. High parity, duration or completeness of pregnancy, and old birth age decrease ovarian cancer risk. Progesterone induces apoptosis of ovarian cancer cells under high concentration as an equivalent level at pregnancy. Because progesterone takes charge for the protection against ovarian cancer, adjuvant progesterone therapy is expected to enhance treatment outcome of ovarian cancer.
Acknowledgments
This research was supported by Basic Science Research Program (No. 2011-0025394), WCU (World Class University) program (No. R31-10056), and Priority Research Centers Program (No. 2009-0093820) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
REFERENCES
- 1.Huggins CB. The Hormone-dependent cancers. Bull N Y Acad Med. 1963;39:752–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Campagnoli C, Abba C, Ambroggio S, Peris C. Pregnancy, progesterone and progestins in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2005;97:441–50. doi: 10.1016/j.jsbmb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184–203. doi: 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 4.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 5.Haring J, Schuler S, Lattrich C, Ortmann O, Treeck O. Role of estrogen receptor beta in gynecological cancer. Gynecol Oncol. 2012;127:673–6. doi: 10.1016/j.ygyno.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Armaiz-Pena GN, Mangala LS, Spannuth WA, Lin YG, Jennings NB, Nick AM, et al. Estrous cycle modulates ovarian carcinoma growth. Clin Cancer Res. 2009;15:2971–8. doi: 10.1158/1078-0432.CCR-08-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer DW, Hutchison GB, Welch WR, Scully RE, Ryan KJ. Determinants of ovarian cancer risk. I. Reproductive experiences and family history. J Natl Cancer Inst. 1983;71:711–6. [PubMed] [Google Scholar]
- 8.Ryan KJ, Smith OW. Biogenesis of Steroid Hormones in the Human Ovary. Recent Prog Horm Res. 1965;21:367–409. [PubMed] [Google Scholar]
- 9.Erickson GF. Physiologic basis of ovulation induction. Semin Reprod Endocrinol. 1996;14:287–97. doi: 10.1055/s-2008-1067974. [DOI] [PubMed] [Google Scholar]
- 10.Baird D, Horton R, Longcope C, Tait JF. Steroid prehormones. Perspect Biol Med. 1968;11:384–421. doi: 10.1353/pbm.1968.0053. [DOI] [PubMed] [Google Scholar]
- 11.Baird DT, Horton R, Longcope C, Tait JF. Steroid dynamics under steady-state conditions. Recent Prog Horm Res. 1969;25:611–64. doi: 10.1016/b978-0-12-571125-8.50017-x. [DOI] [PubMed] [Google Scholar]
- 12.Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313. ; discussion 313–4. [PubMed] [Google Scholar]
- 13.Gronemeyer H, Meyer ME, Bocquel MT, Kastner P, Turcotte B, Chambon P. Progestin receptors: isoforms and anti-hormone action. J Steroid Biochem Mol Biol. 1991;40:271–8. doi: 10.1016/0960-0760(91)90192-8. [DOI] [PubMed] [Google Scholar]
- 14.Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–64. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 16.Conneely OM, Mulac-Jericevic B, Lydon JP. Progester-one-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–8. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 17.Peluso JJ. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med. 2007;25:198–207. doi: 10.1055/s-2007-973432. [DOI] [PubMed] [Google Scholar]
- 18.Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev. 1996;17:587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- 19.Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ. The limits of the cellular capacity to mediate an estrogen response. Mol Endocrinol. 1992;6:157–67. doi: 10.1210/mend.6.2.1569962. [DOI] [PubMed] [Google Scholar]
- 20.Saceda M, Lindsey RK, Solomon H, Angeloni SV, Martin MB. Estradiol regulates estrogen receptor mRNA stability. J Steroid Biochem Mol Biol. 1998;66:113–20. doi: 10.1016/s0960-0760(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 21.Ing NH, Ott TL. Estradiol up-regulates estrogen receptor-alpha messenger ribonucleic acid in sheep endometrium by increasing its stability. Biol Reprod. 1999;60:134–9. doi: 10.1095/biolreprod60.1.134. [DOI] [PubMed] [Google Scholar]
- 22.Guo C, Savage L, Sarge KD, Park-Sarge OK. Gonadotropins decrease estrogen receptor-beta messenger ribonucleic acid stability in rat granulosa cells. Endocrinology. 2001;142:2230–7. doi: 10.1210/endo.142.6.8102. [DOI] [PubMed] [Google Scholar]
- 23.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A. 1999;96:1858–62. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Mellay V, Grosse B, Lieberherr M. Phospholipase C beta and membrane action of calcitriol and estradiol. J Biol Chem. 1997;272:11902–7. doi: 10.1074/jbc.272.18.11902. [DOI] [PubMed] [Google Scholar]
- 25.Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. Eur J Endocrinol. 2003;148:281–92. doi: 10.1530/eje.0.1480281. [DOI] [PubMed] [Google Scholar]
- 26.Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319(Pt 3):657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold SF, Obourn JD, Yudt MR, Carter TH, Notides AC. In vivo and in vitro phosphorylation of the human estrogen receptor. J Steroid Biochem Mol Biol. 1995;52:159–71. doi: 10.1016/0960-0760(94)00166-j. [DOI] [PubMed] [Google Scholar]
- 28.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siiteri PK, Vande Wiele RL, Lieberman S. Occurrence of dehydroisoandrosterone glucuronoside in normal human urine. J Clin Endocrinol Metab. 1963;23:588–94. doi: 10.1210/jcem-23-6-588. [DOI] [PubMed] [Google Scholar]
- 30.Schneider MA, Davies MC, Honour JW. The timing of placental competence in pregnancy after oocyte donation. Fertil Steril. 1993;59:1059–64. doi: 10.1016/s0015-0282(16)55928-7. [DOI] [PubMed] [Google Scholar]
- 31.Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol. 1972;112:1061–7. doi: 10.1016/0002-9378(72)90181-0. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell BF, Challis JR, Lukash L. Progesterone synthesis by human amnion, chorion, and decidua at term. Am J Obstet Gynecol. 1987;157:349–53. doi: 10.1016/s0002-9378(87)80169-2. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev. 1990;11:124–50. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- 34.Carr BR, Simpson ER. Cholesterol synthesis by human fetal hepatocytes: effect of lipoproteins. Am J Obstet Gynecol. 1984;150:551–7. doi: 10.1016/s0002-9378(84)90438-1. [DOI] [PubMed] [Google Scholar]
- 35.Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16:608–48. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Williams obstetrics. New York: The McGraw-Hill Company; 2010. [Google Scholar]
- 37.Perkins SL, Al-Ramahi M, Claman P. Comparison of serum progesterone as an indicator of pregnancy nonviability in spontaneously pregnant emergency room and infertility clinic patient populations. Fertil Steril. 2000;73:499–504. doi: 10.1016/s0015-0282(99)00543-9. [DOI] [PubMed] [Google Scholar]
- 38.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Fetal Medicine Foundation Second Trimester Screening G. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 39.Modugno F. Ovarian cancer and high-risk women-implications for prevention, screening, and early detection. Gynecol Oncol. 2003;91:15–31. doi: 10.1016/s0090-8258(03)00254-3. [DOI] [PubMed] [Google Scholar]
- 40.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 41.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–42. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piek JM, van Diest PJ, Verheijen RH. Ovarian carcinogenesis: an alternative hypothesis. Adv Exp Med Biol. 2008;622:79–87. doi: 10.1007/978-0-387-68969-2_7. [DOI] [PubMed] [Google Scholar]
- 43.Yen ML, Yen BL, Bai CH, Lin RS. Risk factors for ovarian cancer in Taiwan: a case-control study in a low-incidence population. Gynecol Oncol. 2003;89:318–24. doi: 10.1016/s0090-8258(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 44.Yang CY, Kuo HW, Chiu HF. Age at first birth, parity, and risk of death from ovarian cancer in Taiwan: a country of low incidence of ovarian cancer. Int J Gynecol Cancer. 2007;17:32–6. doi: 10.1111/j.1525-1438.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 45.Kumar TR. Multiple ovulations, ovarian epithelial inclusion cysts, and it’Smad two! Endocrinology. 2007;148:3591–4. doi: 10.1210/en.2007-0574. [DOI] [PubMed] [Google Scholar]
- 46.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–21. [PubMed] [Google Scholar]
- 47.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1212–20. doi: 10.1093/oxfordjournals.aje.a116429. [DOI] [PubMed] [Google Scholar]
- 48.Jensen A, Sharif H, Frederiksen K, Kjaer SK. Use of fertility drugs and risk of ovarian cancer: Danish Population Based Cohort Study. BMJ. 2009;338:b249. doi: 10.1136/bmj.b249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–86. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 50.Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA. 1995;274:1926–30. [PubMed] [Google Scholar]
- 51.Cramer DW, Welch WR, Scully RE, Wojciechowski CA. Ovarian cancer and talc: a case-control study. Cancer. 1982;50:372–6. doi: 10.1002/1097-0142(19820715)50:2<372::aid-cncr2820500235>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 52.Gertig DM, Hunter DJ, Cramer DW, Colditz GA, Speizer FE, Willett WC, et al. Prospective study of talc use and ovarian cancer. J Natl Cancer Inst. 2000;92:249–52. doi: 10.1093/jnci/92.3.249. [DOI] [PubMed] [Google Scholar]
- 53.Wong C, Hempling RE, Piver MS, Natarajan N, Mettlin CJ. Perineal talc exposure and subsequent epithelial ovarian cancer: a case-control study. Obstet Gynecol. 1999;93:372–6. doi: 10.1016/s0029-7844(98)00439-6. [DOI] [PubMed] [Google Scholar]
- 54.Ness RB, Grisso JA, Cottreau C, Klapper J, Vergona R, Wheeler JE, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11:111–7. doi: 10.1097/00001648-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, Koenig KL, Berrino F, Lukanova A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:799–810. doi: 10.1158/1055-9965.EPI-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White KL, Schildkraut JM, Palmieri RT, Iversen ES, Jr, Berchuck A, Vierkant RA, et al. Ovarian cancer risk associated with inherited inflammation-related variants. Cancer Res. 2012;72:1064–9. doi: 10.1158/0008-5472.CAN-11-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilailak S, Vipupinyo C, Suraseranivong V, Chotivanich K, Kietpeerakool C, Tanapat Y, et al. Depot medroxyprogesterone acetate and epithelial ovarian cancer: a multicentre case-control study. BJOG. 2012;119:672–7. doi: 10.1111/j.1471-0528.2012.03298.x. [DOI] [PubMed] [Google Scholar]
- 58.Seeger H, Wallwiener D, Mueck AO. Is there a protective role of progestogens on the proliferation of human ovarian cancer cells in the presence of growth factors? Eur J Gynaecol Oncol. 2006;27:139–41. [PubMed] [Google Scholar]
- 59.Franceschi S, La Vecchia C, Helmrich SP, Mangioni C, Tognoni G. Risk factors for epithelial ovarian cancer in Italy. Am J Epidemiol. 1982;115:714–9. doi: 10.1093/oxfordjournals.aje.a113353. [DOI] [PubMed] [Google Scholar]
- 60.Whiteman DC, Siskind V, Purdie DM, Green AC. Timing of pregnancy and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:42–6. [PubMed] [Google Scholar]
- 61.Rossing MA, Tang MT, Flagg EW, Weiss LK, Wicklund KG. A case-control study of ovarian cancer in relation to infertility and the use of ovulation-inducing drugs. Am J Epidemiol. 2004;160:1070–8. doi: 10.1093/aje/kwh315. [DOI] [PubMed] [Google Scholar]
- 62.Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166:894–901. doi: 10.1093/aje/kwm157. [DOI] [PubMed] [Google Scholar]
- 63.Beral V, Fraser P, Chilvers C. Does pregnancy protect against ovarian cancer? Lancet. 1978;1:1083–7. doi: 10.1016/s0140-6736(78)90925-x. [DOI] [PubMed] [Google Scholar]
- 64.Dick ML, Siskind V, Purdie DM, Green AC. Incomplete pregnancy and risk of ovarian cancer: results from two Australian case-control studies and systematic review. Cancer Causes Control. 2009;20:1571–85. doi: 10.1007/s10552-009-9402-3. [DOI] [PubMed] [Google Scholar]
- 65.Gierach GL, Modugno F, Ness RB. Relations of gestational length and timing and type of incomplete pregnancy to ovarian cancer risk. Am J Epidemiol. 2005;161:452–61. doi: 10.1093/aje/kwi069. [DOI] [PubMed] [Google Scholar]
- 66.Pelucchi C, Galeone C, Talamini R, Bosetti C, Montella M, Negri E, et al. Lifetime ovulatory cycles and ovarian cancer risk in 2 Italian case-control studies. Am J Obstet Gynecol. 2007;196:83, e1–7. doi: 10.1016/j.ajog.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 67.Moorman PG, Calingaert B, Palmieri RT, Iversen ES, Bentley RC, Halabi S, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167:1059–69. doi: 10.1093/aje/kwn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989;60:592–8. doi: 10.1038/bjc.1989.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper GS, Schildkraut JM, Whittemore AS, Marchbanks PA. Pregnancy recency and risk of ovarian cancer. Cancer Causes Control. 1999;10:397–402. doi: 10.1023/a:1008960520316. [DOI] [PubMed] [Google Scholar]
- 70.Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344:1250–4. doi: 10.1016/s0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 71.Chiaffarino F, Parazzini F, Negri E, Benzi G, Scarfone G, Franceschi S, et al. Time since last birth and the risk of ovarian cancer. Gynecol Oncol. 2001;81:233–6. doi: 10.1006/gyno.2001.6136. [DOI] [PubMed] [Google Scholar]
- 72.Albrektsen G, Heuch I, Kvale G. Reproductive factors and incidence of epithelial ovarian cancer: a Norwegian prospective study. Cancer Causes Control. 1996;7:421–7. doi: 10.1007/BF00052668. [DOI] [PubMed] [Google Scholar]
- 73.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. “Incessant ovulation” and ovarian cancer. Lancet. 1979;2:170–3. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 74.Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–32. doi: 10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- 75.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res. 1999;59:3658–62. [PubMed] [Google Scholar]
- 76.Wilcox CB, Feddes GO, Willett-Brozick JE, Hsu LC, DeLoia JA, Baysal BE. Coordinate up-regulation of TMEM97 and cholesterol biosynthesis genes in normal ovarian surface epithelial cells treated with progesterone: implications for pathogenesis of ovarian cancer. BMC Cancer. 2007;7:223. doi: 10.1186/1471-2407-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ. Inhibitory effects of progesterone on plasma membrane fluidity and tumorigenic potential of ovarian epithelial cancer cells. Exp Biol Med (Maywood) 2003;228:308–14. doi: 10.1177/153537020322800310. [DOI] [PubMed] [Google Scholar]
- 78.Bu SZ, Yin DL, Ren XH, Jiang LZ, Wu ZJ, Gao QR, et al. Progesterone induces apoptosis and up-regulation of p53 expression in human ovarian carcinoma cell lines. Cancer. 1997;79:1944–50. doi: 10.1002/(sici)1097-0142(19970515)79:10<1944::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 79.Lindgren P, Backstrom T, Mahlck CG, Ridderheim M, Cajander S. Steroid receptors and hormones in relation to cell proliferation and apoptosis in poorly differentiated epithelial ovarian tumors. Int J Oncol. 2001;19:31–8. [PubMed] [Google Scholar]
- 80.Chuaire-Noack L, Sanchez-Corredor MC, Ramirez-Clavijo S. P53 and its role in the ovarian surface epithelium. A review. Invest Clin. 2008;49:561–93. [PubMed] [Google Scholar]
- 81.Schildkraut JM, Bastos E, Berchuck A. Relationship between lifetime ovulatory cycles and overexpression of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1997;89:932–8. doi: 10.1093/jnci/89.13.932. [DOI] [PubMed] [Google Scholar]
- 82.McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ. Effects of progesterone on ovarian tumorigenesis in xenografted mice. Cancer Lett. 2005;221:49–53. doi: 10.1016/j.canlet.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Mukherjee K, Syed V, Ho SM. Estrogen-induced loss of progesterone receptor expression in normal and malignant ovarian surface epithelial cells. Oncogene. 2005;24:4388–400. doi: 10.1038/sj.onc.1208623. [DOI] [PubMed] [Google Scholar]
- 84.Lee JY, Shin JY, Kim HS, Heo JI, Kho YJ, Kang HJ, et al. Effect of combined treatment with progesterone and tamoxifen on the growth and apoptosis of human ovarian cancer cells. Oncol Rep. 2012;27:87–93. doi: 10.3892/or.2011.1460. [DOI] [PubMed] [Google Scholar]
- 85.Syed V, Mukherjee K, Godoy-Tundidor S, Ho SM. Progesterone induces apoptosis in TRAIL-resistant ovarian cancer cells by circumventing c-FLIPL overexpression. J Cell Biochem. 2007;102:442–52. doi: 10.1002/jcb.21304. [DOI] [PubMed] [Google Scholar]
- 86.Syed V, Ho SM. Progesterone-induced apoptosis in immortalized normal and malignant human ovarian surface epithelial cells involves enhanced expression of FasL. Oncogene. 2003;22:6883–90. doi: 10.1038/sj.onc.1206828. [DOI] [PubMed] [Google Scholar]
- 87.Clarke P, Tyler KL. Apoptosis in animal models of virus-induced disease. Nat Rev Microbiol. 2009;7:144–55. doi: 10.1038/nrmicro2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez-Rodilla I, Verna V, Munoz AB, Estevez J, Boix M, Schneider J. Expression of the apoptosis-related genes Bcl-2 and p53 in clinical samples from endometrial carcinoma patients. Anticancer Res. 2011;31:4191–3. [PubMed] [Google Scholar]
- 89.Formby B, Wiley TS. Bcl-2, survivin and variant CD44 v7–v10 are downregulated and p53 is upregulated in breast cancer cells by progesterone: inhibition of cell growth and induction of apoptosis. Mol Cell Biochem. 1999;202:53–61. doi: 10.1023/a:1007081021483. [DOI] [PubMed] [Google Scholar]
- 90.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis-the p53 network. J Cell Sci. 2003;116:4077–85. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 91.Fauvet R, Dufournet Etienne C, Poncelet C, Bringuier AF, Feldmann G, Darai E. Effects of progesterone and anti-progestin (mifepristone) treatment on proliferation and apoptosis of the human ovarian cancer cell line, OVCAR-3. Oncol Rep. 2006;15:743–8. [PubMed] [Google Scholar]
- 92.Murdoch WJ, Martinchick JF. Oxidative damage to DNA of ovarian surface epithelial cells affected by ovulation: carcinogenic implication and chemoprevention. Exp Biol Med (Maywood) 2004;229:546–52. doi: 10.1177/153537020422900613. [DOI] [PubMed] [Google Scholar]
- 93.Nguyen H, Syed V. Progesterone inhibits growth and induces apoptosis in cancer cells through modulation of reactive oxygen species. Gynecol Endocrinol. 2011;27:830–6. doi: 10.3109/09513590.2010.538100. [DOI] [PubMed] [Google Scholar]
- 94.Adler I, Tulassay Z, Stark J, Marczell I, Nagy-Repas P, Varbiro S, et al. The effect of certain steroid hormones on the expression of genes involved in the metabolism of free radicals. Gynecol Endocrinol. 2012;28:912–6. doi: 10.3109/09513590.2012.683067. [DOI] [PubMed] [Google Scholar]
- 95.Morrissy S, Strom J, Purdom-Dickinson S, Chen QM. NAD (P)H: quinone oxidoreductase 1 is induced by progesterone in cardiomyocytes. Cardiovasc Toxicol. 2012;12:108–14. doi: 10.1007/s12012-011-9144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He L, Yang H, Zhai LD, Shao H, Li YS. A preliminary study on progesterone antioxidation in promoting learning and memory of young ovariectomized mice. Arch Med Sci. 2011;7:397–404. doi: 10.5114/aoms.2011.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]