Abstract
Background:
The suppression of abnormal cell proliferation is therapeutic strategies for the treatment of cancer. In this study, we investigated the regulatory mechanism of quercetin-induced apoptosis through regulation of Sestrin 2 and AMPK signaling pathway.
Methods:
After treatment of quercetin to colon cancer cells, intracellular ROS was detected using by DCFH-DA. To examine how quercetin and H2O2 induced apoptosis, we analyzed the change of Sestrin 2, p53 expression and p-AMPKα1, p-mTOR levels by Western blotting. To evaluate the effect of intracellular ROS generated by quercetin on colon cancer cells, NAC, anti-oxidative agent, was co-treated.
Results:
Quercetin increased apoptotic cell death though generating intracellular reactive oxygen species (ROS), and it was responsible for Sestrin 2 expression. Increased Sestrin 2 expression was accompanied by AMPK activation. Interestingly, mTOR activity by Sestirn 2 expression was dependent on AMPK phosphorylation. On the other hand, the expression of Sestrin 2 by quercetin-generated intracellular ROS was independent of p53.
Conclusions:
We suggested that quercetin-induced apoptosis involved Sestrin 2/AMPK/mTOR pathway, which was regulated by increased intracellular ROS by quercetin.
Keywords: Quercetin, ROS, Sestrin2, AMPK, p53
INTRODUCTION
Since 1983, cancer has been regarded to one of the reason of death and the incidence to 180,000 new patients diagnose each of year in South Korea. Particularly colorectal cancer incidence is increase steadily since 1999, and especially the elderly ages, the incidence highest follow by gastric cancer.1 In this reason, it increased that interest to alternative medicine for treatment of colorectal cancer and the cancer prevention study on the effect of plants extract is in progress. Among plant extracts, quercetin has been reported as representing the number of carcinoma and anti-inflammatory effect.2 Especially in tumor cells, quercetin exerts a directly pro-apoptotic effect by regulated caspase -3, -6, -8 and AMPKα1/ASK1 pathway, and it can indeed block the growth of cancer cells at different phases of cell cycle by controlled transcription factors such as p53.3–5
p53, the tumor suppressor protein, plays a central role in cancer prevention. p53 could be regulated cell cycle arrest and pro-apoptotic effect by controlled downstream molecules in cancer cells. The major downstream effectors of p53 include p21Waf1, which participate in cell cycle arrest, and Bax as well as PUMA, which trigger apoptosis.6 Recent report has suggested that the activated p53 by cell stress lead to suppressed mTOR C1 through AMPKα1/TSC2 pathway.7
Sestrin 2 is first revealed by the downstream effector of p53, and it involved in regulation of cell viability in variety cellular stress. Sestrin 2 expression is induced upon DNA damages such as UV-irradiation and doxorubicin, dependent on p53, and oxidative stress like a hypoxia, independent on p53. In addition, it leaded to apoptosis in cancer.8 Previous study has shown that Sestrin 2 expression in cancer cell suppressed cell growth and proliferation by lead to negative control of mTOR through AMPKα1 phosphorylaton.9,10
AMPK, serine/threonine protein kinase, participates in an energy sensing cascade that response to deletion of ATP. AMPK, which was activated by various up-stream factors such as LKB1 or CaMKK, inhibited mTOR for lead to apoptosis by activation of TSC2.11 Previous research has shown that AMPK activation through quercetin-generated ROS is induced apoptosis via ASK1/p38 MAPK pathway in MCF-7 breast cancer cells.4
mTOR is representative protein that regulate cell growth and proliferation, and depending on the role and component, separated mTOR Complex 1 (C1) and mTOR Complex 2 (C2). mTOR C1 is known that very important factor for cell growth and proliferation in cancer cells.12 mTOR C1 is activated by various growth factors such as Akt or IGF-1, especially Akt-mTOR C1 pathway is over activation in cancer cells. That pathway, which activated mTOR C1 by Akt, is reported that one of the important mechanism for tumorigenesis.13 In previously study, be inhibited mTOR C1 by AMPK activation leaded to decrease of cancer cell growth and proliferation.7
In this study, we investigated the anti-proliferation and induced apoptosis effects by Quercetin via increased intracellular ROS in HCT 116 colon cancer cells. And involvement of Sestrin 2 in the apoptotic process of cancer cells treated with a quercetin. Previously report has that quercetin-induced apoptosis through the generation of ROS, which was responsible for the activation of AMPKα1.4 Here, we demonstrated that quercetin-generated ROS induced apoptosis were involved to expression of Sestrin 2 and it accompanied by AMPK phosphorylation. And expression of Sestrin 2 is p53 independently on ROS.
MATERIALS AND METHODS
1. Reagent
Quercetin and hydrogen peroxide, NAC (N-acetyl cystein), MTT [(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide], DCFH-DA are purchased from Sigma Aldrich (Sigma Aldrich, St. Luice, MO, USA). Compound C are purchased from Calbiochem (Calbiochem, San Diego, CA, USA). FITC-Annexin V apoptosis detection kit is obtained from BD Pharmingen (BD Pharmingen, San Diego, CA, USA). Specific anti-bodies that recognized p-AMPKα1, p-mTOR, p53, β-actin are obtained from Cell Signaling technology (Cell Signaling Technology, Beverly, MA, USA) and Sestrin 2 are purchased from Proteintech (Proteintech, Chicago, IL, USA).
2. Cell culture
HCT116 and HT-29 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were grown in RPMI 1640 medium (HyClone, Waltham, MA, USA) containing 10% fetal bovine serum (HyClone, Waltham, MA, USA) and 1% antibiotics (100 mg/L streptomycin, 100 U/ml penicillin) at 37°C in a 5% CO2 atmosphere. Cells were suspended by Trypsin-EDTA (HyClone, Waltham, MA, USA) and separated 1.5×105/ml at each plates, every 48 h.
3. Measurement of intracellular ROS levels
Cells were seeded 1×106/ml in 100 mm plate and incubated for 24 h. After incubation, cells treated with test compound for 6 h at 37°C in a 5% CO2 atmosphere. Cells were incubated with 40 μM of DCFH-DA for 30 min and harvested by trypsinization, collected by centrifugation, washed with PBS twice, and resuspended in PBS. Fluorescence intensity were analyzed by using flow cytometer (Becton-Dickinson Biosciences, Drive Frankline Lages, NJ, USA).
4. Determination of apoptosis by Annexin V/PI staining analysis
Cells were seeded at 1×106/ml in 100 mm plate and incubated for 24 h. After incubation, cells treated with test compound for 24 h at 37°C in a 5% CO2 atmosphere. Total cells were harvested by trypsinization, collected by centrifugation, washed with PBS, and resuspended in binding buffer. Cells were stained with Annexin V and PI for 15 min. Fluorescence intensity were analyzed by using flow cytometer (Becton-Dickinson Biosciences, Drive Frankline Lages, NJ, USA).
5. Cell proliferation assay (MTT)
Cell viability was measured by MTT assay. Cells were seeded at 4,000/ml each well in 96 well plate, and incubated 24 h. After the incubation, treated with test compound and incubate at 37°C in a 5% CO2 atmosphere. After 24 h, cells were incubated with 30 μl MTT (5 mg/ml with PBS) solution for 1 hr. Optical densities of solution, in each well, were determined by Microplate reader (BIO-RAD Laboratories, Inc., Tokyo, Japan) at 595 nm.
6. Western blotting
Cells were seeded at 1×105/ml in 6-well plate and incubated for 24 h. After the incubation, treated with test compound for 6 h at 37°C in a 5% CO2 atmosphere. Cells were rinsed twice with ice-cold PBS and scraped with lysis buffer [50 mM Tris-HCl (pH 8.0, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 1 mM PMSF)] and subjected to the Western blot analysis. 1st anti-body reacted for overnight at 4°C and 2nd anti-body reacted for 75 min at room-temperature with agitation slowly.
7. Statistical analysis
Cell viability was statistically analyzed using ANOVA test (SPSS, Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULT
1. Quercetin-generated ROS in HCT116 colon cancer cells
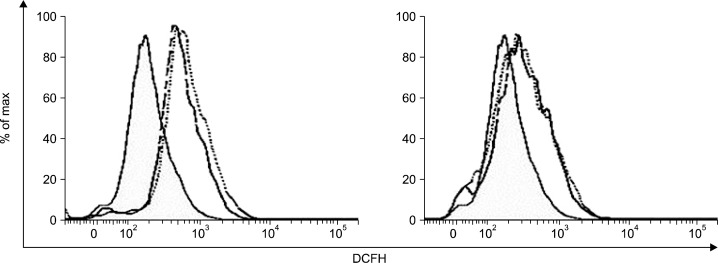
To examine whether quercetin generates ROS in HCT116 colon cancer cells, we measured the intracellular ROS levels after treatment of quercetin (25 μM, 50 μM) for 6 h. As shown in Fig. 1, quercetin increased ROS levels at the indicate concentration (left panel). These effects were completely blocked by combined treatment with NAC, a ROS scavenger (right panel).
Fig. 1.
Quercetin increased intracellular ROS. Cells were treated with indicate concentration of quercetin for 6 h (left panel), and pre-treated with 5 mM NAC for 30 min and then exposed to quercetin (right panel). After 6 h, Cells were treated with 40 μ M DCFH-DA for 30 min, and fluorescence intensity was measured by flow cytometer. Dashed line, quercetin 25 μ M; dotted line, quercetin 50 μM.
2. Quercetin and H2O2 induce apoptosis
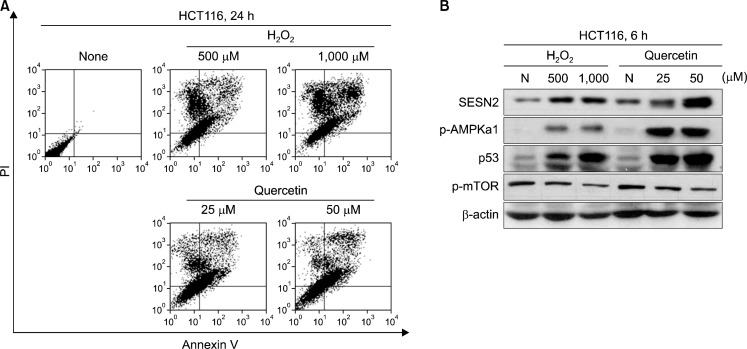
We investigated the apoptotic effect by quercetin via increase intracellular ROS. In this reason, we treated with quercetin (25 μM, 50 μM) and H2O2 (500 μM, 1,000 μM) for 24 h. Cells were stained with Annexin V/PI and measured apoptotic cells by flow cytometer. As we shown in Fig. 2A, be treated with quercetin groups increased annexin-V positive cell population on treated groups, too.
Fig. 2.
Quercetin-generated ROS strongly induced apoptosis and expressed Sestrin 2, p53 as well as activated AMPKα 1. (A) Cells were treated with indicated concentration of H2O2 and quercetin for 24 h. Cells were stained with Annexin V/PI and fluorescence intensity was measured by flow cytometer. (B) Cells were treated with indicate concentration of H2O2 and quercetin for 6 h. Next, expression of Sestein 2, p53 and activate of AMPKα1, mTOR were analyzed by Western blotting.
3. Quercetin and H2O2 regulate expression of Sestrin 2, p53 and AMPKα1, mTOR activation
To examine how quercetin and H2O2 induced apoptosis, we first examined quercetin expresses Sestrin 2, p53 and activate AMPKα1, whereas inhibited mTOR. We analyzed the change of Sestrin 2, p53 expression and p-AMPKα1, p-mTOR levels after treatment of quercetin (25–50 μM) and H2O2 (500–1,000 μM) in different concentration by Western blotting. Our results showed that quercetin strongly expressed Sestin 2, p53 and activated AMPK, whereas inhibited mTOR in dose dependently. In addition, H2O2 treated groups were induced that expression of Sestrin 2, p53 and AMPK phosphorylation, but mTOR activity was decreased (Fig. 2B).
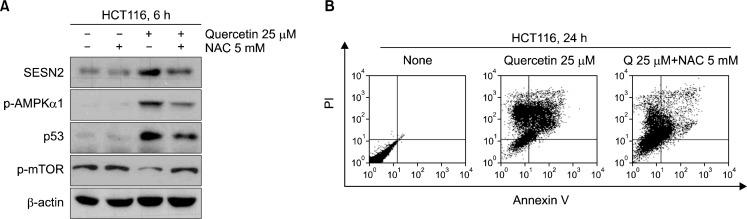
4. Quercetin modulates expression of Sestrin 2, p53 and AMPK, mTOR activation through generation of intracellular ROS
For make sure that quercetin regulated signal proteins and induced apoptosis were involved with increased intracellular ROS levels, we co-treated with NAC, anti-oxidative agent, and analyzed proteins level and Annexin V-positive cells. The group co-treated quercetin decreased Sestrin 2, p53 expression level and AMPK phosphorylation, whereas induced mTOR activity (Fig. 3A). And unlikely quercetin treatment group, Annexin V-positive cells were decreased in NAC co-treatment group (Fig. 3B).
Fig. 3.
(A) Cells were pre-treated with 5 mM NAC and treatment of quercetin 25 μM for 6 h. The expression of Sestein 2, p53 and activate of AMPKα1, mTOR were analyzed by Western blotting. (B) Cells were treated with 20 μM quercetin for 24 h after pre-treated with 5 mM NAC and cells were stained with Annexin-V/PI and fluorescence intensity was measured by flow cytometer.
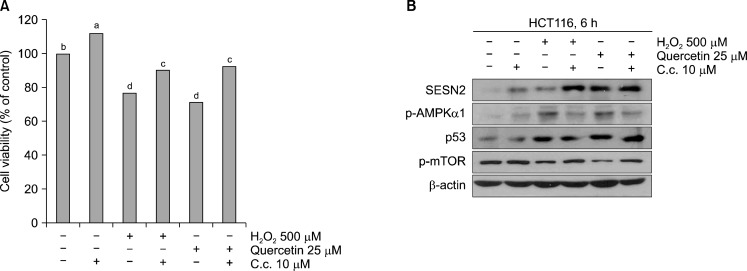
5. Sestrin 2 down-regulated proliferation and mTOR activity through AMPK phosphorylation
We investigated quercetin and H2O2 decreased mTOR phosphorylation by Sestrin 2 were involved AMPK activation. In order to do, we co-treated quercetin and H2O2 with Compound C, AMPK inhibitor, and analyzed cell viability by MTT assay and proteins level by Western blotting. As we shown that, despite the co-treated with AMPK inhibitor group were not changed cell viability, quercetin and H2O2 treated group were reduced cell viability (Fig. 4A). Moreover, quercetin and H2O2 induced expression of Sestrin 2 without AMPK inhibition, whereas mTOR acitivity was not decreased with AMPK inhibition (Fig. 4B).
Fig. 4.
(A) Querctin-generated ROS reduced cell viability though activation of AMPKα1. Cells were pre-treated with 10 μ M Compound C for 30 min, and then treated with H2O2 500 μM and quercetin 25 μ M. Cell viability were measured by MTT assay. a–dP<0.05 (each experiment’s n=3). (B) The expression of Sestrin 2, p53 and activation of AMPKα 1, mTOR were analyzed by Western blotting.
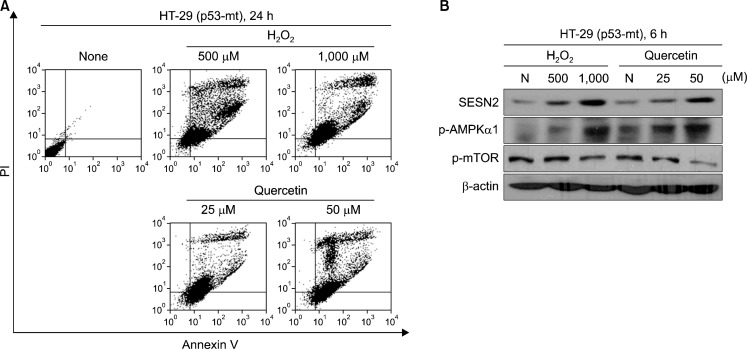
6. Sestrin 2 is expressed and induced apoptosis in p53 negative cells
To examine the expression of Sestrin 2 is p53-independent manner. We treated quercetin and H2O2 in HT-29 colon cancer cells, which were p53 mutant. Typically, Sestrin 2 is known that p53-dependent manner except hypoxia condition. Our result shown that Sestrin 2 expressed as well as AMPK phosphorylation levels were induces in HT-29 cells. Moreover, quercetin induced apoptotic cell death in HT-29 cells (Fig. 5).
Fig. 5.
Quercetin-generated ROS induced apoptosis and regulated proteins in p53 mutant cells. (A) Cells were treated with indicate concentration of H2O2 and quercetin for 24 h. And then stained with Annexin-V/PI and fluorescence intensity was measured by flow cytometer. (B) Cells were treated with indicate concentration of H2O2 and quercetin for 6 h. The expression of Sestrin 2 and activation of AMPKα1, mTOR were analyzed by Western blotting.
DISCUSSION
Pathological cell proliferation and division is involved in various diseases such as abnormal angiogenesis, tumor formation. Thus, suppression of this abnormal cell proliferation may provide therapeutic strategies for the treatment of those diseases. That come to the fore in the field of cancer treatment, particularly alternative medicine such as treatment through plant extracted compound newly attention. These plant extracts compound induced apoptosis and cell cycle arrest through regulated intracellular protein signals in cancer cells.14 A various plant extracts compound are used in the experiment for chemotheraphy, above all thing, quercetin, which was polyphenolic compound extracted from red onion and green tea, is known to have diverse pharmacological activities, including anti-cancer, anti-inflammation, and anti-proliferation.2 Recently study has shown that quercetin generats intracellular ROS and induces apoptosis through controlled AMPK/ASK1 pathway in MCF-7 breast cancer cells.4 Moreover, it induced apoptosis that treated with quercetin in HT-29 colon cancer cells via regulated AMPK/COX-2 pathway.15 Quercetin induced apoptosis through the activation of AMPK, however, AMPK activity by any pathway is not clear. According to some studies, the transcription of p53 induced AMPK phosphorylation, but these results could not explain that phosphorylation of AMPK in p53 mutant cells.7 In recent study, however, a specific stress such as oxygen stress or DNA damage stimulates Sestrin 2 transcription in cancer cells, and it induced the phosphorylation of AMPK through interaction with AMPK.9 According to this paper, cell does not inhibit mTOR activity and cell cycle arrest through AMPK phosphorylation when it suppressed Sestrin 2 transcription. Thus, we hypothesized that quercetin-generated intracellular ROS induced apoptosis through the inhibition of mTOR via Sestrin 2 transcription, and it accompanied by AMPK phosphorylation. Our result shown that quercetin suppressed proliferation by expression of Sestrin 2 and AMPK phosphorylation, whereas inhibited mTOR activity. Moreover, quercetin by increasing intracellular ROS can transcript Sestrin 2 directly, and it was confirmed that the upstream function in the AMPK signaling pathway.
Importantly, a known of the cancer cells in approximately 50% are p53 mutant.16 Thus, p53-independent manner to induce apoptosis is very important. Our results shown that not only quercetin inhibited mTOR through Sestrin 2 transcription and AMPK phosphorylation, but it induced apoptosis in HT-29 colon cancer cells, which were p53 mutant cells.
In conclusion, we demonstrate that Sestrin 2 is co-factor for AMPK/mTOR pathway, and the signaling pathway of Sestrin 2/AMPK/mTOR is responsible for cancer cells to undergo apoptosis initiated by ROS when the cancer cells were treated with quercetin. Furthermore, quercetin-generated intracellular ROS induces apoptosis and regulates Sestrin 2/AMPK/mTOR pathway in p53 mutant cells.
Acknowledgments
This paper has been supported by 2013 Hannam University Research Fund.
REFERENCES
- 1.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, et al. Quercetin-induced apoptosis acts through mitochondrial- and capase-2-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2009;28:493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 4.Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ. Induction of apoptosis by quercetin is mediated through AMPKalpha1/ASK1/p38 pathway. Cancer Lett. 2010;292:228–36. doi: 10.1016/j.canlet.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Tanigawa S, Fujii M, Hou DX. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem. 2008;72:797–804. doi: 10.1271/bbb.70680. [DOI] [PubMed] [Google Scholar]
- 6.Amol J. Levine, p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 7.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. PNAS. 2005;102:8204–9. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–31. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 9.Alexander A, Walker CL. The p53-regulated Sestrin gene product inhibit mTOR signaling. Cell. 2008;134:451–60. [Google Scholar]
- 10.Sanli T, Linher-Melville K, Tsakiridis T, Singh G. Sestrin2 modulates AMPK subunit expression and Its response to Ionizing Radiation in breast cancer cells. PLoS One. 2012;7:e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budanov AV, Karin M. The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett. 2011;585:952–7. doi: 10.1016/j.febslet.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway-Beyond rapalogs. Oncotarget. 2010;1:530–43. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, et al. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondria cytochrome C release and apoptosis. Int J Cancer. 2002;98:761–9. doi: 10.1002/ijc.10202. [DOI] [PubMed] [Google Scholar]
- 15.Lee YK, Park SY, Kim YM, Lee WS, Park OJ. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41:201–7. doi: 10.3858/emm.2009.41.3.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]