Abstract
Background:
A greater intake of vegetables and fruits has been linked to a reduced incidence of colon cancer. Flavonoids are polyphenolic compounds which are broadly distributed in fruits and vegetables and display a remarkable spectrum of physiological activities, including anti-carcinogenic effects. The objective of this study was to determine the mechanisms by which kaempferol, a flavonol present in tea, apples, strawberries, and beans, inhibits the growth of HT-29 human colon cancer cells.
Methods:
To examine the effects of kaempferol on cell cycle progression in HT-29 cells, cells were treated with various concentrations (0–60 μmol/L) of kaempferol. Cell proliferation and DNA synthesis were evaluated by MTT assay and [3H]thymidine incorporation assay, respectively. Fluorescence-activated cell sorting analyses were conducted to calculate cell cycle phase distribution. Western blot analyses and in vitro kinase assays were used to estimate the expression of proteins involved in the regulation of cell cycle progression and the activity of cyclin-dependent kinase (CDK)s, respectively.
Results:
Kaempferol decreased viable cell numbers and [3H]thymidine incorporation into DNA of HT-29 cells in a dose-dependent manner. Kaempferol induced G1 cell cycle arrest within 6 h and G2/M arrest at 12 h. Kaempferol inhibited the activity of CDK2 and CDK4 as well as the protein expression of CDK2, CDK4, cyclins D1, cyclin E, and cyclin A, and suppressed the phosphorylation of retinoblastoma protein. Additionally, kaempferol decreased the levels of Cdc25C, Cdc2, and cyclin B1 proteins, as well as the activity of Cdc2.
Conclusions:
The present results indicate that kaempferol induces G1 and G2/M cell cycle arrest by inhibiting the activity of CDK2, CDK4, and Cdc2. The induction of cell cycle arrest may be one of the mechanisms by which kaempferol exerts anti-carcinogenic effects in colon cancer cells.
Keywords: Kaempferol, Colon cancer, Cell cycle progression, Chemoprevention
INTRODUCTION
Colon cancer is one of the principal causes of cancer- related deaths in Western countries,1 and the incidence of this disease is increasing in developing countries due to changing dietary habits and the continuous increase in life expectancy. Despite the many progresses in cancer treatment, chemotherapy of solid tumors remains considerably restricted, with significant shortcomings such as low selectivity of anti-cancer drugs and the reappearance of drug-resistant tumors. Therefore, there is increasing urgency to find a source of innovative chemopreventive and chemotherapeutic agents.
Epidemiological studies have shown that colorectal cancer risk decreases with higher intake of vegetables, fruits, and grains.2,3 Flavonoids are a class of more than 4,000 phenylbenzopyrones which occur in many edible plants, including vegetables and fruits, and are receiving considerable attention for their contribution to the anti- carcinogenic properties associated with these foods.4,5 Previous studies have shown that several flavonoids exhibit potent anti-tumor activity against several rodent and human colon cancer cell lines.6,7 Kaempferol, a flavonol, is commonly found in many types of fruits and vegetables including beans, apples, broccoli, and strawberries,8 and epidemiological studies have provided evidence that there is a negative correlation between kaempferol intake and cancer risk.9
In the present study we examined whether kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. The results of this study indicate that kaempferol inhibits the proliferation of HT-29 cells, and that the inhibition of both G1 and G2/M cell cycle progression is responsible, at least in part, for kaempferol-induced growth inhibition of HT-29 cells.
MATERIALS AND METHODS
1. Materials
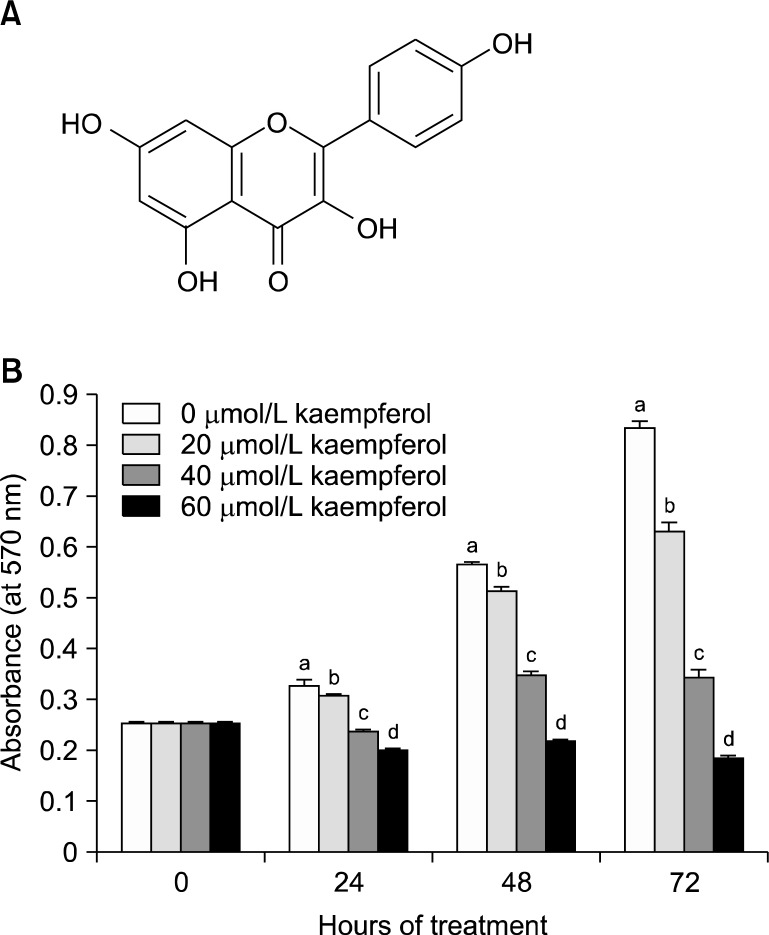
We purchased reagents from the following suppliers: ka-empferol (3,4’,5,7-tetrahydroxyflavone, Fig. 1A), 3-[4,5-di-methylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), anti-β-actin antibody, essentially fatty acid- free bovine serum albumin (BSA), and transferrin (Sigma, St. Louis, MO, USA); selenium and DMEM/Ham’s F-12 nutrient mixture (DMEM/F-12; Gibco BRL, Gaithersburg, MD, USA); horse- radish peroxidase (HRP)-conjugated anti-rabbit and anti- mouse IgG (Amersham, Arlington Heights, IL, USA); antibodies against cyclin-dependent kinase (CDK)2, CDK4, cell cycle controller (Cdc2), cyclin E, and cyclin A (Santa Cruz Biotechnology, Santa Cruz, CA, USA); antibodies against phospho-retinoblastoma protein (P-Rb) (Ser807/811), Rb, Cdc25C, and cyclin B1 (Cell Signaling, Beverly, MA, USA); anti-cyclin D1 antibody (NeoMarkers, Fremont, CA, USA); [γ-32P] ATP (PerkinElmer Life and Analytical Sciences, Inc., Boston, MA, USA).
Fig. 1.
Kaempferol inhibits viable HT-29 cell numbers. (A) Structure of kaempferol. (B) HT-29 cells were plated and treated with various concentrations of kaempferol for 24, 48, or 72 h. Cell numbers were estimated by MTT assay. Each bar represents the mean±SEM (n=6). Means at a time without the same letter differ, P<0.05.
2. Cell culture and MTT assay
HT-29 is a human colon cancer cell line derived from the tumor in a female patient with colon adenocarcinoma.10 We purchased the HT-29 cells (passage number: 129) from the American Type Culture Collection (Manassas, VA, USA) and used these cells between the 135th and 145th passage for these experiments. Cells were maintained in DMEM/F12 supplemented with 100 ml/L fetal bovine serum (FBS), 100,000 U/L penicillin, and 100 mg/L streptomycin. To examine the effects of kaempferol on cell viability, cells in the monolayer culture were serum-starved in DMEM/F12 supplemented with 5 mg/L transferrin, 5 μg/L selenium, and 0.1 g/L BSA (serum-free medium) for 24 h. After serum-starvation, cells were cultured in serum-free medium containing various concentrations of kaempferol. Media were changed every two days. Viable cell numbers were estimated by MTT assay.11
3. [3H]Thymidine incorporation
Cells were plated in 96-well plates at a density of 6,000 cells/well, and were then serum starved, and treated with kaempferol for 21 h, as described above. [3H]Thymidine (0.5 μCi/well) was added to the cell monolayers, which were then incubated for a further 3 h in order to measure the incorporation into DNA as previously described.11
4. Fluorescence-activated cell sorting (FACS) analysis
Cells were serum-starved and treated with kaempferol as described above. To estimate cell cycle phase distribution, cells were separated by trypsin-EDTA, fixed in ethanol, treated with RNase, and the cellular DNA was then stained with propidium iodide, as previously described.12 The percentage of cells in the G1, S and G2/M phases of the cell cycle were analyzed by flow cytometry (Becton Dickinson, Franklin Lake, NJ, USA).
5. Immunoprecipitation & Western blot analyses
Total cell lysates were prepared as previously described.13 Total cell lysates (1 mg protein) were incubated with anti-CDK2, anti-CDK4, or Cdc2 antibody for 1 h, and were then immunoprecipitated by protein A-Sepharose beads as previously described.12 The immunoprecipitates or total cell lysates (50 μg protein) were analyzed by Western blot analysis with their relevant antibodies, as previously described.13
6. In vitro kinase assay
We estimated CDK activity as previously described.14 In brief, CDK2, CDK4, or Cdc2 immunoprecipitates were incubated with [γ32P] ATP and the individual substrates (CDK2 and Cdc2: Histone H1; CDK4: Rb). The 32P-labeled Histone H1 or Rb was subjected to SDS-PAGE, and the gels were visualized by autoradiography.
7. Statistical analysis
The results were expressed as means±SEM and analyzed by analysis of variance. Differences between groups were assessed using the Student’s t-test or Duncan’s multiple range test, utilizing SAS statistical software version 8.12 (SAS Institute, Cary, NC, USA).
RESULTS
1. Kaempferol inhibits the proliferation of HT-29 cells
To examine whether kaempferol inhibits HT-29 cell growth, cells were cultured in serum-free medium with or without various concentrations of kaempferol for 24, 48, or 72 h, and viable cell numbers were estimated by MTT assay. Kaempferol decreased the viable cell numbers in a dose-dependent manner with a 75% decrease in the viable cell number within 72 h after the addition of 60 μmol/L kaempferol (Fig. 1B).
2. Kaempferol induces G1 and G2/M cell cycle arrest in HT-29 cells
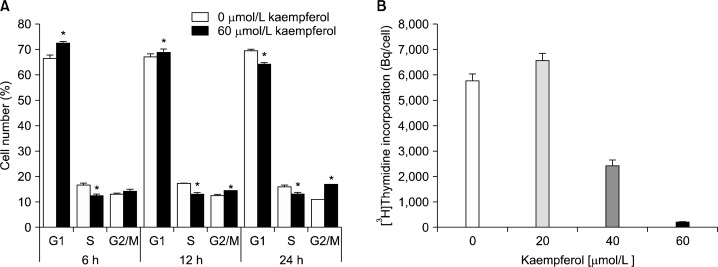
To determine whether kaempferol regulates the cell cycle progression of HT-29 cells, cells were treated with or without 60 μmol/L kaempferol, and DNA was stained with propidium iodide followed by fluorescence-activated cell sorting analysis. Upon exposure of cells to kaempferol for 6 h we observed an increase in the proportion of cells in G1, and the G1 phase accumulation was ensued with an equivalent decrease in the proportion of cells in S phase. After a 12 h exposure to kaempferol, the fraction in G2/M phase was increased. Twenty four hours after kaempferol treatment the delay in G2/M became more apparent (Fig. 2A). Additionally, results of [3H]thymidine incorporation assay revealed that kaempferol decreased the incorporation of thymidine into the DNA of HT-29 cells (Fig. 2B).
Fig. 2.
Kaempferol suppresses the cell cycle progression in HT-29 cells. (A) Cells were treated with 0 or 60 μmol/L kaempferol for 6, 12, or 24 h. Cells were trypsinized, fixed, and treated with RNase. Cellular DNA was then stained with propidium iodide. The percentages of cells in G1, S, and G2/M phases of the cell cycle were analyzed by flow cytometry. * indicates significantly different from the control group (0 μmol/L Kaempferol), P<0.05. (B) Cells were treated with various concentrations of kaempferol for 21 h. [3H]Thymidine was added and the incubation was continued for another 3 h to measure the incorporation into DNA. Each bar represents the mean±SEM (n=6). Means with different letters are significantly different, P<0.05.
3. Kaempferol inhibits the phosphorylation of Rb in HT-29 cells
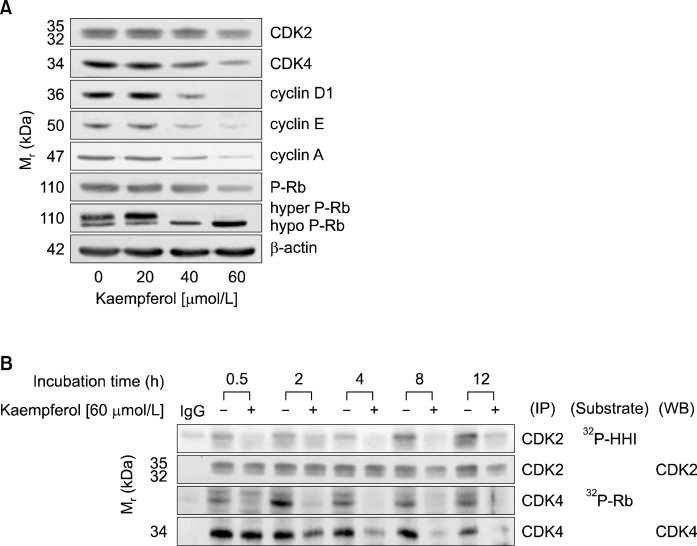
We next examined the effect of kaempferol on the levels of proteins involved in the regulation of G1-S transition by Western blot analyses. The cell cycle transition is regulated by CDKs, and CDKs activities are regulated by cyclins and CDK inhibitors.15 As shown in Fig. 3A, kaempferol decreased the protein levels of CDK2 and CDK4, as well as those of cyclin D1, cyclin E, and cyclin A. However, p21 and p27 levels were not increased by kaempferol treatment (data not shown). Western blot analysis with an antibody specific to P-Rb revealed that kaempferol treatment reduced the levels of P-Rb. When the Western blots were probed using the total Rb antibody, kaempferol decreased the hyper-phosphorylated Rb levels and increased the hypo-phosphorylated Rb levels (Fig. 3A). To detect CDK activity, CDK2 and CDK4 enzyme complexes were immunoprecipitated with CDK2 and CDK4 antibody, respectively, and the in vitro kinase assay was performed using histone H1 (HH1) or Rb as a substrate. The results showed that kaempferol decreased CDK2 and CDK4 activities within 30 min of 60 μmol/L kaempferol treatment (Fig. 3B). These data indicate that kaempferol induced G1 phase arrest by inhibition of CDK2 and CDK4 activities.
Fig. 3.
Kaempferol suppresses the activity of CDKs and phosphorylation of Rb in HT-29 cells. (A) Cells were treated with various concentrations of kaempferol for 6 h. Cells were lysed, and the ly-sates were analyzed via Western blotting with indicated antibodies. (B) Cells were treated with 0 or 60 μmol/L kaempferol for the indicated periods. Cell lysates were incubated with an antibody raised against CDK2 or CDK4, and the immune complexes were precipitated with protein A-Sepharose. The immunoprecipitated proteins were analyzed by an in vitro kinase assay using Histone H1 (HH1, CDK2) or Rb (CDK4) as a substrate or by Western blotting. The photographs of results, which are representative of two or three independent experiments, are shown.
4. Kaempferol inhibits Cdc2 activity in HT-29 cells
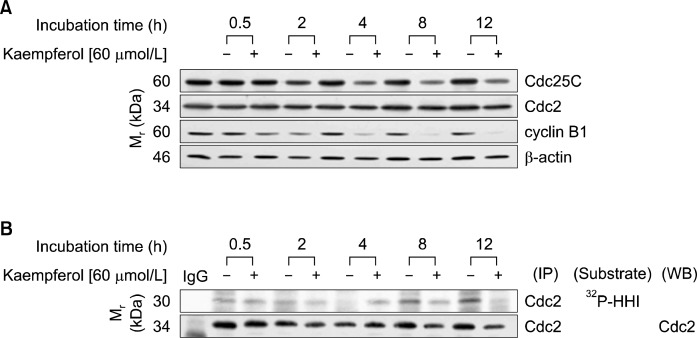
Western blot analysis revealed that the expression of Cdc25C was reduced upon treatment with 60 μmol/L kaempferol for 2 h, and the trend continued until 12 h after the addition of kaempferol. Small decreases in the expression of Cdc2 were detected 4 h after the addition of kaempferol. The expression of cyclin B1 was markedly reduced in cells treated with kaempferol for 4 h (Fig. 4A). However, Cdc2 activity, as estimated by immunopreciptation and in vitro kinase assay, was decreased 8 h after the addition of kaempferol (Fig. 4B).
Fig. 4.
Kaempferol suppresses the activity of Cdc2 in HT-29 cells. Cells were treated with 0 or 60 μmol/L kaempferol for the indicated periods. (A) Cell lysates were analyzed via Western blotting with the indicated antibodies. (B) Cell lysates were incubated with anti-Cdc2 antibody, and the immunoprecipitated proteins were prepared as described in Fig. 3B. The precipitated proteins were analyzed by an in vitro kinase assay using Histone H1 (HH1) as a substrate or by Western blotting. The photographs of results, which are representative of two or three independent experiments, are shown.
DISCUSSION
Over the past several decades, considerable attempts have been made to iderntify food components which inhibit, retard or reverse multi-stage carcinogenesis.16 Flavonoids, commonly found in fruits and vegetables, display remarkable anti-carcinogenic properties.5,17 Kaempferol, a member of flavonoids, is abundantly present in beans, apples, broccoli, and strawberries,8 and exhibits cancer preventive properties.9 Epidemiological evidence indicates that a high intake of kaempferol is negatively correlated with risk of advanced colorectal adenoma recurrence.18 Because previous studies by other investigators have shown that kaempferol inhibits the growth of the colon cancer cell lines HT-29 and Caco-2,19 the present study was performed to identify the mechanisms by which kaempferol inhibits HT-29 human colon cancer cell growth. The present results have revealed that kaempferol 1) inhibits DNA synthesis; 2) induces G1 cell cycle arrest; 3) decreases the expression of cyclin D1, E, and A; 4) decreases the expression of CDK2 and 4; 5) suppresses the activity of CDK2 and 4; 6) suppresses the phosphorylation of RB; 7) induces G2/M phase arrest; 8) suppresses the expression of Cdc25C, cyclin B1, and Cdc2; and 9) suppresses Cdc2 activity.
The present results suggest that kaempferol may be used as an agent for the prevention of colon cancer. However, kaempferol’s efficacy against tumor development and promotion in vivo will depend chiefly upon its bioavailability to the various tissues, an area that is yet to be systematically researched. In German healthy female students the mean intake estimate (7-day period) of kaempferol was 4.7 mg/d and the mean plasma concentration was 10.7 nmol/L.20 The concentrations used in the present in vitro cell culture experiments were 20-60 μmol/L, which are much higher than those found in human blood samples. However, unabsorbed dietary kaempferol may be presented to the epithelium of large intestine in higher concentrations than to cells in peripheral tissues if people consume high amounts of the flavonoid. Future studies are needed to establish the absorption, metabolism, and distribution of kaempferol as well as its toxicity in animals and humans.
Mammalian cell cycle is composed of 4 phases; G1, S, G2, and M phases, and dysregulation of cell cycle progression is one of the hallmarks of cancer.21 In the present study kaempferol treatment induced G1 arrest within 6 h (Fig. 2A). A wide variety of cell types respond to many growth factors by the activation of CDK4. Kinase activity of CDK4 requires the binding of the positive regulatory subunit, known as cyclin D1.22 In the present experiment, kaempferol decreased the levels of CDK4 and cyclin D1 proteins within 6 h in a dose-dependent manner (Fig. 3A). CDK4 activity was drastically decreased within 0.5 h after kaempferol treatment (Fig. 3B). In addition to the inhibition of CDK4 activity, kaempferol decreased the levels of CDK2, cyclin A and cyclin E proteins, leading to a decreased CDK2 activity. These results clearly showed that kaempferol induces a reduction in the protein levels of CDKs and cyclins, thereby reducing the activity of these enzymes in HT-29 cells.
Members of the Rb protein are phosphorylated by CDKs, leading to the stimulation of gene expression necessary for G1-S cell cycle progression.23 In the present experiment, Western blot analysis of total cell lysates with P-Rb antibody revealed that kaempferol decreased P-Rb levels. When the immunoblot was probed using total Rb antibody, two bands were detected with increased intensity of hypo-phosphorylated Rb being noted in cells treated with kaempferol (Fig. 3A). Hypo-phosphorylated Rb tightly associates with a family of transcription activators the E2F proteins, and thereby represses transcription.23 Together, these results suggest that a reduction in CDK activity leads to increased hypo-phosphorylated Rb (decreased phosphorylation of Rb) in cells treated with kaempferol. As a result, E2F cannot activate those genes necessary for the completion of the G1 to S phase transition.
In addition to the inhibition of the G1/S cell cycle transition, treatment of HT-29 cells with kaempferol for 12 h led to G2/M arrest (Fig. 2A).The G2/M checkpoint blocks cells from dividing when DNA is damaged, providing a chance for restoration and the prevention of the proliferation of damaged cells.24 Inhibitors of the G2/M may be useful for the prevention and treatment of cancer. Cdc2 (or CDK-1) is activated by interaction with cyclin B1, and the activation of Cdc2 is required for G2/M transition.25 Cdc2/cyclin B1 is dephosphorylated, and is thereby activated by active Cdc25C phosphatase, leading to the G2/M transition of cell cycle.26 Kaempferol decreased the levels of Cdc25C, Cdc2 and cyclin B1 proteins, and the activity of Cdc2 was also decreased in HT-29 cells treated with kaempferol (Fig. 4). These results indicate that kaempferol inhibits the activation of Cdc2/cyclin B1 complex via the downregulation of Cdc25C, leading to the G2/M cell cycle arrest.
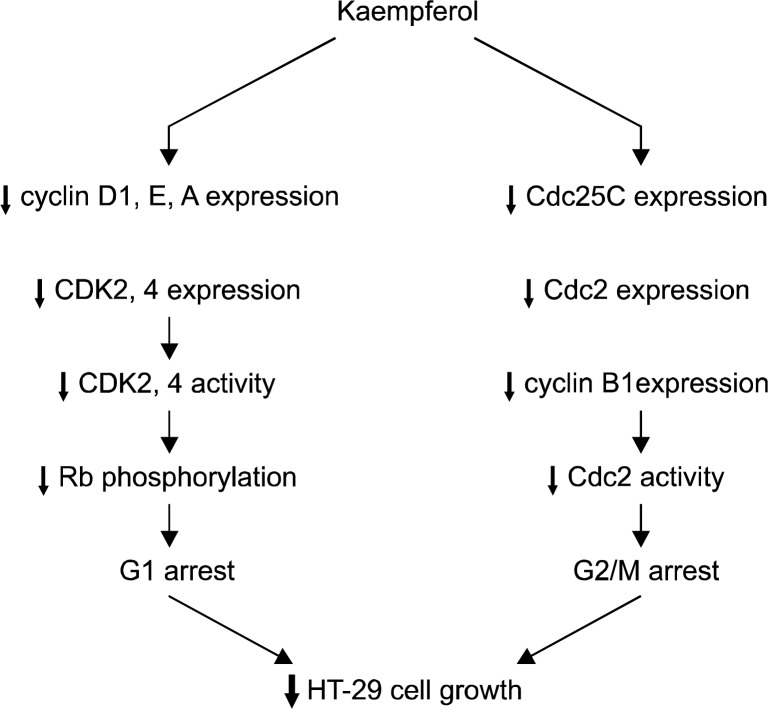
In summary, kaempferol reduces the levels of cyclin A, D1, and E, as well as those of CDK2 and 4 in HT-29 cells, which results in decreased activities of CDK2 and 4, leading to decreased Rb phosphorylation. Increased hypo- phosphorylated Rb may suppress the E2F-dependent transcription of genes involved in the regulation of the G1-S phase transition. Kaempferol also decreases both Cdc2 and cyclin B1 protein levels, which results in decreased Cdc2 activity, leading to a G2/M arrest (Fig. 5).
Fig. 5.
Proposed schemata of the mechanisms by which kaemp-ferol induces cell cycle arrest in HT-29 cells.
Downregulation of Cdc25C may also contribute to the decreased Cdc2 activity in cells treated with kaempferol. Because kaempferol can block both G1 and G2 checkpoints it can potentially be used to prevent the proliferation of colon cancer cells. Further efforts should be made to study the effect of kaempferol on animal and human models of colon cancer.
Acknowledgments
This research was supported by Basic Science research Program through the National Research Foundation of Korea (NRF), as funded by the Ministry of Education (NRF-2012R1A1A2006607) and by the NRF grant funded by the Korea government (MSIP) (NRF-2013R1A2A2A05 004533). The authors declare that they have no competing interest.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993;15:499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 3.Witte JS, Longnecker MP, Bird CL, Lee ER, Frankl HD, Haile RW. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol. 1996;144:1015–25. doi: 10.1093/oxfordjournals.aje.a008872. [DOI] [PubMed] [Google Scholar]
- 4.Denis L, Morton MS, Griffiths K. Diet and its preventive role in prostatic disease. Eur Urol. 1999;35:377–87. doi: 10.1159/000019912. [DOI] [PubMed] [Google Scholar]
- 5.Kuo SM. Dietary flavonoid and cancer prevention: evidence and potential mechanism. Crit Rev Oncog. 1997;8:47–69. doi: 10.1615/critrevoncog.v8.i1.30. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel U, Kuntz S, Brendel MD, Daniel H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000;60:3823–31. [PubMed] [Google Scholar]
- 7.Hoensch HP, Kirch W. Potential role of flavonoids in the prevention of intestinal neoplasia: a review of their mode of action and their clinical perspectives. Int J Gastrointest Cancer. 2005;35:187–95. doi: 10.1385/IJGC:35:3:187. [DOI] [PubMed] [Google Scholar]
- 8.Somerset SM, Johannot L. Dietary flavonoid sources in Australian adults. Nutr Cancer. 2008;60:442–9. doi: 10.1080/01635580802143836. [DOI] [PubMed] [Google Scholar]
- 9.Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogh J, Trempe G. New Human tumor cell lines. In: Fogh J, editor. Human tumor cells in vitro. New York: Plenum Publishing Corp; 1975. pp. 115–59. [Google Scholar]
- 11.Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JHY. Trans-10, cis-12 conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002;283:G357–G67. doi: 10.1152/ajpgi.00495.2001. [DOI] [PubMed] [Google Scholar]
- 12.Cho HJ, Kim EJ, Lim SS, Kim MK, Sung MK, Kim JS, et al. Trans-10, cis-12, not cis-9, trans-11, conjugated linoleic acid inhibits G1-S progression in HT-29 human colon cancer cells. J Nutr. 2006;136:893–8. doi: 10.1093/jn/136.4.893. [DOI] [PubMed] [Google Scholar]
- 13.Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, et al. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003;284:G996–1005. doi: 10.1152/ajpgi.00347.2002. [DOI] [PubMed] [Google Scholar]
- 14.Lu X, Jung J, Cho HJ, Lim do Y, Lee HS, Chun HS, et al. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–90. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 15.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–60. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein IB. Cancer prevention: recent progress and future opportunities. Cancer Res. 1991;51:5080s–5s. [PubMed] [Google Scholar]
- 17.Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev. 2011;5:1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bobe G, Sansbury LB, Albert PS, Cross AJ, Kahle L, Ashby J, et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2008;17:1344–53. doi: 10.1158/1055-9965.EPI-07-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–42. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 20.Radtke J, Linseisen J, Wolfram G. Fasting plasma concentrations of selected flavonoids as markers of their ordinary dietary intake. Eur J Nutr. 2002;41:203–9. doi: 10.1007/s00394-002-0377-z. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Baker SJ, Reddy EP. CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer. 2012;3:658–69. doi: 10.1177/1947601913478972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdonald JI, Dick FA. Posttranslational modifications of the retinoblastoma tumor suppressor protein as determinants of function. Genes Cancer. 2012;3:619–33. doi: 10.1177/1947601912473305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–9. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 25.Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin- dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 2002;9:1287–93. doi: 10.1038/sj.cdd.4401130. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya EK. G2 cell cycle arrest-a direct link between PKA and Cdc25C. Cell Cycle. 2003;2:39–41. doi: 10.4161/cc.2.1.291. [DOI] [PubMed] [Google Scholar]