Abstract
Background:
Oxidative stress damages to cells or tissues, however, cellular defense systems including heme oxygenase-1 (HO-1) protects them against oxidative stress. Flavonoid compounds can activate cellular defense mechanisms against oxidative stress and it can reduce cell damages. In the present study, the cytoprotective effects of morin (3,5,7,2’,4’-pentahydroxyflavone), in terms of HO-1 enzyme, against the oxidative stress and its involved mechanisms was elucidated.
Methods:
RT-PCR and western blot analysis were assessed to detect the mRNA and protein expression, respectively. Cell viability was measured by using MTT test. The immunocytochemistry was performed to define location of target protein. Electrophoretic mobility shift assay performed to measure transcription factor-promoter site binding activity.
Results:
Morin elevated mRNA and protein levels of HO-1 in human lens epithelial cells (HLE-B3). HO-1 inhibitor ZnPP attenuated the protective effect of morin against H2O2-induced cytotoxicity. Morin increased the protein level of transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which up-regulates HO-1 expression by binding to the antioxidant response element (ARE) within the HO-1 gene promoter. Moreover, morin induced the translocation of Nrf2 from the cytosol into the nucleus. Morin activated extracellular-regulated kinase (ERK), while ERK inhibitor attenuated morin-enhanced Nrf2 and HO-1 expression.
Conclusions:
Morin activates ERK-Nrf2 signaling cascades in HLE-B3 cells, leading to the up-regulation of HO-1 and cytoprotection against oxidative stress.
Keywords: Morin, HO-1, Nrf2, Oxidative stress
INTRODUCTION
Reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical and peroxide are implicated in oxidative stress and it has been strongly linked with the formation of various degenerative diseases including cancer1 and cataract.2–4 In cancer cells, ROS-induced hyper-phosphorylation of JNK can translate oncogenic signals, thus supporting cellular proliferation by activation of AP-1, in addition to the proliferation signals mediated by ERK.1 Therefore, ROS may play an important role in the promotion phase of tumor generation and also, ROS is suggested to implicate damage to the lens.5 Cellular defense system against oxidative stress contains active antioxidant defense system such as thioredoxin reductase, glutathione, catalase, NADH: quinone oxidoreductase 1, superoxide dismutase and heme oxygenase-1 (HO-1).6,7 HO-1 catalyzes the oxygen-dependent degradation of heme to biliverdin, iron, and carbon monoxide using reducing equivalents. HO-1 is highly expressed in spleen and liver and inducible by various substances. Since HO-1 is induced as a protective mechanism in response to various stimuli, targeted induction of this enzyme may be considered as an important therapeutic strategy for the protection against oxidative tissue damage.7 A number of intracellular signaling molecules have been identified to be involved in regulating the induction of HO-1. A major transcription factor of HO-1 is nuclear factor erythroid 2-related factor 2 (Nrf2).8 Nrf2, a member of the cap’n’ collar family of bZIP transcription factor, has been known to play an important role in the antioxidant response element (ARE)-mediated expression of phase II detoxifying, antioxidant enzymes.
Flavonoids including flavone, flavanone, flavonol, and isoflavone are polyphenolic compounds which are widespread in food and beverages and possess a wide range of biological activities. It has recently attracted a great interest as potential therapeutic agents against a large variety of disease. Morin (3,5,7,2’,4’-pentahydroxyflavone) has been used as herbal medicines.9 Morin contains wide range of biological actions including antioxidant properties.10,11 Recently, we have reported that morin protected cells against oxidative stress induced by hydrogen peroxide and γ-ray radiation.12,13 In the present study, we examined the cytoprotective effects of morin, in terms of HO-1 enzyme, against the oxidative stress and its involved mechanisms.
MATERIALS AND METHODS
1. Cell culture
Human lens epithelial cells (HLE-B3) were grown in Dulbecco’s modified eagle medium (DMEM) supplemented with 20% fetal bovine serum in a humidified 5% CO2 atmosphere at 37°C.
2. Materials
Morin was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The phospho ERK and ERK antibodies were provided from Cell Signaling Technology (Beverly, MA, USA). Nrf2 antibody was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). HO-1 antibody was supplied by Stressgen Biotechnologies (Victoria, BC, Canada).
3. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from HLE-B3 cells using TRIzol® reagent (Invitrogen, Carlbad, CA, USA) according to the manufacturer’s instructions. RT-PCR was performed following standard procedures. Amplification products were resolved by 1.2% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light. Primers were purchased from Bionics (Seoul, Korea). PCR conditions for HO-1 and for the house- keeping gene, glyceraldehyde-3-phophate dehydrogenase (GAPDH) were as follow: HO-1, 25 cycles of 95°C for 1 min; 60°C for 1 min, 72°C for 2 min, GAPDH, 26 cycles of 94°C for 1 min; 56°C for 2 min; 72°C for 2 min. The pairs of primers were as follows: HO-1 sense 5’-CAGGCAGAGAATGCTGAGTT C-3’ and antisense 5’-GATGTTGAGCAGGAACGCAGT-3’, GAPDH sense 5’-AAGGTCGGAGTCAACGGATTT-3’ and antisense 5’-GCAGTGAGGGTCTCTCTCCCT-3’.
4. Western blot analysis
Cell or nuclear lysates were collected, and protein concentrations were determined using the Bradford reagent. Aliquots of the lysates (40 μg of protein) were boiled for 5 min and electrophoresed on 10% SDS-polyacrylamide gels. Gels were transferred onto nitrocellulose membranes. Membranes were then incubated with the indicated primary antibodies and further incubated with secondary immunoglobulin G-horseradish peroxidase conjugates. Protein bands were visualized by developing the blots using an enhanced chemiluminescence western blotting detection kit (Amersham, Buckinghamshire, UK) and exposing the membranes to X-ray film.
5. Cell viability
The effect of morin on the cell viability was determined by the MTT assay, which is based on the reduction of a tetrazolium salt by mitochondrial dehydrogenase in viable cells.14 Cells were seeded in a 96 well plate at a density of 1×105 cells/ml and treated with 50 μM of morin and 10 μM ZnPP (an inhibitor of HO-1), followed 1 h later by 1 mM of H2O2. After incubating for 48 h at 37°C, 50 μl of the MTT stock solution (2 mg/ml) was then added to each well to attain a total reaction volume of 250 μl. After incubation for 2.5 h, the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 μl dimethyl sulfoxide, and the OD540 was read on a scanning multi-well spectrophotometer.
6. Immunocytochemistry
Cells plated on coverslips were fixed with 1% paraformaldehyde for 30 min and permeabilized with 2% Triton X-100 in phosphate buffered-saline (PBS) for 2.5 min. Cells were treated with blocking medium (1% bovine serum albumin in PBS) for 1 h and incubated with Nrf2 antibody diluted in blocking medium for 2 h. Immuno-reacted primary Nrf2 antibody was detected by a 1 : 200 dilution of FITC-conjugated secondary antibody (Jackson Immuno-Research Laboratories, West Grove, PA, USA) for 1 h. After washing with PBS, stained cells were mounted onto microscope slides in mounting medium with DAPI (Vector, Burlingame, CA, USA). Images were collected using the LSM 510 program on a Zeiss confocal microscope.
7. Electrophoretic mobility shift assay (EMSA)
Synthetic double strand oligonucleotide containing the Nrf2 binding domain (ARE) was labeled with [γ-32P] ATP using T4 polynucleotide kinase and separated from unincorporated [γ-32P] ATP by gel filtration using a nick spin column (Pharmacia Biotech, Bjorkgatan, Sweden). Prior to addition of the 32P-labeled oligonucleotide (100,000 cpm), 10 μg of the nuclear extract was incubated on ice for 15 min in gel shift binding buffer [20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl and 50 mM Tris-HCl, pH 7.5 with 0.25 μg/ml poly (dI-dC)] DNA-protein complexes were resolved by 6% polyacryl amide gel electrophoresis at 200 V for 2 h followed by autoradiography.
8. Statistical analysis
All measurements were made in triplicate, and all values are expressed as the mean±the standard error. The results were subjected to an analysis of variance (ANOVA) followed by the Tukey test to analyze differences between means. A P-value of <0.05 was considered significant.
RESULTS
1. Induction in protein and mRNA of HO-1 in mo-rin-treated HLE-B3 cells
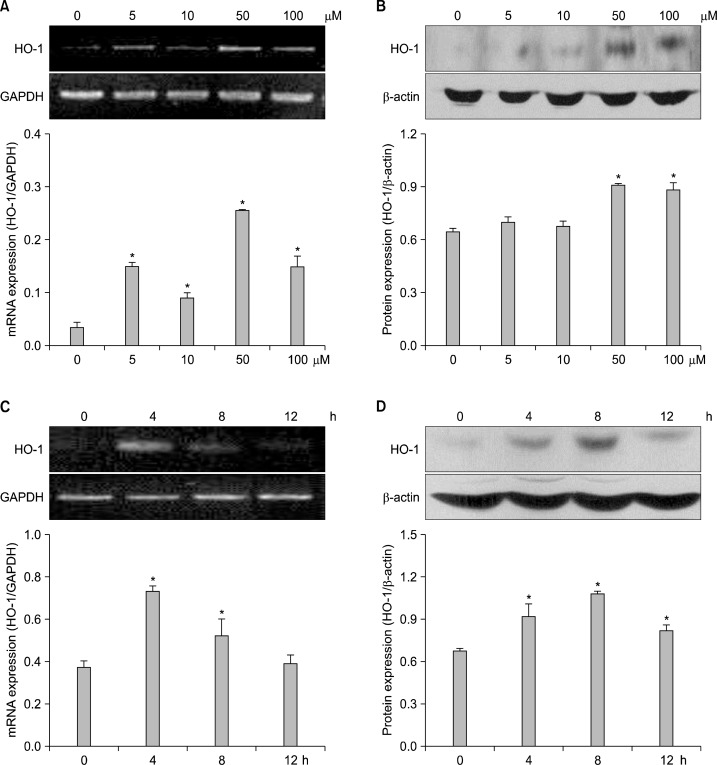
To investigate whether morin has an ability to induce HO-1 expression in HLE-B3 cells, the cells were treated with morin at various concentrations. Morin at 50 μM significantly increased HO-1 mRNA and protein expression (Fig. 1A and B). And morin at 50 μM significantly increased expression of HO-1 mRNA at 4 h (Fig. 1C). 1n parallel with elevated expression of the mRNA transcript, the protein level of HO-1 in morin-treated cells was also increased at 4 h (Fig. 1D). These results suggested that morin induced HO-1 expression.
Fig. 1.
mRNA and protein expression of HO-1 in morin-treated HLE-B3 cells. Cells were treated with various concentrations of morin at 8 h. (A) RT-PCR analysis was conducted to measure induction of mRNA transcript of HO-1. *Significantly different from control cells (P<0.05). (B) Western blot analysis was conducted to measure induction of HO-1 protein. *Significantly different from control cells (P<0.05). Cells were treated with 50 μM of morin for various times. (C) RT-PCR analysis was performed. *Significantly different from control cells (P<0.05). (D) Western blot analysis was performed. *Significantly different from control cells (P<0.05).
2. Involvement of HO-1 in cell damage induced by oxidative stress
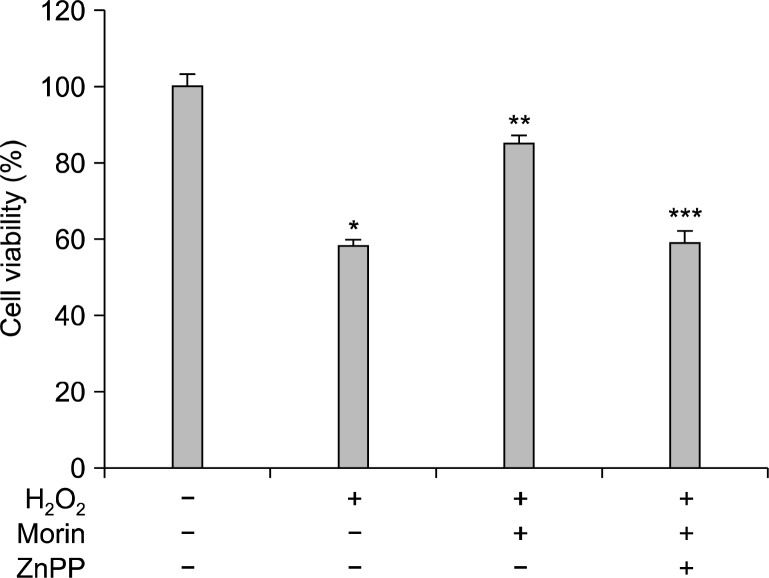
To determine whether the HO-1 enhanced by morin confers cytoprotection against oxidative stress, cells were pretreated with the HO-1 inhibitor ZnPP. ZnPP attenuated the protective effect of morin against H2O2-induced cytotoxicity (Fig. 2). Therefore, the cytoprotective effect of morin was mediated through HO-1 induction.
Fig. 2.
The cytoprotective effect of morin against H2O2-induced cell death. Cells were pre-incubated with ZnPP at 10 μM for 2 h, followed by 1 h of incubation with morin and exposure to 1 mM of H2O2 for 48 h. Cell viability was measured using the MTT assay. *Significantly different from control cells (P<0.05), **Significantly different from H2O2-treated cells (P<0.05), and ***Significantly different from morin plus H2O2-treated cells (P<0.05).
3. Increase of nuclear translocation and ARE binding activity of Nrf2 in morin-treated HLE-B3 cells
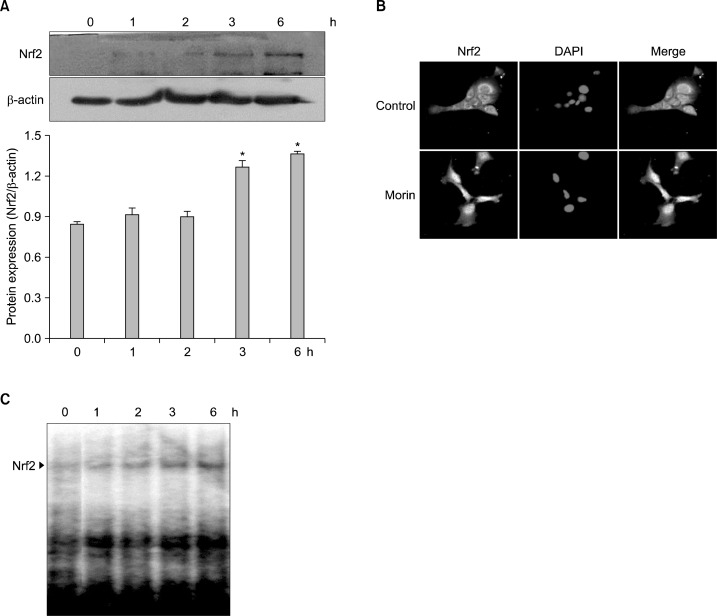
To evaluate the ability of morin to induce Nrf2, a transcription factor modulating HO-1 expression, western blot, immunocytochemistry, and EMSA performed with nuclear extracts. The induction of Nrf2 expression after 3 h was observed in morin-treated cells (Fig. 3A), and also the translocation of Nrf2 to nucleus was observed (Fig. 3B). Furthermore, EMSA data showed that morin-treated cells increased the ability of Nrf2 binding to ARE (Fig. 3C). These results suggested that morin increased nuclear translocation and ARE binding activity of Nrf2.
Fig. 3.
Morin activates Nrf2 in HLE-B3 cells. Cells were treated with 50 μM of morin for various times. (A) The nuclear lysates from morin-treated cells were immune-blotted with Nrf2 specific antibody. *Significantly different from control cells (P<0.05). (B) Cells were treated with morin for 6 h and immunocytochemistry was performed to define nuclear translocation of Nrf2. (C) Nuclear extracts were subjected to EMSA for measurement of NRf2-ARE binding activity.
4. Increase of ARE-binding activity of Nrf2 and HO-1 expression by ERK signaling in morin-treated cells
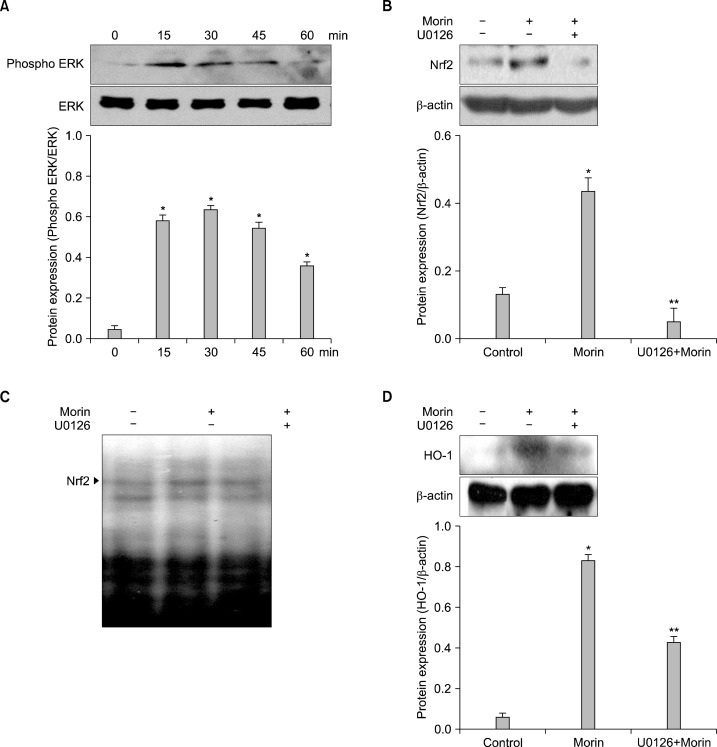
To investigate the upstream of Nrf2-ARE signaling, the activation of ERK was monitored by western blot analysis using the phopho-ERK antibody. Morin induced the expression of active ERK1/2 (phospho-ERK1/2) (Fig. 4A). Because ERK is considered to be critical for the inducible expression of Nrf2, it was examined whether the ERK inhibitor can suppress the activation of Nrf2 in morin-treated cells. Morin-induced nuclear Nrf2 expression and ARE binding activity of Nrf2 were inhibited by U0126 (Fig. 4B and C). And HO-1 level increased by morin was also inhibited by the U0126 (Fig. 4D). These results suggested that morin-induced HO-1 expression was partially regulated via ERK pathway.
Fig. 4.
Morin up-regulates HO-1 via phosphorylation of ERK. Cells were treated with morin for various times. (A) Western blot were performed to detect expression of ERK. *Significantly different from control cells (P<0.05). Cells were pre-incubated with inhibitor-U0126 (2.5 μM) for 1 h and the protein was analyzed by (B) western blot and (C) DNA binding activity was detected by EMSA. *Significantly different from control cells (P<0.05). **Significantly different from morin-treated cells (P<0.05). (D) Cells were pre-incubated with U0126 for 1 h, and after incubation of 8 h, the HO-1 expression was analyzed by western blot. *Significantly different from control cells (P<0.05). **Significantly different from morin-treated cells (P<0.05).
DISCUSSION
Oxidative stress via excess ROS plays a key role in the initiation and progression of carcinogenesis including retinoblastoma.15–18 Aerobic organisms including mammals generate ROS as part of normal aerobic metabolism, and have developed elaborate antioxidant mechanisms for combating oxidative stress. The effective antioxidant defense systems for combating the toxicities of ROS is a family of antioxidant enzymes.3,6,7,19 The chemo-preventive effects of many synthetic and natural substances that reduce the cancer risk could be attributed to induction of antioxidant enzymes.20,21
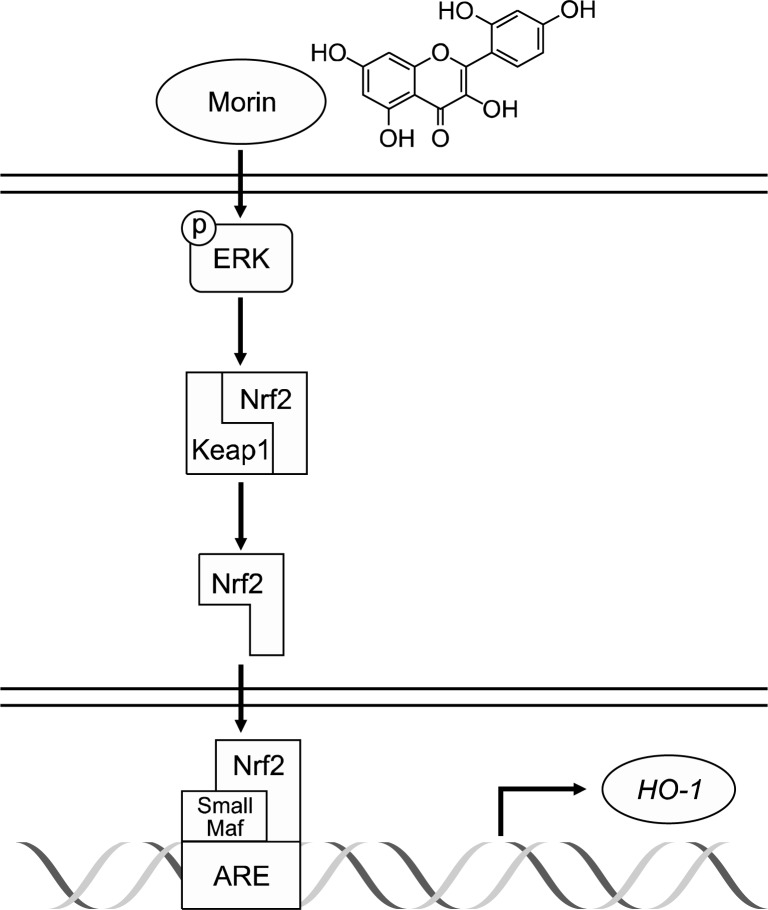
Morin has a phenolic structure with hydroxyl groups at C2’ and C4’. Some studies has revealed that morin have anti-hypertensive effect,9,22 anti-cancer effect,23,24 and anti-hepatocellular transformation effect.25 It has reported that morin exhibited cytoprotection against oxidative stress induced by hydrogen peroxide via inhibiting ROS generation and inducing catalase activation;12 morin protected cells against oxidative stress induced by γ-ray radiation via reduction of ROS level and attenuation of SEK1-JNK-AP 1 pathway.13 The present study showed that morin increased HO-1 expression in human lens epithelial cells and HO-1 inhibitor attenuated the cytoprotective effect of morin against oxidative stressed-cell damage. A number of intracellular signaling molecules have been identified to be involved in regulating the induction of HO-1 including AP-1,26 NF-kB,27 and Nrf2.28 A transcription factor Nrf2 is kept in cytosol fraction as an inactive form by inhibitor protein-Keap1. Release of Nrf2 from Keap1 leads to nuclear translocation and form heterodimer with small Maf protein,29 and then binds to ARE elements resulting in expression of HO-1.8,29 Morin increased the nuclear levels of the Nrf2 and its binding to the ARE. It is reported that ERK phosphorylates Nrf2 which may facilitate the release of Nrf2 from the Keap1-Nrf2 complex, allowing activated Nrf2 to translocate into the nucleus where it forms a heterodimer with the small Maf protein.30 Our present studies also showed that the morin induced the activation of ERK and ERK inhibitor attenuated the morin-induced Nrf2 expression, DNA binding activity, and HO-1 level. It suggests that morin induces HO-1 expression via ERK-Nrf2 signaling pathway (Fig. 5). In conclusion morin enhanced cellular defense against oxidative stress and might develop a possible chemo-preventive substance.
Fig. 5.
A proposed pathway for morin-induced HO-1 via up-regulation of ERK, and Nrf2, explaining the cytoprotective effect against oxidative stress.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1120340).
REFERENCES
- 1.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress-signaling in cancer. EMBO Reports. 2002;3:420–5. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long AC, Colitz CM, Bomser JA. Apoptotic and necrotic mechanisms of stress-induced human lens epithelial cell death. Exp Biol Med (Maywood) 2004;229:1072–80. doi: 10.1177/153537020422901012. [DOI] [PubMed] [Google Scholar]
- 3.Lin D, Barnett M, Grauer L, Robben J, Jewell A, Takemoto L, et al. Expression of superoxide dismutase in whole lens prevents cataract formation. Mol Vis. 2005;11:853–8. [PubMed] [Google Scholar]
- 4.Shichi H. Cataract formation and prevention. Expert Opin Investig Drugs. 2004;13:691–701. doi: 10.1517/13543784.13.6.691. [DOI] [PubMed] [Google Scholar]
- 5.Vinson JA. Oxidative stress in cataracts. Pathophysiology. 2006;13:151–62. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Padgaonkar VA, Leverenz VR, Dang L, Chen SC, Pelliccia S, Giblin FJ. Thioredoxin reductase may be essential for the normal growth of hyperbaric oxygen-treated human lens epithelial cells. Exp Eye Res. 2004;79:847–57. doi: 10.1016/j.exer.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–91. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 8.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 9.Kang DG, Moon MK, Sohn EJ, Lee DH, Lee HS. Effects of morin on blood pressure and metabolic changes in fructose-induced hypertensive rats. Biol Pharm Bull. 2004;27:1779–83. doi: 10.1248/bpb.27.1779. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb M, Leal-Campanario R, Campos-Esparza MR, San-chez-Gomez MV, Alberdi E, Arranz A, et al. Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis. 2006;23:374–86. doi: 10.1016/j.nbd.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53:8537–41. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH, Chang WY, et al. Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem Biol Interact. 2009;177:21–7. doi: 10.1016/j.cbi.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Kang KA, Kang SS, Park JW, Hyun JW. Morin (2’,3,4’,5,7-pentahydroxyflavone) protected cells against γ- radiation-induced oxidative stress. Basic Clin Pharmacol Toxicol. 2011;108:63–72. doi: 10.1111/j.1742-7843.2010.00629.x. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–42. [PubMed] [Google Scholar]
- 15.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 16.Deepa PR, Nalini V, Mallikarjuna K, Vandhana S, Krishna-kumar S. Oxidative stress in retinoblastoma: correlations with clinicopathologic features and tumor invasiveness. Curr Eye Res. 2009;34:1011–8. doi: 10.3109/02713680903291139. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay S, Dutta J, Raut R, Datta H, Bhattacharyay AK. Expression of oxidative stress in metastatic retino-blastoma-a comparative study. Nepal J Ophthalmol. 2012;4:271–6. doi: 10.3126/nepjoph.v4i2.6543. [DOI] [PubMed] [Google Scholar]
- 18.Vandhana S, Lakshmi TS, Indra D, Deepa PR, Krishnakumar S. Microarray analysis and biochemical correlations of oxidative stress responsive genes in retinoblastoma. Curr Eye Res. 2012;37:830–41. doi: 10.3109/02713683.2012.678544. [DOI] [PubMed] [Google Scholar]
- 19.Fan YJ, Rong Y, Li PF, Dong WL, Zhang DY, Zhang L, et al. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyltransferase and antioxidant enzymes. Food Chem Toxicol. 2013;55:172–81. doi: 10.1016/j.fct.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Chow JM, Shen SC, Huan SK, Lin HY, Chen YC. Querce-tin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem Pharmacol. 2005;69:1839–51. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Jrah-Harzallah H, Ben-Hadj-Khalifa S, Almawi WY, Maaloul A, Houas Z, Mahjoub T. Effect of thymoquinone on 1, 2- dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur J Cancer. 2013;49:1127–35. doi: 10.1016/j.ejca.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Herrera MD, Zarzuelo A, Jimenez J, Marhuenda E, Duarte J. Effects of flavonoids on rat aortic smooth muscle contractility: structure-activity relationships. Gen Pharmacol. 1996;27:273–7. doi: 10.1016/0306-3623(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 23.Brown J, O’Prey J, Harrison PR. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: Involvement of the akt and stress kinase pathways. Carcinogenesis. 2003;24:171–7. doi: 10.1093/carcin/24.2.171. [DOI] [PubMed] [Google Scholar]
- 24.Kuo HM, Chang LS, Lin YL, Lu HF, Yang JS, Lee JH, et al. Morin inhibits the growth of human leukemia HL-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res. 2007;27:395–405. [PubMed] [Google Scholar]
- 25.Hsiang CY, Wu SL, Ho TY. Morin inhibits 12-O-tetradecano-ylphorbol-13-acetate-induced hepatocellular transformation via activator protein 1 signaling pathway and cell cycle progression. Biochem Pharmacol. 2005;69:1603–11. doi: 10.1016/j.bcp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Hartsfield CL, Alam J, Choi AM. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. FASEB J. 1998;12:1675–82. doi: 10.1096/fasebj.12.15.1675. [DOI] [PubMed] [Google Scholar]
- 27.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–61. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 28.Morimitsu Y, Nakagawa Y, Hayashi K, Fujii H, Kumagai T, Nakamura Y, et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J Biol Chem. 2002;277:3456–63. doi: 10.1074/jbc.M110244200. [DOI] [PubMed] [Google Scholar]
- 29.Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA. 2004;101:6379–84. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zipper LM, Mulcahy RT. Erk activation is required for Nrf2 nuclear localization uring pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci. 2003;73:124–34. doi: 10.1093/toxsci/kfg083. [DOI] [PubMed] [Google Scholar]