Abstract
Helicobacter pylori (H. pylori) induced DNA damage which may be related to gastric cancer development. The DNA damage response coordinates DNA repair, cell-cycle transition, and apoptosis through activation of DNA damage response molecules. The damaged DNA is repaired through non-homologous end joining (NHEJ) or homologous recombination (HR). In the present study, we investigated the changes of HR DNA repair proteins (ataxia-telangiectasia-mutated; ATM, ATM and Rad3-related; ATR), NHEJ repair proteins (Ku70/80), cell cycle regulators (Chk1, Chk2), and apoptosis marker (p53/p-p53) were determined in H. pylori-infected Mongolian gerbils. In addition, the effect of an antioxidant N-acetylcysteine (NAC) on H. pylori-induced DNA damage response was determined to assess the involvement of oxidative stress on DNA damage of the animals infected with H. pylori. One week after intragastric inoculation with H. pylori, Mongolian gerbils were fed with basal diet with or without 3% NAC for 6 weeks. After 6 week, the expression levels of DNA repair proteins (Ku70/80, ATM, ATR), cell cycle regulators (Chk1, Chk2) and apoptosis marker (p-p53/p53) were increased in gastric mucosa of Mongolian gerbils, which was suppressed by NAC treatment. In conclusion, oxidative stress mediates H. pylori-induced DNA damage response including NHEJ and HR repairing processes, cell cycle arrest and apoptosis in gastric mucosa of Mongolian gerbils.
Keywords: Helicobacter pylori, DNA damage response, Oxidative stress, Mongolian gerbil
INTRODUCTION
Helicobacter pylori (H. pylori) infection leads to gastroduodenal inflammation, peptic ulceration, and gastric carcinoma.1,2 A characteristic event in the infected tissue is the infiltration of the subepithelial gastric lamina propria by phagocytes, mainly neutrophils and macrophages, that produce large amounts of reactive oxygen species (ROS). ROS activate the oxidant-sensitive transcription factor NF-κB, which induces expression of oncogenes and cell-cycle regulators.3,4 H. pylori-elicited neutrophils produce ROS, which subsequently injure gastric mucosal cells including DNA damage.5
Cell death linked to DNA damage has been implicated in various diseases caused by environmental stress and infection. Severe DNA damage, which is beyond the capacity of the DNA repair proteins, triggers apoptosis.6 Accumulation of DNA damage has been proposed to be a principal mechanism of infection, inflammation, cancer, and aging. Ataxia-telangiectasia-mutated (ATM) and ATM and Rad3-related (ATR) are the main transducers of the DNA strand break signal. Once the DNA is damaged, the DNA repair protein Ku70/80 translocates into the nucleus, a process which may be mediated by ATM, a member of the phosphoinositide-3-kinase-like family.7–11 The damaged DNA is repaired through non-homologous end joining (NHEJ) or homologous recombination (HR). ATM and ATR are critical molecules initiating HR repair process while Ku70/80 initiate NHEJ DNA repair process. Therefore, the activation of both ATM/ATR and Ku70/80 are important in DNA repair process.12–15
However, DNA repair proteins interact with other molecules to repair the damaged DNA through NHEJ or HR. For instance, ATM phosphorylates key proteins (Ku70/80 and Artemis) to repair the damaged DNA by NHEJ. On the other hand, ATM temporarily arrests the cell cycle by phosphorylating CHK2, which in turn phosphorylates p53, while the damage is being repaired. ATM repairs the damaged DNA through HR by interacting with ATR.
In the present study, we investigated the changes of HR DNA repair proteins (ATM, ATR), NHEJ repair proteins (Ku70/80), cell cycle regulators (Chk1, Chk2), and apoptosis marker (p53/p-p53) were determined in H. pylori-infected Mongolian gerbils. In addition, the effect of an antioxidant N-acetylcysteine (NAC) on H. pylori-induced DNA damage response (DDR) was determined to assess the involvement of oxidative stress on DNA damage of the animals infected with H. pylori.
MATERIALS AND METHODS
1. Animals
Five-week-old male specific-pathogen-free Mongolian gerbils (MGS/Sea) with an average weight of approximately 40 g were purchased from Charles River Laboratories (Wilmington, MA, USA). Gerbils were housed in polypropylene cages on hard wood chip bedding in groups of five per cage. Food and water were provided ad libitum. The animals were maintained in a temperature-controlled room (22±2°C) with a 12-h light-dark cycle. The animal experiments were performed in accordance with institutional guidelines. Protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Yonsei University Medical Center (YUMC; Permit #: 10–107). 10 gerbils are included in each group. All animals were maintained in the specific pathogen-free facility at YUMC.
2. Bacterial inoculation
H. pylori strain 7.13 was maintained as frozen stock at −80°C in brain-heart infusion medium that was supplemented with 20% glycerol and 10% fetal bovine serum. Bacteria was grown on horse blood agar plates containing 4% Columbia agar base (Oxoid, Hampshire, UK), 5% defibrinated horse blood (HemoStat Labs, Dixon, CA), 0.2% β-cyclodextrin, 10 μg/ml vancomycin, 5 μg/ml cefsulodin, 2.5 U/ml polymyxin B, 5 μg/ml trimethoprim, and 8 μg/ml amphotericin B at 37°C under microaerophilic conditions. A microaerobic atmosphere was generated using a CampyGen sachet (Oxoid) in a gas pack jar. For liquid culture, H. pylori was grown in brucella broth (Difco & BBL Diagnostics, Franklin Lakes, NJ, USA) containing 10% FBS (Gibco-BRL, Grand Island, NY, USA). Cultures were shaken in a microaerobic environment. According to the growth curve, 108 bacteria were collected and resuspended in 500 μl of brucella broth for the infection of each animal.
3. Experimental design
One week after inoculation with H. pylori, Mongolian gerbils were fed AIN76A diet (Research Diets, Inc, New Brunswick, NJ, USA) with 3% NAC (w/w) for 6 weeks. As a negative control, Mongolian gerbils that were not inoculated with H. pylori were fed the AIN76A diet. Gerbils that were inoculated with H. pylori were fed the diet AIN76A and considered as a positive H. pylori control without NAC.
The level of NAC supplementation (3%, w/w) was adapted from previous study showing the inhibitory effect of NAC against benzo [α] pyrene-induced skin tumor.16 Body weight and food intake were measured every week during the experimental period. There was no difference among groups in body weight and food intake during the experimental periods (data not shown). Gastric mucosal samples were homogenized in 10 mM Tris buffer (pH 7.4). The homogenates were used for determining protein levels of ATM, ATR, Ku70 Ku80, Chk1, Chk2, p53 and p-p53.
4. Western blot analysis
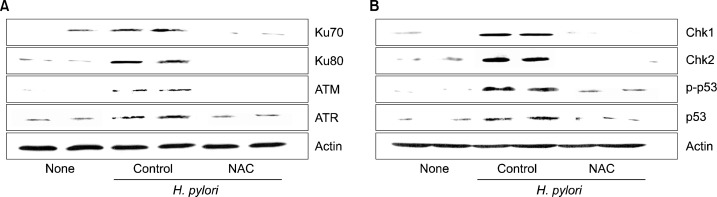
Total cell extracts were prepared from gastric mucosa and separated by SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions. Samples were then transferred onto membranes (Amersham Inc., Arlington Heights, IL, USA) by electroblotting. After blocking using 5% nonfat dry milk, the membranes were incubated with antibodies for ATM, ATR, Ku70 Ku80, p53 and p-p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), antibodies for Chk1 and Chk2 (Cell Signaling Technology, Inc., Beverly, MA, USA) and anti-actin antibody (Santa Cruz Biotechnology). The immunoreactive proteins were visualized using anti-mouse secondary antibody conjugated to horse-radish peroxidase, followed by enhanced chemiluminescence (Amersham). Actin was used as a loading control. Among 10 gerbils for each group, the representative two protein bands of two animals for each group were shown in Fig. 1.
Fig. 1.
Protein levels of DDR molecules in H. pylori-infected gastric mucosal tissues of Mongolian gerbils. (A) The levels of ATM, ATR, Ku70, and Ku80 as well as (B) Chk1, Chk2, p53, and p-p53 in gastric mucosal tissues were measured by western blotting. Two representative bands per group are shown. Abbreviations: None, animals without H. pylori infection that were fed the control diet; H. pylori control, animals with H. pylori infection that were fed the control diet; H. pylori+NAC, animals with H. pylori infection that were fed a diet supplemented with NAC.
RESULTS
To determine whether H. pylori infection induces DDR, we determined the changes of HR DNA repair proteins (ataxia-telangiectasiamutated; ATM, ATM and Rad3-related; ATR), NHEJ repair proteins (Ku70/80), cell cycle regulators (Chk1, Chk2), and apoptosis marker (p53/p-p53) in H. pylori-infected Mongolian gerbils. As shown in Fig. 1, ATM, ATR, Ku70 and Ku80 were increased by H. pylori-infected tissues. Down-stream regulators such as Chk1 and Chk2 as well as p-53 were induced by H. pylori infection.
To investigate the inhibitory effects of NAC against H. pylori-induced DNA damage, the expression levels of DNA repair proteins (ATM, ATR, Ku70/80) as well as Chk1, Chk2, p53/p-p53 were determined in the gastric mucosal tissues of animals infected with H. pylori that were and were not supplemented with NAC. Protein levels of DDR molecules induced by H. pylori infection were lower in the NAC-treatment group than in the control-diet group, as determined by western blotting, respectively.
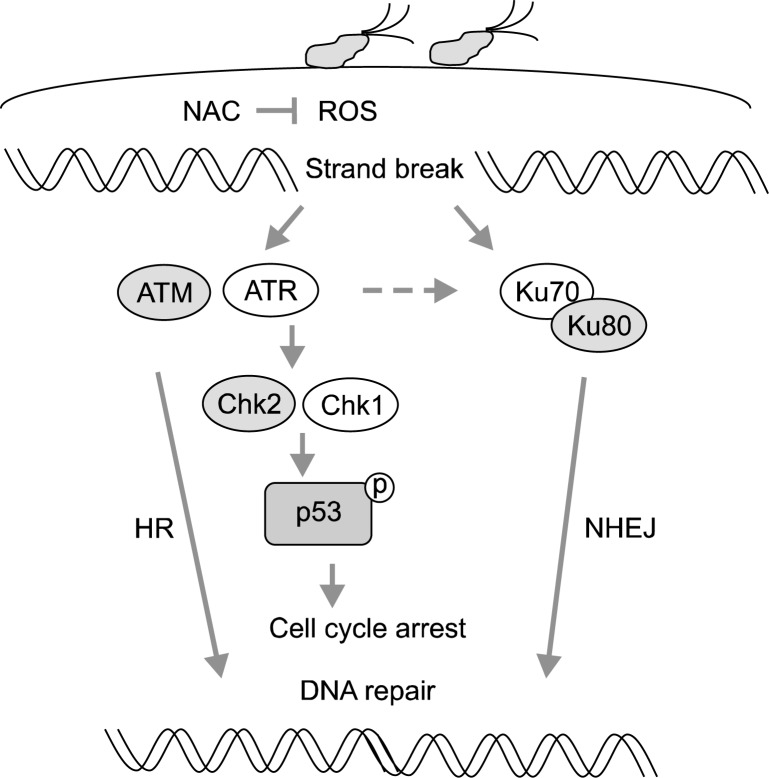
The level of phospho-p53 was greater in the H. pylori-infected groups than in the non-infected group, and was lower in the NAC-treatment group than in the control-diet group. Since p53 is activated by H. pylori infection, apoptosis may be occurred in the infected tissues. Inhibitory effect of NAC on H. pylori-induced expression of DDR molecules and activation of p53 indicates that oxidative stress induces DNA damage in the infected tissues. The results suggest that suppression of oxidative stress may prevent H. pylori-induced carcinogenesis by inhibiting DNA damage and related DDR in the gastric mucosal tissues. Fig. 2 shows the proposed scheme of H. pylori-induced DDR. H. pylori infection increases oxidative stress in the infected tissue which triggers DDR by inducing DNA repair proteins (HR, NHEJ) and cell cycle regulators (Chk1, Chk2) and activate/induce p53. During DNA repair occurs, cell cycle transitions induced and then the damaged DNA may be repaired. On the other hand, the cells may proliferate with the unrepaired DNA, which may contribute the development of carcinogenesis.
Fig. 2.
Proposed scheme of H. pylori-induced DDR. H. pylori infection increases oxidative stress in the infected tissue which triggers DDR by inducing DNA repair proteins (HR, NHEJ) and cell cycle regulators (Chk1, Chk2) and activate/induce p53. During DNA repair occurs, cell cycle is arrested and the damaged DNA may be repaired. On the other hand, the cells with the unrepaired DNA may proliferate, which may contribute to the development of cancer. DDR, DNA damage response; HR, homologous recombination; NHEJ, non-homologous end joining.
DISCUSSION
Animal models for H. pylori infection have been developed to replicate many features of human gastric carcinogenesis in order to test potential therapeutic agents for the prevention and treatment of H. pylori-associated gastric disease. The Mongolian gerbil model is the best animal model for this purpose because H. pylori infection induces chronic gastritis, gastric ulcers, and intestinal metaplasia in these animals. Mongolian gerbils develop gastric neoplasia and gastric cancer after chronic infection by H. pylori strain 7.13,17,18 as used in the present study.
DNA repair proteins interact with other molecules to repair the damaged DNA through NHEJ or HR. ATM signal is critical in cell cycle control and in cellular apoptosis via the p53 pathway.7–11 Ku proteins translocate into the nucleus upon the occurrence of DNA damage, and their nuclear transports are possibly controlled by phosphorylation. Present study shows that the damaged DNA induces the induction of ATM, ATR, Ku proteins and cell cycle transition as well as activation of p53 in H. pylori-infected tissues. Previously, we showed that nuclear loss of Ku proteins or ATM may be the underlying mechanism of oxidative stress-induced apoptotic cell death.19 However, in the present study, Ku proteins were induced by oxidative DNA damage. Therefore, Ku loss may not be the cause of H. pylori-associated gastric cancer development. Since ATM is activated by phosphorylation and nuclear transports of Ku proteins are possibly controlled by phosphorylation, further study should be performed to determine phosphorylation of Ku proteins or ATM in in gastric mucosa of H. pylori-infected Mongolian gerbils treated with or without NAC.
ATM plays a role in DNA double strand break repair in concert with the meiotic recombination 11 (Mre11)/Rad50/Nijmegen breakage syndrome 1 (NBS1) (M/R/N complex). DNA-protein kinase (DNA-PK) and ATM share several substrates as phosphorylation targets, including Artemis, p53, and histone H2AX.6 Interplays among Ku70/80, Artemis, DNA-PK, and ATM are involved in DNA damage responses. In addition, Ku70/80 also affects ATM-dependent ATR activation. In the present study, H. pylori infection induces the expression of Ku proteins and ATM as well as induction of p53 and phosphorylation of P53. Therefore, induction of DNA damage molecules may be defensive mechanism of the cells against DNA damage to repair the damaged DNA.
In conclusion, oxidative stress mediates H. pylori-induced DDR including NHEJ and HR repairing processes, cell cycle arrest, and apoptosis in gastric mucosa of Mongolian gerbils.
Acknowledgments
This study was supported by a 2012 grant from NRF Korea (NRF-2012R1A1A2043423).
REFERENCES
- 1.Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–33. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. J Am Med Assoc. 1994;272:65–9. [PubMed] [Google Scholar]
- 3.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Rad Biol Med. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 4.Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997–1003. doi: 10.1007/s00011-010-0245-x. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Miura S, Mori M, Kai A, Suzuki H, Fukumura D, et al. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–8. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morio T, Kim H. Ku, Artemis, and ataxia-telangiectasia-mutated: signalling networks in DNA damage. Int J Biochem Cell Biol. 2008;40:598–603. doi: 10.1016/j.biocel.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Kim KH, Morio T, Kim H. Ataxiatelangiectasia-mutated-dependent activation of Ku in human fibroblasts exposed to hydrogen peroxide. Ann N Y Acad Sci. 2006;1090:542–8. doi: 10.1196/annals.1378.056. [DOI] [PubMed] [Google Scholar]
- 8.Arrington ED, Caldwell MC, Kumaravel TS, Lohani A, Joshi A, Evans MK, et al. Enhanced sensitivity and long-term G2 arrest in hydrogen peroxide-treated Ku80-null cells are unrelated to DNA repair defects. Free Rad Biol Med. 2000;29:1166–76. doi: 10.1016/s0891-5849(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 9.Chechlacz M, Vemuri MC, Naegele JR. Role of DNA dependent protein kinase in neuronal survival. J Neurochem. 2001;78:141–54. doi: 10.1046/j.1471-4159.2001.00380.x. [DOI] [PubMed] [Google Scholar]
- 10.Difilippantonio MJ, Zhu J, Chen HT, Meffre E, Nussenzweig MC, Max EE, et al. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature. 2000;404:823–5. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Jin S, Gao Y, Weaver DT, Alt FW. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V (D) J recombination. Proc Natl Acad Sci USA. 1997;94:8076–81. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamsler A, Daily D, Hochman A, Stern N, Shiloh Y. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–54. [PubMed] [Google Scholar]
- 13.Tomimatsu N, Tahimic CG, Otsuki A, Burma S, Fukuhara A, Sato K, et al. Ku70/80 modulates ATM and ATR signaling pathways in response to DNA double strand breaks. J Biol Chem. 2007;282:10138–45. doi: 10.1074/jbc.M611880200. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Pluth JM, Cooper PK, Cowan MJ, Chen DJ, Yannone SM. Artemis deficiency confers a DNA doublestrand break repair defect and Artemis phosphorylation status is altered by DNA damage and cell cycle progression. DNA Repair (Amst) 2005;4:556–70. doi: 10.1016/j.dnarep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Succi J, Feng Z, Prithivirajsingh S, Stpry MD, Legerski RJ. Artemis is a phosphorylation target of ATM and ATR and is involved in the G2/M DNA damage checkpoint response. Mol Cell Biol. 2004;24:9207–20. doi: 10.1128/MCB.24.20.9207-9220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin KR, Trempus C, Saulnier M, Kari FW, Barrett JC, Freanch JE. Dietary N-acetyl-L-cysteine modulates benzo [a] pyrene-induced skin tumors in cancer-prone p53 haploin-sufficient Tg. aC (v-Ha-ras) mice. Carcinogenesis. 2001;22:1373–8. doi: 10.1093/carcin/22.9.1373. [DOI] [PubMed] [Google Scholar]
- 17.Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–87. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JY, Lim JW, Kim H, Morio T, Kim KH. Oxidative stress induces nuclear loss of DNA repair proteins, Ku70 and Ku80, and apoptosis in pancreatic acinar AR42J cells. J Biol Chem. 2003;278:36676–87. doi: 10.1074/jbc.M303692200. [DOI] [PubMed] [Google Scholar]