Abstract
Ovarian cancer is a lethal gynecological cancer causing cancer-related deaths in women worldwide. It is difficult to diagnosis at an early stage when more than 90% patients can be cured because of lack of specific symptoms and early detection markers. Most of malignant ovarian tumors are originated from the germinal epithelium of the ovary. For investigation with animal models of epithelial-derived ovarian cancer (EOC), laying hens are the most relevant animal models because they spontaneously develop EOC as occurs in women through ovulating almost every day. As in women, EOC in the hen is age-related and grossly and histologically similar to that in women. However, domesticated animals are inappropriate for research human EOC due to multiple pregnancies and lactating or seasonally anestrous. In addition, the non-spontaneous nature of rodents EOC limits clinical relevance with human EOC. Recent studies have shown that ovarian cancer could arise from epithelium from the oviduct as oviduct-related genes are up-regulated in EOC of hens. Therefore, we showed in the review: 1) characterization and classification of EOC; 2) chicken models for EOC; 3) relationship estrogen with EOC; 4) candidate prognostic factors for EOC including serpin peptidase inhibior, clade B (ovalbumin), member 3 (SERPINB3), SERPINB11, gallicin 11 (GAL11), secreted phosphoprotein 1 (SPP1) and alpha 2 macroglobulin (A2M) in normal and cancerous ovaries of laying hens; 5) biological roles of microRNAs in development of EOC. Collectively, the present reviews indicate that expression of SERPINB3, SERPINB11, GAL11, SPP1 and A2M is clearly associated with the development of ovarian carcinogenesis. These results provide new insights into the prognostic biomarkers for EOC to diagnose and to evaluate responses to therapies for treating EOC of humans.
Keywords: Epithelial-derived ovarian cancer, Prognostic factors, Hen, Estrogen, microRNA
INTRODUCTION
Ovarian cancer is the most fatal gynecological carcinoma even though it is the 8th most commonly diagnosed cancer and the 7th leading cause of cancer-related deaths in women worldwide.1 There are two hypothesis linked to carcinogenesis of ovaries, which indicate ‘incessant ovulation’ and ‘gonadotropin’ hypothesis. Incessant ovulation causes increase of epithelial ovarian cancer with the number of ovulation repeating ovarian rupture and repair.2 And gonadotropin-related hypothesis provides that incidence rate of the ovarian cancer is increased by high levels of gonadotropin such as FSH and LH through stimulating the ovarian epithelium surface.3 In a variety of previous studies, the first hypothesis related with spontaneous incessant ovulation has been reported having strong relationship with malignant transformation of ovaries. Recently several studies have suggested alternative theory that aggressive ovarian carcinomas arise from the fallopian tube in women.4,5
Ovarian cancer can be classified to three cancerous types, epithelial carcinoma, sex-cord stromal carcinoma and germ cell carcinoma.6,7 More than 90% of human ovarian cancers are originated from ovarian surface epithelium. And there are mainly four subtypes of epithelial-derived ovarian cancer that are serous (70%), endometrioid (10–20%), mucinous (3%) and clear cell (10%) carcinomas based on tumor cell morphology.8 Furthermore, staging of human ovarian cancer is described by FIGO system from Stage I to IV with presence or absence of metastasis and ascites. Due to the lack of specific symptom and prognostic factors to diagnose ovarian cancer, most patients with this disease present advanced stage (Stage III or IV).9,10 This fact causes approximately 70% of patients with ovarian cancer to death. Therefore, it is important to develop the valuable early detection marker to diagnosis or treatment for ovarian cancers.
To investigate the mechanisms of target genes to develop as a biomarker, laying hens are relevant models because they spontaneously induce EOC at a high rate after stop egg production as occur in women whereas other animals including mammals and rodents are not develop spontaneous in nature for ovarian cancer.11 Moreover, commonly used biomarkers to detect ovarian cancer clinically such as CA125 (also known as MUC16), epididymis protein 4 (HE4), proliferation markers including proliferating cell nuclear antigen (PCNA), vimentin, a proto-oncogene (ERBB2), a growth factor receptor (EGFR), a cell cycle inhibitor (p27), oncofetal tumor markers (CEA, Lewis Y and Tag 72) and TGF-α are expressed in chicken ovarian cancer, too.12–14 In addition, histological appearance of EOC of chicken is similar to those of humans.15 Therefore, avian model is the best for determination of oncogenic mechanisms.
In this regard, to discover the prognostic factors for diagnosis and treatment of EOC, we reviewed the characteristics, classification and experimental models for EOC and relationship estrogen and genetic regulation including SERPINB3, SERPINB11, GAL11, SPP1, and A2M genes with development of female reproductive tract and those of disease. In addition, we determined the biological roles of microRNA in development of EOC.
CHARACTERIZATION AND CLASSIFICATION OF OVARIAN CANCER
The histologic classification categorized ovarian carcinomas according to derivation from coelomic surface epithelium, germ cells, and sex-cord stromal cells.6,7 Among the rest, the majority human malignant ovarian cancers are germinal epithelium of the ovary. The etiology of EOC is not well known. The likelihood of developing EOC is associated with several factors such as age, genetics, epigenetics, hormones and others. Previous studies suggest that the major causative factor of EOC is incessant ovulation which contributes to increased risk for genetic aberrations to the ovarian surface epithelium in response to repeated rupture and repair of the epithelial surface of the ovary.2,16 According to this hypothesis, taking oral contraceptives for more than five years and multiparity can reduce the incidence of ovarian cancer by suppressing ovulation and controlling hormone levels.17,18
EOC is classified as follows: serous, endometrioid, mucinous and clear cell tumors based on tumor cell morphology and histology.8 Serous carcinoma is the most common of EOC with specific characteristics that include multiple cysts, solid areas, glands and parts of papillae. Malignant serous carcinomas account for approximately 30% of ovarian serous carcinomas and nearly 70% of all EOCs. Most serous carcinomas are large and form bilaterally.19,20 In development of ovarian serous carcinoma, a few gene mutations have been identified. For example, mutations in tumor protein 53 gene is frequently associated with malignant serous carcinomas.21 And V-KI-RAS2 kirsten rat sarcoma viral oncogene homolog (KRAS) and V-RAF murine sarcoma viral oncogene homolog B1 (BRAF) gene mutations exist in the early grade serous carcinomas and they lead to activation of the mitogen activated protein kinase (MAPK) signaling pathway.22
The next most common EOC is endometrioid carcinomas that make up 10–20% of all ovarian cancers. This type of cancer is composed of epithelial and stromal cells that resemble those of the endometrium. These tumors are associated with endometriosis due to genetic alterations and hyperplasia of the endometrium.23,24 In addition, endometrioid carcinomas have glands, solid masses or a fibrous consistency. The endometrioid carcinomas are related to various alterations in molecular genetics including the mutation of oncogenes, tumor suppressor genes and other genes associated with DNA repair. For example, activating mutations of a key effectors of the wingless-type MMTY integration site family (WNT) signaling pathway and, catenin beta 1 (CTNNB1), as well as inactivating mutations of tumor suppressor gene, phosphatase and tensin homolog (PTEN), have been detected mainly in endometrioid carcinomas. Both of them are rare in the other types of ovarian cancers.25,26
The third most common EOC is the mucinous carcinomas which occur in a small percentage (3%) of primary ovarian carcinomas. Mucinous carcinomas are composed of papillae and solid areas, mucin-riched cytoplasm and large areas of necrosis and hemorrhage. Histologically, mucinous carcinomas are characterized with glands and cysts including abundant cytoplasmic mucins.27 The mechanism responsible for development of mucinous carcinomas has not been established; however mutations in the KRAS gene are commonly associated with mucinous ovarian tumors. This analysis indicates that KRAS mutations might be early events in the development of mucinous tumors.28
Clear cell carcinomas account for approximately 10% of EOC. Most ovarian clear cell carcinomas are malignant, as benign and borderline tumors are uncommon. Clear cell carcinomas are composed of clear cells that develop as tubular, papillary, solid or mixed types and hobnail cells which contain apical nuclei. Most of tumors are solid or cystic masses with one or more nodules protruding into the lumen.20,29,30 In clear cell carcinomas of the ovary, the following genetic mutations have been found as follows: mutations of PIK3CA (20–25%), TP53 (8.3%), PTEN (8%) and BRAF (6.3%).31–34 In addition, these type of tumor are associated with over-expression of numerous genes such as HFF1 homeobox 1B (HNF-1B), SPP1, neuraminidase 3 (NEU3) and annexin A4.35,36
Recent studies based on clinicopathologic and molecular genetic characteristics have suggested dualistic model for ovarian carcinogenesis, which indicates type I and type II tumors.37–39 Type I tumor features low-grade serous and endometrioid carcinomas, well-differentiated clear cell and mucinous carcinomas and Brenner tumors. This type of EOC exists in the early stage (stage I) and grows slowly from precursor lesions, such as borderline tumors and endometriosis. In addition, type I tumors are associated with specific genetic mutations, including ARID1A, BRAF, CTNNB1, ERBB2, KRAS, PIK3CA, PPP2R1A, PTEN, Raf, and Ras.40–44 On the other hand, type II tumors present papillary, glandular, and solid morphologies and consist of high-grade serous and endometrioid carcinomas, malignant mingled mesodermal carcinosarcomas, and undifferentiated carcinomas. Type II epithelial ovarian cancers present in advanced stage (stage II-IV) and grow aggressively occurring to more than 75% of all EOC patients. They show high frequency of TP53 gene mutations, which are indicated rarely in the type I tumors.45 Furthermore, approximately 50% of high-grade serous carcinomas is related in molecular alteration of BRCA by the gene mutation or by methylation of BRCA promoter.46
CHICKEN MODEL FOR EPITHELIAL-DERIVED OVARIAN CANCER
The majority of women diagnosed at an advanced stage of EOC have a high probability of dying from the disease. EOC is associated with complex genetic and epigenetic alterations leading to ovarian cancer. Thus, it is very important to identify mechanism leading to initiation, promotion and progression of EOC. It is difficult to establish etiologies and pathogenesis of EOC in women; therefore, exploitation of animal models for EOC is essential.
The laying hen is a valuable model for investigation of EOC because they develop EOC spontaneously at a high rate after producing eggs when more than two years of age. Similarly, natural menopause usually arises between 40-and 55-years of age in women when production of female steroid hormones, estrogen and progesterone, is decreasing with advancing age of their ovaries. Incessant ovulation in laying hens (almost every day) and women (once a month) is considered the major causative factor of EOC.11,15,47
Ovarian carcinomas of the laying hen model presents histopathologically with serous, endometrioid, mucinous and clear cell carcinomas as occurs in women. Furthermore, the stages of ovarian cancer in laying hens are similar to that for EOC in women based on the following FIGO system classifications.10,15 Stage I of EOC in laying hens indicates tumor growth limited to the ovary, firm nodules and little or no ascites. For stage II EOC in laying hens, ovarian tumors are larger and have metastasized to the oviduct with moderate ascites. Next, Stage III ovarian cancer in laying hens shows metastasis of the tumor to the pelvic organs, as well as peritoneal and abdominal organs including small and large intestine and mesentery and surface of the liver with copious ascites. Stage IV EOC in laying hens is characterized by severe metastasis to distant organs such as liver, lung and spleen with multiple solid tumors and copious ascites.15 Therefore, the laying hen is the only animal model that develops EOC spontaneously from surface epithelium of the ovaries at an incidence rate due to incessant ovulations and can be used for investigations to develop therapeutic agents for prevention and or treatment of EOC.
Genetically manipulated rodent models of each subtype of ovarian cancer have been used to improve knowledge of the etiologies and pathogenesis of EOC and confirm effects in preclinical tests of signal transduction inhibitors as potential therapeutic agents.20,48,49 On the other hand, the fact that EOC does not occur spontaneously in rodent models limits their clinical relevance.15
ESTROGEN ACTION IN THE EPITHELIAL-DERIVED OVARIAN CANCER
Estrogen is the most important steroid hormone in the avian female reproductive tract as a primary sex hormone. In general, estrogen plays crucial roles in the modification of several cell-types with respect to development and differentiation, altering expression of specific genes in a variety of organs, and regulation of various biological events including protection against apoptosis, osteoporosis, diabetes and Alzheimer’s disease.50–52 For these biological actions, estrogen binds two classical nuclear receptors, estrogen alpha (ESR1) and beta (ESR2).53
Reproductive hormones, including gonadotropins and steroids hormones, affect the risk for development of ovarian cancer.54 Estrogen, in particular, has long been implicated as a factor inducing ovarian cancer. For instance, menopausal women who have taken estrogen as hormone replacement therapy have an increased risk of ovarian cancer55 whereas women who have taken oral contraceptives for more than 5 years have a reduced incidence of ovarian cancer during premenopausal years. 56,57
High levels of estrogen can change immune response, phagocytic activity, growth factor levels and differentiation of cancer cells.58 For example, estrogen increases angiogenesis that is one key feature of cancer development by promoting secretion of vascular endothelial growth factor (VEGF) and endothelial cell migration.59,60 In addition, estrogen regulates expression of hepatocyte growth factor (HGF)61 and epidermal growth factor (EGF), both of which activate proliferation of ovarian surface epithelial cells.62 In animals, incessant exposure of the reproductive tract and mammary glands to estradiol induces development of papillary ovarian carcinomas in guinea pigs and rabbits that are similar to human benign serous carcinomas.63,64 Also, estrogen can increase proliferation of ovarian surface epithelial cells in ewes.65
Both ESR1 and ESR2 have been reported to be expressed in human ovarian cancers.66 In the four subtypes of EOC, ESR1 was expressed abundantly in endometrioid carcinomas (100%) and detected in serous (97%) and mucinous (70%) carcinomas by immunohistochemal analysis. Moreover, expression of ESR1 was higher in malignant EOC than in ovaries with benign tumors and normal ovaries.16,67 In contrast, ESR2 is expressed in all types of EOC in sequence as follows: endometrioid, serous, clear cell, mucinous carcinomas.67,68
On the other hand, the exact mechanisms of estrogen action are unknown regarding development of ovarian cancer. Therefore, advanced studies are required to verify the relationship between estrogenic activity and expression of its receptors and the etiology and pathogenesis of EOC.
CANDIDATE BIOMARKERS FOR EPITHELIAL-DERIVED OVARIAN CANCER
1. A2M
The alpha 2 macroglobulins (A2M) function as protease inhibitors in serum of mammals and are able to bind a variety of cytokines and growth factors.69–72 Proteases and their inhibitors take part in various biological events such as oncogenesis and metastasis because of their capacity to degrade extracellular matrix proteins.73 Similar to other protease inhibitors, A2M is increased in plasma of women with inflammatory and neoplastic lesions of the ovary.74 In addition, A2M increases in blood of laying hens more than 6 months prior to detection of advanced-stage EOC whereas A2M suppresses DNA synthesis in mouse ovarian tumor cells as a cytotoxic factor in serum.75–77 These results suggest that increased levels of A2M in plasma of laying hens develop in the late-stages of ovarian cancer as compared with its concentration in serum of normal laying hens.77 According to various lines of evidence, A2M might be a novel biomarker for improvements in early detection of ovarian cancer.
2. GAL11
GAL11 (also known as beta-defensin 11; DEFB11) belongs to avian defensins that are members of the beta-defensin subfamily members that exhibit antimicrobial activity against microbes including gram-positive/-negative bacteria or fungi.24,78–80 Avian beta defensing genes identified in chicken leukocytes can be subdivided into 14 classes.81 Among them, GAL11 expression increases significantly in response to lipopolysaccharides82 and DES83 in chicken.
In mammals, there are several reports on identification of the role of beta-defensins in carcinogenesis. First of all, the low expression of human beta-defensin 1 (DEFB1) is involved in renal cell carcinomas, prostate cancer, basal cell carcinomas and oral squamous cell carcinomas as a tumor suppressor.84–86 And overexpression of DEFB3 increases development of oral cancer through recruitment of macrophages via EGF that induces DEFB3 expression.87 In addition, DEFB2 and DEFB3 function as proto-oncogenes in oral squamous cell carcinomas, whereas DEFB1 works as a tumor suppressor gene.88 Moreover, GAL11 was induced in the cancerous ovaries compared with normal ovaries of chicken. With these results, it is possible to suggest that beta-defensins influence carcinogenesis through alteration of inflammation and cytokine production.
3. SERPINB3
SERPINB3, also known as squamous cell carcinoma 1 (SCCA1), was discovered originally in squamous cell carcinoma of the cervix.89 It belongs to the serpin superfamily of protease inhibitors related to apoptosis, immune response, blood coagulation, cell migration and invasiveness of cells.90,91 SERPINB3 regulates programmed cell death through different biological process in diverse cancer types and over-expression of this gene is one characteristic of epithelial-derived cancerous cells. SERPINB3 decreases apoptosis mediated by carcinostatis substances and by TNFA -induced cell death by suppressing cytochrome c release from the mitochondria.92,93 In addition, in apoptosis mechanisms, SERPINB3 is upstream of caspase-3, one of its molecular targets, which attenuates caspase-3 activity and apoptosis.94 Moreover, SERPINB3 specifically modulates activity of c-Jun NH2-terminal kinase-1 (JNK-1).95 In chicken ovarian cancer, SERPINB3 mRNA and protein were localized in the glandular epithelium of cancerous ovaries. And it was abundant in the nucleus of both chicken and human ovarian cancer cell lines. Moreover, in 109 human patients with EOC, SERPINB3 protein was showed weak (13.8%), moderate (60.6%), and strong (25.7%) expression respectively.96 Therefore, SERPINB3 may play a crucial role in EOC and be a novel biomarker for prognosis for EOC.
4. SERPINB11
SERPINBs are one of group in the serpin superfamily of serine and cysteine proteinase inhibitors having crucial roles in various biological events such as blood coagulation, angiogenesis, inflammation and fibrinolysis.97 Most clade B serpin genes are intracellular proteins that primarily suppress target proteases whereas SERPINB5 and SERPINB11 are intracellular non-inhibitory proteins.97–99 SERPINB5 is a class II tumor suppressor gene called as maspin (mammary serine protease inhibitor). This gene was demonstrated to induce apoptosis of breast and prostate cancer cells.99,100 Moreover, methylation of the 5’ flanking region of SERPINB5 causes gene silencing in colorectal, ovarian, skin and thyroid carcinomas.101–103 Unlike SERPINB5, SERPINB11 functions as an inhibitor of angiogenesis through repressing endothelial cell migration and controlling mitogenesis.104 SERPINB11 expression was induced in cancerous ovaries in chickens. And in human ovarian cancer cells such as OVCAR-3, SKOV-3 and PA-1 cells, immunoreactive SERPINB11 protein was predominant in the cytoplasm and had a similar expression pattern to that in chicken ovarian cancer cells. These results suggest that SERPINB11 is a biomarker for chicken ovarian endometrioid carcinoma that could be used for diagnosis and monitoring effects of therapies for the disease in women.105
5. SPP1
SPP1 (also called as osteopontin), is a highly phosphorylated integrin-binding ligand and N-linked glycoprotein originally isolated from bones of rats.106 This gene has crucial functions in a variety of physiological processes including cell to cell interactions, inflammatory responses, wound healing, calcification, morphogenesis of organs and tumorigenesis.107 In blood, increases in SPP1 are associated with several types of cancers.108,109 Especially, in development of ovarian cancer, SPP1 expression increased abundantly as compared with normal ovaries. In addition, its expression was localized predominantly to serous carcinoma which is one of subtype of EOC.110 Results of clinical experiments with postoperative patients also indicated that SPP1 is a biomarker for not only detecting specific types of ovarian cancer, but also a marker for examination of responses to primary treatments for cancer in place or in addition to the use of CA125 as a biomarker for cancer.
BIOLOGICAL ROLEES OF MICRORNAS IN DEVELOPMENT AND DIFFERENTIATION
MicroRNAs (miRNAs) are small and non-coding single stranded RNAs. They consist of 18–23 nucleotides that are post-transcriptional regulators and transform cell fate through modulation of target-mRNA translation in various cells and tissues by binding partial sequences in the 3’ untranslated region of target genes. In other words, miRNAs are known to control a variety of biological events such as growth, development, differentiation, oncogenesis, angiogenesis and cell cycle by regulating gene expression. They function through diverse mechanisms including inhibition of translation elongation and degradation of target mRNAs.111–114
Mechanisms of oncogenesis are very complex with genetic and epigenetic processes changing expression of oncogenic and tumor suppressor genes via various mechanisms. An example of one epigenetic factor is miRNAs involved in the initiation and progression of tumors through effects on oncogenes and tumor suppressor genes.115,116 For example, the deletion and down- regulation of miR-15a and miR-16-1 causes overexpression of BCL2 gene that is frequently shown to increase in level of expression in various human cancers through actions as an anti-apoptotic gene.117 In addition, let-7 family members, first demonstrated to be onco- miRNAs, regulate the expression of the RAS oncogene that usually shows highly increased levels in lung cancer cells as compared to normal cells due to mutations in RAS genes.118 So, transfection of let-7 in lung cancer cells can protect from development of lung cancer or reduce tumor size if cells have RAS mutations.116 Moreover, the MYC oncogene which regulates cell proliferation and apoptosis induces B-cell cancer through correlation with miR-155.119,120 Also collaboration between MYC oncogene and miR-17-92 causes amplification of B-cell tumorigenesis.121 Furthermore, it is remarkable that several miRNAs (miR-20, miR-92a, miR-93, miR-126, miR-132, miR-218 and miR-221) control intracellular signaling pathways downstream of vascular endothelial growth factors (VEGFs) that are remarkable regulators of vascular development and maintenance of carcinogenesis.114
The miRNAs also regulate gene expression at post-transcription levels in EOC. Compared with normal ovaries, abnormal expression of miRNAs has been demonstrated in human EOC. For example, miR-200a, miR-200b, miR-200c and miR-141, miR-429 are expressed in the epithelial phenotype of cancer cells by targeting ZEB1 and ZEB2 that are E-cadherin repressor proteins and overex-pressed in human endometrioid ovarian tumors.122,123 In addition, the expression of miR-21, miR-203 and miR-205 is up-regulated in EOC as compared to normal ovaries of women and the abundance of these miRNAs increase considerably after treatment of 5-aza-2’-deoxycytidine to demethylate OVCAR3 cells. These results suggest that DNA hypomethylation might be involved in the mechanism for over-expression of oncogenic miRNAs.123–125 On the other hand, there are down-regulated miRNAs leading to an increase in cellular events. For instance, miR-9, miR-15a, miR-22, miR-152 are suppressed in ovarian cancer cell lines and this repression is associated with increases invasion, migration and proliferation of the cancer cells.126–129 In chickens, several microRNAs were reported to regulate expression of their target genes that are related in the development of EOC (Table 1).130–132
Table 1.
Post-transcriptional regulatory microRNAs for prognostic factors in epithelial-derived ovarian cancer in chickens
| Gene | Target miRNA | Gene ID | Accession No. |
|---|---|---|---|
| GAL11 | gga-mir-1615 | 100315962 | NR_035103.1 |
| SERPINB3 | gga-mir-101 | 777874 | NR_031494.1 |
| gga-mir-1668 | 100315917 | NR_035161.1 | |
| gga-mir-1681 | 100315975 | NR_035174.1 | |
| SPP1 | gga-mir-140 | 777833 | NR_031453.1 |
In accordance with previous studies, the cancer-related miRNAs expressed aberrantly or mutated in various cancers might have crucial roles as modifiers of expression of oncogenes or tumor suppressor genes that regulate their target genes.
CONCLUSION
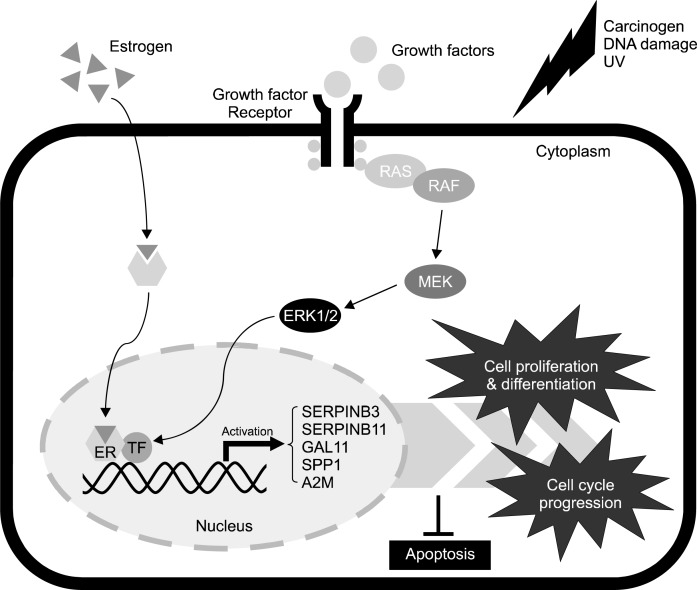
Ovarian carcinogenesis leads to dynamic alterations in morphology, physiology and function of the female reproductive tract. Present review demonstrates general characteristics and animal model of EOC, and the function of prognostic factors (SERPINB3, SERPINB11, GAL11, SPP1 and A2M) which are associated with and may be essential for development of EOC in women and laying hens. In addition indicated genes might be regulated by mechanisms affecting both the genome and epigenome including post-transcriptional regulation via miRNAs and methylation or demethylation of CpG sites of target genes. In addition, most suggested genes for detection of ovarian cancer are also related in the development of the chicken oviduct in response to estrogen which can act via its receptors to induce malignant transformations in cells of the ovaries. These results support the recently suggested hypothesis that oviduct developmental regulatory genes are critical regulators for development and differentiation of epithelial cells of the ovaries as they transition from the normal to the cancerous state during oncogenesis in women and laying hens. Collectively, present study revealed regulation of expression and function of five selected genes during progression of development of EOC and that their expression depends on transactivation of estrogen via estrogen receptors as shown in Fig. 1.133 However, further studies are required to elucidate the clinical application of discoveries of these target genes in the diagnosis and treatment of EOC.
Fig. 1.
Schematic illustrating mechanism for expression and function of regulatory genes for development of the oviduct and for development of epithelial-derived ovarian cancer. Carcinogens, DNA damage, estrogen and ultra-violet light (UV) likely activate estrogenand MAPK cascade signaling pathway that regulate cell proliferation and differentiation, cell cycle progression and apoptosis in EOC through stimulation of expression of SERPINB3, SERPINB11, GAL11, SPP1 and A2M genes. Legend: RAS, synaptic Ras-GTPase-activating protein; RAF, mitogen-activated protein kinase (MAPK) kinase kinase; MEK, MAPK kinase; ERK1/2, extracellular signal-regulated kinase; ER, estrogen receptor; TF, transcription factor.
Acknowledgments
We appreciate Dr. Jae Yong Han (Seoul National University, Korea) for providing tissue samples. This research was funded by the Ministry of Education, Science, and Technology, and also by a grant from the Next- Generation BioGreen 21 Program (No. PJ008142), Rural Development Administration, Republic of Korea.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Fathalla MF. Incessant ovulation-a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 3.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–21. [PubMed] [Google Scholar]
- 4.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 5.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Shih IM. Pathogenesis of ovarian cancer: Lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–60. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih Ie M. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198:351–6. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, et al. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36:9–17. doi: 10.1007/s007950300002. [DOI] [PubMed] [Google Scholar]
- 9.Goodman MT, Correa CN, Tung KH, Roffers SD, Wu XC, Young JL, et al. Stage at diagnosis of ovarian cancer in the United States, 1992–1997. Cancer. 2003;97:2648–59. doi: 10.1002/cncr.11347. [DOI] [PubMed] [Google Scholar]
- 10.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 11.Fredrickson TN. Ovarian tumors of the hen. Environ Health Perspect. 1987;73:35–51. doi: 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson E, Anderson K, Ashwell C, Petitte J, Mozdziak PE. CA125 expression in spontaneous ovarian adenocarcinomas from laying hens. Gynecol Oncol. 2007;104:192–8. doi: 10.1016/j.ygyno.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Burford C, Barnes MN, Berry W, Partridge EE, Grizzle WE. Immunohistochemical expression of molecular markers in an avian model: a potential model for pre-clinical evaluation of agents for ovarian cancer chemoprevention. Gynecol Oncol. 2001;81:373–9. doi: 10.1006/gyno.2001.6191. [DOI] [PubMed] [Google Scholar]
- 14.Li JP, Dowdy S, Tipton T, Podratz K, Lu WG, Xie X, et al. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev Mol Diagn. 2009;9:555–66. doi: 10.1586/erm.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, et al. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 2009;19:531–9. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 17.Hippisley-Cox J, Coupland C. Identifying women with suspected ovarian cancer in primary care: derivation and validation of algorithm. BMJ. 2012;344:d8009. doi: 10.1136/bmj.d8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–32. doi: 10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- 19.Chen VW, Ruiz B, Killeen JL, Cote TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–42. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 20.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–24. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 22.Singer G, Oldt R, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 23.Depriest PD, Banks ER, Powell DE, Vannagell JR, Gallion HH, Puls LE, et al. Endometrioid carcinoma of the ovary and endometriosis-the association in postmenopausal women. Gynecol Oncol. 1992;47:71–5. doi: 10.1016/0090-8258(92)90079-x. [DOI] [PubMed] [Google Scholar]
- 24.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–55. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 25.Catasus L, Bussaglia E, Rodriguez I, Gallardo A, Pons C, Irving JA, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: Similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–8. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Wright K, Wilson P, Morland S, Campbell I, Walsh M, Hurst T, et al. beta-catenin mutation and expression analysis in ovarian cancer: Exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer. 1999;82:625–9. doi: 10.1002/(sici)1097-0215(19990827)82:5<625::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005;24:4–25. [PubMed] [Google Scholar]
- 28.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–6. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary - A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–9. [PubMed] [Google Scholar]
- 30.Tammela J, Geisler JP, Eskew PN, Geisler HE. Clear cell carcinoma of the ovary: poor prognosis compared to serous carcinoma. Eur J Gynaecol Oncol. 1998;19:438–40. [PubMed] [Google Scholar]
- 31.Campbell IG, Russell SE, Choong DYH, Montgomery KG, Ciavarella ML, Hooi CSF, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 32.Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: A comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–7. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: Possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 34.Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, et al. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–13. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, et al. Clear cell carcinoma of the ovary: Potential pathogenic mechanisms (Review) Oncol Rep. 2010;23:1193–203. doi: 10.3892/or_00000750. [DOI] [PubMed] [Google Scholar]
- 36.Zorn KK, Bonome T, Gangi L, Chandramouli GVR, Awtrey CS, Gardner GJ, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 37.Kurman RJ, Shih Ie M. Molecular pathogenesis and extra-ovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcazar JL, Utrilla-Layna J, Minguez JA, Jurado M. Clinical and ultrasound features of type I and type II epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23:680–4. doi: 10.1097/IGC.0b013e31828bdbb6. [DOI] [PubMed] [Google Scholar]
- 39.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. American J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auner V, Kriegshauser G, Tong D, Horvat R, Reinthaller A, Mustea A, et al. KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer. 2009;9:111–8. doi: 10.1186/1471-2407-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, et al. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–92. [PubMed] [Google Scholar]
- 45.Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senturk E, Cohen S, Dottino PR, Martignetti JA. A critical re-appraisal of BRCA1 methylation studies in ovarian cancer. Gynecol Oncol. 2010;119:376–83. doi: 10.1016/j.ygyno.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Damjanov I. Ovarian tumours in laboratory and domestic animals. Curr Top Pathol. 1989;78:1–10. doi: 10.1007/978-3-642-74011-4_1. [DOI] [PubMed] [Google Scholar]
- 48.Stakleff KD, Von Gruenigen VE. Rodent models for ovarian cancer research. Int J Gynecol Cancer. 2003;13:405–12. doi: 10.1046/j.1525-1438.2003.13317.x. [DOI] [PubMed] [Google Scholar]
- 49.Vanderhyden BC, Shaw TJ, Ethier JF. Animal models of ovarian cancer. Reprod Biol Endocrinol. 2003;1:67. doi: 10.1186/1477-7827-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6:180–5. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 51.Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the women’s health initiative. Endocr Rev. 2005;26:308–12. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- 52.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 53.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 54.Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: An overview with emphasis on hormonal factors. J Toxicol Environ Health B Crit Rev. 2008;11:301–21. doi: 10.1080/10937400701876095. [DOI] [PubMed] [Google Scholar]
- 55.Lacey JV, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–41. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 56.Schildkraut JM, Calingaert B, Marchbanks PA, Moorman PG, Rodriguez GC. Impact of progestin and estrogen potency in oral contraceptives on ovarian cancer risk. J Natl Cancer Inst. 2002;94:32–8. doi: 10.1093/jnci/94.1.32. [DOI] [PubMed] [Google Scholar]
- 57.Spillman MA, Manning NG, Dye WW, Sartorius CA, Post MD, Harrell JC, et al. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Res. 2010;70:8927–36. doi: 10.1158/0008-5472.CAN-10-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hrushesky WJ, Gruber SA, Sothern RB, Hoffman RA, Lakatua D, Carlson A, et al. Natural killer cell activity: age, estrous- and circadian-stage dependence and inverse correlation with metastatic potential. J Natl Cancer Inst. 1988;80:1232–7. doi: 10.1093/jnci/80.15.1232. [DOI] [PubMed] [Google Scholar]
- 59.Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–90. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- 60.Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology. 1993;133:829–37. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]
- 61.Liu YH, Lin L, Zarnegar R. Modulation of hepatocyte growth-factor gene-expression by estrogen in mouse ovary. Mol Cell Endocrinol. 1994;104:173–81. doi: 10.1016/0303-7207(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 62.Hsueh AJ, Welsh TH, Jones PB. Inhibition of ovarian and testicular steroidogenesis by epidermal growth factor. Endocrinology. 1981;108:2002–4. doi: 10.1210/endo-108-5-2002. [DOI] [PubMed] [Google Scholar]
- 63.Silva EG, Tornos C, Deavers M, Kaisman K, Gray K, Gershenson D. Induction of epithelial neoplasms in the ovaries of guinea pigs by estrogenic stimulation. Gynecol Oncol. 1998;71:240–6. doi: 10.1006/gyno.1998.5153. [DOI] [PubMed] [Google Scholar]
- 64.Bai WL, Oliveros-Saunders B, Wang Q, Acevedo-Duncan ME, Nicosia SV. Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell Dev Biol Anim. 2000;36:657–66. doi: 10.1290/1071-2690(2000)036<0657:ESOOSE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 65.Murdoch WJ, Van Kirk EA. Steroid hormonal regulation of proliferative, p53 tumor suppressor, and apoptotic responses of sheep ovarian surface epithelial cells. Mol Cell Endocrinol. 2002;186:61–7. doi: 10.1016/s0303-7207(01)00675-x. [DOI] [PubMed] [Google Scholar]
- 66.Karlan BY, Jones JL, Greenwald M, Lagasse LD. Steroid-Hormone effects on the proliferation of human ovarian surface epithelium in-vitro. Am J Obstet Gynecol. 1995;173:97–104. doi: 10.1016/0002-9378(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 67.Fujimura M, Hidaka T, Kataoka K, Yamakawa Y, Akada S, Teranishi A, et al. Absence of estrogen receptor-alpha expression in human ovarian clear cell adenocarcinoma compared with ovarian serous, endometrioid, and mucinous adenocarcinoma. Am J Surg Pathol. 2001;25:667–72. doi: 10.1097/00000478-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 68.Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armstrong PB. Proteases and protease inhibitors: a balance of activities in host-pathogen interaction. Immunobiology. 2006;211:263–81. doi: 10.1016/j.imbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem. 1989;264:11539–42. [PubMed] [Google Scholar]
- 71.Tayade C, Esadeg S, Fang Y, Croy BA. Functions of alpha 2 macroglobulins in pregnancy. Mol Cell Endocrinol. 2005;245:60–6. doi: 10.1016/j.mce.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Vanleuven F, Marynen P, Cassiman JJ, Vandenberghe H. Proteolysis of human alpha-2-macroglobulin without hydrolysis of the internal thiolesters or expression of the receptor recognition site. J Biol Chem. 1988;263:468–71. [PubMed] [Google Scholar]
- 73.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 74.Zbroja-Sontag W. Defense proteins and immune complexes in the blood serum of women with inflammatory and neoplastic lesions of the ovary. Am J Reprod Immunol. 1983;4:11–20. doi: 10.1111/j.1600-0897.1983.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 75.Koo PH. Human alpha 2-macroglobulin: a major serum factor cytotoxic for tumor cells. Cancer Lett. 1983;18:169–77. doi: 10.1016/0304-3835(83)90064-2. [DOI] [PubMed] [Google Scholar]
- 76.Koo PH. Tumor inhibition by human alpha 2-macroglobulin. Ann N Y Acad Sci. 1983;421:388–90. doi: 10.1111/j.1749-6632.1983.tb18129.x. [DOI] [PubMed] [Google Scholar]
- 77.Hawkridge AM, Wysocky RB, Petitte JN, Anderson KE, Mozdziak PE, Fletcher OJ, et al. Measuring the intra-individual variability of the plasma proteome in the chicken model of spontaneous ovarian adenocarcinoma. Anal Bioanal Chem. 2010;398:737–49. doi: 10.1007/s00216-010-3979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harwig SSL, Swiderek KM, Kokryakov VN, Tan L, Lee TD, Panyutich EA, et al. Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 1994;342:281–5. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 79.van Dijk A, Veldhuizen EJ, Haagsman HP. Avian defensins. Vet Immunol Immunopathol. 2008;124:1–18. doi: 10.1016/j.vetimm.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao Y, Hughes AL, Ando J, Matsuda Y, Cheng JF, Skinner-Noble D, et al. A genome-wide screen identifies a single beta-defensin gene cluster in the chicken: implications for the origin and evolution of mammalian defensins. BMC Genomics. 2004;5:56. doi: 10.1186/1471-2164-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdel Mageed AM, Isobe N, Yoshimura Y. Immunolocalization of avian beta-defensins in the hen oviduct and their changes in the uterus during eggshell formation. Reproduction. 2009;138:971–8. doi: 10.1530/REP-09-0181. [DOI] [PubMed] [Google Scholar]
- 82.Mageed AM, Isobe N, Yoshimura Y. Expression of avian beta-defensins in the oviduct and effects of lipopolysaccharide on their expression in the vagina of hens. Poult Sci. 2008;87:979–84. doi: 10.3382/ps.2007-00283. [DOI] [PubMed] [Google Scholar]
- 83.Song G, Seo HW, Choi JW, Rengaraj D, Kim TM, Lee BR, et al. Discovery of candidate genes and pathways regulating oviduct development in chickens. Biol Reprod. 2011;85:306–14. doi: 10.1095/biolreprod.110.089227. [DOI] [PubMed] [Google Scholar]
- 84.Donald CD, Sun CQ, Lim SD, Macoska J, Cohen C, Amin MB, et al. Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas. Lab Invest. 2003;83:501–5. doi: 10.1097/01.lab.0000063929.61760.f6. [DOI] [PubMed] [Google Scholar]
- 85.Gambichler T, Skrygan M, Huyn J, Bechara FG, Sand M, Altmeyer P, et al. Pattern of mRNA expression of beta-defensins in basal cell carcinoma. BMC Cancer. 2006;6:163–8. doi: 10.1186/1471-2407-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joly S, Compton LM, Pujol C, Kurago ZB, Guthmiller JM. Loss of human beta-defensin 1, 2, and 3 expression in oral squamous cell carcinoma. Oral Microbiol Immunol. 2009;24:353–60. doi: 10.1111/j.1399-302X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 87.Kesting MR, Loeffelbein DJ, Hasler RJ, Wolff KD, Rittig A, Schulte M, et al. Expression profile of human beta-defensin 3 in oral squamous cell carcinoma. Cancer Invest. 2009;27:575–81. doi: 10.1080/07357900802620851. [DOI] [PubMed] [Google Scholar]
- 88.Winter J, Pantelis A, Reich R, Martini M, Kraus D, Jepsen S, et al. Human beta-defensin-1,-2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Invest. 2011;29:196–201. doi: 10.3109/07357907.2010.543210. [DOI] [PubMed] [Google Scholar]
- 89.Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–8. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 90.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 91.Suminami Y, Kishi F, Sekiguchi K, Kato H. Squamous-cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun. 1991;181:51–8. doi: 10.1016/s0006-291x(05)81380-4. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto K, Kiyoshima T, Matsuo K, Ozeki S, Sakai H. Effect of SCCA1 and SCCA2 on the suppression of TNF alpha-induced cell death by impeding the release of mitochondrial cytochrome c in an oral squamous cell carcinoma cell line. Tumour Biol. 2005;26:165–72. doi: 10.1159/000086949. [DOI] [PubMed] [Google Scholar]
- 93.Kato H. Expression and function of squamous cell carcinoma antigen. Anticancer Res. 1996;16:2149–53. [PubMed] [Google Scholar]
- 94.Suminami Y, Nagashima S, Vujanovic NL, Hirabayashi K, Kato H, Whiteside TL. Inhibition of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br J Cancer. 2000;82:981–9. doi: 10.1054/bjoc.1999.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katagiri C, Nakanishi J, Kadoya K, Hibino T. Serpin squamous cell carcinoma antigen inhibits UV-induced apoptosis via suppression of c-JUN NH2-terminal kinase. J Cell Biol. 2006;172:983–90. doi: 10.1083/jcb.200508064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim W, Ahn SE, Jeong W, Kim JH, Kim J, Lim CH, et al. Tissue specific expression and estrogen regulation of SERPINB3 in the chicken oviduct. Gen Comp Endocrinol. 2012;175:65–73. doi: 10.1016/j.ygcen.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 97.Askew DJ, Cataltepe S, Kumar V, Edwards C, Pace SM, Howarth RN, et al. SERPINB11 is a new noninhibitory intracellular serpin - Common single nucleotide polymorphisms in the scaffold impair conformational change. J Biol Chem. 2007;282:24948–60. doi: 10.1074/jbc.M703182200. [DOI] [PubMed] [Google Scholar]
- 98.Bird CH, Blink EJ, Hirst CE, Buzza MS, Steele PM, Sun J, et al. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol Cell Biol. 2001;21:5396–407. doi: 10.1128/MCB.21.16.5396-5407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–4. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 100.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 101.Bettstetter M, Woenckhaus M, Wild PJ, Rummele P, Blaszyk H, Hartmann A, et al. Elevated nuclear maspin expression is associated with microsatellite instability and high tumour grade in colorectal cancer. J Pathol. 2005;205:606–14. doi: 10.1002/path.1732. [DOI] [PubMed] [Google Scholar]
- 102.Boltze C, Schneider-Stock R, Quednow C, Hinze R, Mawrin C, Hribaschek A, et al. Silencing of the maspin gene by promoter hypermethylation in thyroid cancer. Int J Mol Med. 2003;12:479–84. [PubMed] [Google Scholar]
- 103.Khalkhali-Ellis Z. Maspin: the new frontier. Clin Cancer Res. 2006;12:7279–83. doi: 10.1158/1078-0432.CCR-06-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–9. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 105.Lim W, Kim JH, Ahn SE, Jeong W, Kim J, Bazer FW, et al. Avian SERPINB11 gene: a marker for ovarian endometrioid cancer in chickens. Exp Biol Med (Maywood) 2012;237:150–9. doi: 10.1258/ebm.2011.011250. [DOI] [PubMed] [Google Scholar]
- 106.Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–36. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 107.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 108.Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer. 2002;95:506–12. doi: 10.1002/cncr.10709. [DOI] [PubMed] [Google Scholar]
- 109.Le QT, Sutphin PD, Raychaudhuri S, Yu SCT, Terris DJ, Lin HS, et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res. 2003;9:59–67. [PubMed] [Google Scholar]
- 110.Kim JH, Skates SJ, Uede T, Wong KK, Schorge JO, Feltmate CM, et al. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA. 2002;287:1671–9. doi: 10.1001/jama.287.13.1671. [DOI] [PubMed] [Google Scholar]
- 111.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580–7. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 113.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–40. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 114.Dang LT, Lawson ND, Fish JE. MicroRNA control of vascular endothelial growth factor signaling output during vascular development. Arterioscler Thromb Vasc Biol. 2013;33:193–200. doi: 10.1161/ATVBAHA.112.300142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calin GA, Croce CM. MicroRNA-cancer connection: The beginning of a new tale. Cancer Research. 2006;66:7390–4. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 116.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 117.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 MicroRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 119.Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, et al. BIC and miR-155 are highly expressed in Hodgkin, priniary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207:243–9. doi: 10.1002/path.1825. [DOI] [PubMed] [Google Scholar]
- 120.Eis PS, Tam W, Sun LP, Chadburn A, Li ZD, Gomez MF, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A. 2005;102:3627–32. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy Y, Mariani O, et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17:1627–35. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- 123.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 124.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 125.Lou YH, Yang XS, Wang FL, Cui ZM, Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int J Mol Med. 2010;26:819–27. doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- 126.Bhattacharya R, Nicoloso M, Arvizo R, Wang EF, Cortez A, Rossi S, et al. MiR-15a and MiR-16 Control Bmi-1 Expression in Ovarian Cancer. Cancer Res. 2009;69:9090–5. doi: 10.1158/0008-5472.CAN-09-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M, et al. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kappa B1. FEBS J. 2009;276:5537–46. doi: 10.1111/j.1742-4658.2009.07237.x. [DOI] [PubMed] [Google Scholar]
- 128.Li J, Liang SH, Yu HL, Zhang J, Ma DA, Lu X. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2010;119:543–8. doi: 10.1016/j.ygyno.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 129.Zhou X, Zhao F, Wang ZN, Song YX, Chang H, Chiang YP, et al. Altered expression of miR-152 and miR-148a in ovarian cancer is related to cell proliferation. Oncol Rep. 2012;27:447–54. doi: 10.3892/or.2011.1482. [DOI] [PubMed] [Google Scholar]
- 130.Lim W, Jeong W, Kim J, Ka H, Bazer FW, Han JY, et al. Differential expression of secreted phosphoprotein 1 in response to estradiol-17beta and in ovarian tumors in chickens. Biochem Biophys Res Commun. 2012;422:494–500. doi: 10.1016/j.bbrc.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 131.Lim W, Jeong W, Kim J, Yoshimura Y, Bazer FW, Han JY, et al. Expression and regulation of beta-defensin 11 in the oviduct in response to estrogen and in ovarian tumors of chickens. Mol Cell Endocrinol. 2013;366:1–8. doi: 10.1016/j.mce.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 132.Lim W, Kim HS, Jeong W, Ahn SE, Kim J, Kim YB, et al. SERPINB3 in the chicken model of ovarian cancer: a prognostic factor for platinum resistance and survival in patients with epithelial ovarian cancer. PLoS One. 2012;7:e49869. doi: 10.1371/journal.pone.0049869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simpkins F, Garcia-Soto A, Slingerland J. New insights on the role of hormonal therapy in ovarian cancer. Steroids. 2013;78:530–7. doi: 10.1016/j.steroids.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]