Abstract
Cancer remains a lethal disease, and many scientists are currently trying to develop more effective therapies. Natural compounds are potential sources of anti-cancer therapies and are obtained from diverse sources including marine organisms, microorganisms and plants. In this paper, we evaluated natural compounds from non-edible plant sources, which is a neglected area of research despite the promising future of these compounds. In addition, we assessed the function and mechanism of action of these compounds in relation to cancer chemoprevention.
Keywords: Chemoprevention, Chemotherapy, Natural compounds
INTRODUCTION
Cancer caused 7.6 million deaths in 2008, and it is estimated that it will cause 13.1 million deaths in 2030 (GLOBOCAN 2008, IARC). Accordingly, the cancer drug market is rapidly evolving to keep up with the increasing numbers of cancer patients. Interestingly, 18% of all global sales of the top 100 drugs, which totaled $51 billion, involved the sale of 20 cancer drugs in 2009 (22nd Annual Cancer Progress Conference 2011, KANTAR HEALTH).
Natural products serve as a novel source for the development of anti-cancer drugs because of their unique structural diversity.1 The current sources of natural products are more diversified than they have been in the past and include plants, marine organisms and microorganisms.2–4 Since recently, natural compounds from marine organisms have been investigated actively and already improved cancer therapy.5,6 However, many scientists focus particularly on the effects of natural products in cancer chemoprevention, because of the difficulty to treat advanced forms of cancer.
Plants remain a prominent source of natural products for cancer chemoprevention, which is supported by the fact that 25% of current therapies on the market are derived from plants.4,7 Unfortunately, however, while many papers discuss the cancer prevention potential of food-related plants, very little is known about compounds from non-food plants including terrestrial and marine sources. The aim of this review is to document the molecular mechanisms by which these less investigated compounds interfere with the initiation and promotion of cancer to prevent the development of cancer.
THE CATEGORIZATION OF NATURAL COMPOUNDS FROM PLANTS
We categorized various natural compounds with anti-cancer effects and distinguished edible and non-edible sources. To do this, we first gathered data related to natural compounds from plants containing terrestrial and marine organisms. Next, we divided these natural compounds into two categories, edible or not. The source of each compound was determined from the literature or, when the source was not provided, from well-known databases, such as Super Natural II, NPACT and DrugBank. Edible sources of compounds included fruits, vegetables, teas, or oils, and non-edible sources included various medicinal plants as a whole or their parts, such as the bark, stems and roots. In the past 2 years, 60% of all natural compounds from plants were derived from edible sources, and the remaining 40% were derived from non-edible sources based upon 165 review papers. Because many of these compounds have been investigated multiple times worldwide, the actual ratio of natural compounds from edible sources to those from non-edible source may, in fact, be much larger. These data demonstrate that many scientists prefer to research with edible plants as a source of natural compounds rather than non-edible plants.
THE RELATIONSHIP BETWEEN APPROVED ANTI-CANCER DRUGS AND THE SOURCE OF THE NATURAL COMPOUNDS
Once anti-cancer compounds have been identified in natural products, an essential final step is to develop the actual drug. Accordingly, we wanted to identify the number of natural compounds with anti-cancer effects that were obtained from edible or non-edible plant sources and that have been approved as an anti-cancer drug. Since David J. Newman and Gordon M. Cragg published an article dealing with natural products and new drugs in 1997, their article has been updated regularly and is currently in the 4th edition.8 Their paper contains invaluable statistics related to natural products and drugs, making it a valuable paper for many scientists in this field. Additional data were collected from the Food and Drug Administration (FDA) and European Medicines Agency (EMEA). After collecting information from Newman and Cragg’s review paper, we searched for the source of each approved drug manually because the authors simply divided drug sources into B (biological), N (natural product), ND (derived from a natural product), S (synthetic drug) and V (vaccine). We identified the source of each approved anti-cancer drug as an edible or non-edible plant using DrugBank and Drug information portal sites. We finally categorized all approved anti-cancer drugs by dividing them into edible and non-edible plants sources (Table 1). Unlike previous results about the contribution of natural compounds in many articles, approved anti-cancer drugs are derived from 11 non-edible plant sources (65% of all approved anti-cancer drugs from plants) and from only six edible plant sources (35% of all approved anti-cancer drugs from plants). These statistics suggest that natural compounds from non-edible plant sources have a higher potential of being developed into an anti-cancer drug, indicating that scientists should focus their research on these sources to develop effective cancer therapies.
Table 1.
The categorization of approved anticancer drugs that are derived from natural compounds based on edible or non-edible plant sources. The process of data collection is described in detail in the methods
| Year introduced | Generic name | Brand name | Lead compound | Source | Edible/Non-edible | Involved stage of cancer
|
|
|---|---|---|---|---|---|---|---|
| Chemoprevention | Chemotherapy | ||||||
| 1963 | Vincristine | Oncovin | Vincristine | Vinca Rosea. | Edible | O | |
| 1965 | Vinblastine | Alkaban-AQ, Velban | Vinblastine | Vinca rosea. | Edible | O | |
| 1967 | Teniposide | Vumon, VM-26 | Podophyllotoxin | Podophyllum | Non-edible | O | |
| 1979 | Vindesine | Eldisine | Vinblastine | Vinca Rosea. | Edible | O | |
| 1980 | Etoposide | Toposar, VePesid | Podophyllotoxin | Podophyllum peltatum | Non-edible | O | |
| 1983 | Elliptinium acetate | Celiptium | Ellipticine | Apocynaceae | Non-edible | O | |
| 1989 | Solamargines | Curaderm | Solasodine | Solanaceae | Edible | O | |
| 1989 | Vinorelbine | Navelbine | Vinblastine | Catharanthus roseus | Edible | O | |
| 1993 | Paclitaxel | Taxol | Paclitaxel | Taxus brevifolia | Non-edible | O | |
| 1995 | Docetaxel | Taxotere | Taxane | Taxus brevifolia | Non-edible | O | |
| 1996 | Etoposide phosphate | Etopophos | Podophyllotoxin | Podophyllum peltatum | Non-edible | O | |
| 1996 | Topotecan HCl | Hycamtin | Camptothecin | Camptotheca acuminata | Non-edible | O | |
| 2004 | Belotecan hydrochloride | Camtobell | Camptothecin | Camptotheca acuminata | Non-edible | O | |
| 2005 | Paclitaxel nanoparticles | Abraxane | Paclitaxel | Taxus brevifolia | Non-edible | O | |
| 2007 | Paclitaxel nanoparticles | Nanoxel | Paclitaxel | Taxus brevifolia | Non-edible | O | |
| 2010 | Cabazitaxel | Jevtana | Paclitaxel | Taxus baccata | Non-edible | O | |
| 2010 | Vinflunine | Javlor | Vinblastine | Vinca Rosea. | Edible | O | |
THE EFFECT OF NATURAL COMPOUNDS FROM NON-EDIBLE PLANT SOURCES ON CANCER CHEMOPREVENTION
The process of tumorigenesis consists of at least three steps: initiation, promotion, and progression9 suggesting that cancer progresses over a long period of time. Initial efforts of many groups to cure cancer were mainly focused on the terminal stages of cancer. However, this strategy was ineffective because cancer cells have already become wide-spread throughout the body and are very resistant to anti-cancer drugs. Accordingly, the trend in the treatment of cancer is moving from cancer chemotherapy to chemoprevention, which focuses on the initiation or promotion steps of cancer. Cancer chemoprevention may also reverse chemo- and radio-resistance in cancer patients.10 Therefore, cancer chemoprevention is not only valuable alone but can also be used as an adjuvant for chemotherapy. Table 1 shows that anti-cancer drugs from plant sources were essentially used the field of cancer chemotherapy. Thus, natural compounds from non-edible plant sources should be evaluated for their efficacy in chemoprevention (Fig. 1).
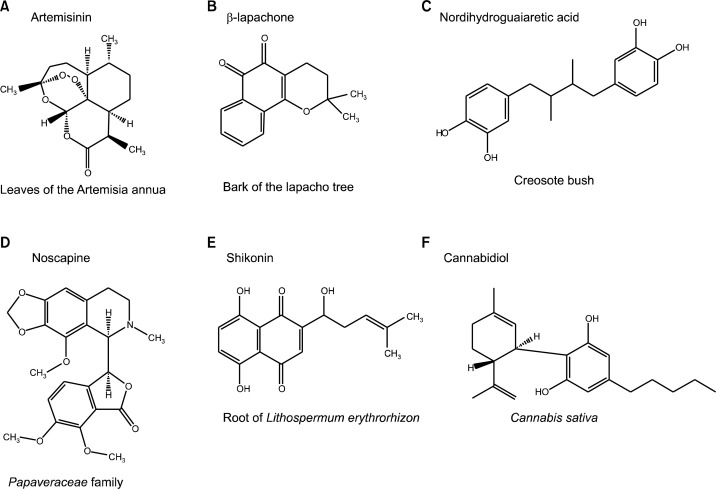
Fig. 1.
Chemical structure of natural compounds with chemopreventive effects from non-edible plants. This figure was generated using ChemDraw software.
Artemisinin (Fig. 1A) is isolated from the leaves of the Artemisia annua, which is a common type of wormwood, and this compound inhibits transferrin receptors in cancer cells. Cancer cells uptake large amounts of ions because of their rapid metabolic rates.11 Transferrin receptors are expressed at especially high levels in breast cancer and leukemia compared to normal cells. Accordingly, artemisinin may have prominent anticancer effects in the early stages of these types of cancer. An early study showed that artemisinin prevents and delays the development of breast cancer and leukemia by interrupting ion absorption.12 In addition, artemisinin has no known side effects at high dose concentrations, so it would be worthwhile to study its effects on the early stages of cancer.
β-lapachone (Fig. 1B) is found in the bark of the lapacho tree, and it has been shown to inhibit tumor necrosis factor-α (TNF-α)-induced nuclear factor κ B (NF- κ B) and Activator Protein 1 (AP-1) in U937 leukemic cells.13 NF-κ B is a well-known transcription factor that induces inflammation and inhibits apoptosis. AP-1 is also activated by TNF-α and is involved in growth modulation and apoptosis. Previous data showed that β-lapachone negatively regulates NF-κ B by participating in TNF- α -induced NF-κ B activation, I κ B degradation and p65 translocation. However, β -lapachone does not affect p50–p65 binding to DNA. β-lapachone also attenuated the level of AP1 and its related kinases, c-Jun N-terminal kinase (JNK) and mitogen activated protein kinase (MAPK).
Nordihydroguaiaretic acid (NDGA) (Fig. 1C) is abundant in the creosote bush and has been shown to epigenetically modify cancer cells to inhibit cancer cell growth. The term “epigenetics” means the modulation of gene expression without gene sequence alteration, and it involves DNA methylation, histone modification and micro RNAs.14 Because DNA methylation prevents the transcription of target genes, methylation of tumor suppressor genes can lead to cancer. First, previous reports on the effects of NDGA in cancer showed that NDGA reduced global DNA methylation in malignant glioma cells.15 Subsequently, it was discovered that NDGA lowers the methylation levels of many important tumor suppressor genes including E-cadherin and p16.15,16
Noscapine (Fig. 1D) is isolated from the Papaveraceae family and has been shown to induce apoptosis by down-regulating survivin expression. Survivin negatively regulates apoptosis or programmed cell death by inhibiting caspase activation.17 In previous studies, noscapine induced apoptosis of neuroblastoma cell lines without affecting p53. Instead, noscapine decreased the expression of survivin sensitizing neuroblastoma cells to apoptosis, suggesting a novel molecular mechanism.
Shikonin (Fig. 1E) can be purified from root of Lithospermum erythrorhizon, which is native to America. Shikonin affects the “Warburg effect” of cancer, when cancer cells produce energy through increased rates of glycolysis followed by lactic acid fermentation.18 Pyruvate kinase M2 (PKM2) is one of the most important metabolic enzymes that regulate this pathway. According to a previous study, levels of PKM2 are much higher in skin tumor tissues than in normal tissues.19 In this research, shikonin inhibited PKM2, which led to cancer cell death and also suppressed the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA), which results in restoring mitochondrial malfunction. This means that shikonin may have a chemopreventive effect by targeting PKM2 involved in the “Warburg effect”.
Cannabidiol (Fig. 1F) is found in Cannabis sativa and has a chemopreventive effect in cancer.20 First, cannabidiol induces fragmentation of caspase-3, which leads to apoptosis of colon cancer cells. Cannabidiol can also down-regulate the expression of Akt, which functions in cell growth, migration and differentiation. Finally, cannabidiol has an anti-inflammatory effect on gut cells by down-regulating inducible nitric oxide synthase (iNOS) but has no anti-inflammatory effect on colon cancer cells.
CONCLUSION
Using natural compounds as candidates for drugs is advantageous over using synthetic compounds for many reasons. First, the sources of natural compounds are abundant and include plants, marine organisms and microorganisms. Thus, these compounds also have unique structures. Developing drugs from natural compounds takes less time and money, and the drugs also have fewer side effects than synthetic compounds. Because of these features, many laboratories worldwide primarily study natural compounds. Nevertheless, research in this field, which focuses on the specific sources, is limited. Specifically, researchers are focusing on dietary or edible sources such as resveratrol, curcumin and genistein rather than nonedible sources. This field should be widened to focus on diverse sources to find valuable natural compounds. As previously mentioned, natural compounds from non-edible plant sources have been turned into effective anticancer drugs. Additionally, there are numerous references to medicinal plants, which predict the effects of the natural compounds isolated from those medicinal plants.
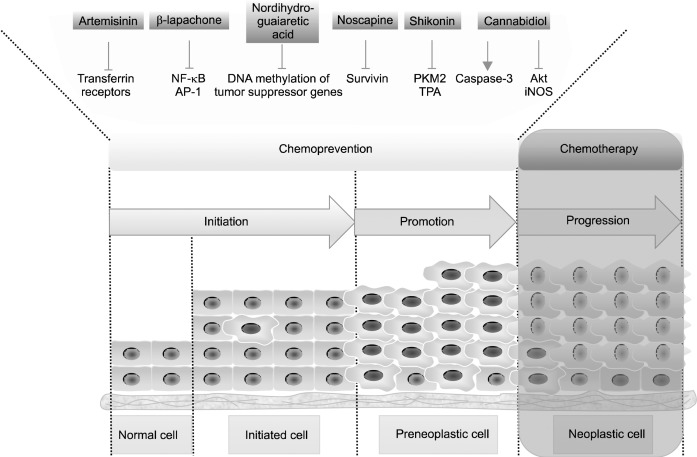
Today, research in oncology is moving away from chemotherapy and toward chemoprevention. Researchers in this field are further encouraged by the success of 10 FDA-approved anti-cancer drugs with chemopreventive effects.21 Natural compounds from non-edible plants have sufficient potential to be developed into chemopreventive drugs. Natural compounds with cancer chemopreventive effects that were obtained from non-edible plant sources inhibit a variety of pro-tumorigenic pathways (Fig. 2). Because of their mechanism of action, cancer chemopreventive drugs function synergistically when administered with chemotherapeutic drugs, providing even more support for the need for continued research in this field.
Fig. 2.
Schematic representation of natural compounds from non- edible plants for cancer chemoprevention acting on multiple stages of carcinogenesis. This figure shows 6 natural compounds and their specific targets in the early steps of carcinogenesis. This figure was generated using Science-Slides software.
Acknowledgments
Research in MD’s lab is supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korea government, Ministry of Science, ICT & Future Planning (MSIP) (No.2011-0030001).
REFERENCES
- 1.Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–36. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–78. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Sawadogo WR, Schumacher M, Teiten MH, Cerella C, Dicato M, Diederich M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules. 2013;18:3641–73. doi: 10.3390/molecules18043641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlikova B, Diederich M. Power from the garden: plant compounds as inhibitors of the hallmarks of cancer. Curr Med Chem. 2012;19:2061–87. doi: 10.2174/092986712800228998. [DOI] [PubMed] [Google Scholar]
- 5.Folmer F, Jaspars M, Schumacher M, Dicato M, Diederich M. Marine natural products targeting phospholipases A2. Biochem Pharmacol. 2010;80:1793–800. doi: 10.1016/j.bcp.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher M, Kelkel M, Dicato M, Diederich M. Gold from the sea: marine compounds as inhibitors of the hallmarks of cancer. Biotechnol Adv. 2011;29:531–47. doi: 10.1016/j.biotechadv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Ramawat K, Goyal S.Natural products in cancer chemoprevention and chemotherapy Herbal Drugs: Ethnomedicine to Modern Medicine: Springer, 2009:153–71. [Google Scholar]
- 8.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 10.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Karin M, Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981;256:3245–52. [PubMed] [Google Scholar]
- 12.Lai H, Singh NP. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–8. doi: 10.1016/j.canlet.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Manna SK, Gad YP, Mukhopadhyay A, Aggarwal BB. Suppression of tumor necrosis factor-activated nuclear transcription factor-kappaB, activator protein-1, c-Jun N-terminal kinase, and apoptosis by beta-lapachone. Biochem Pharmacol. 1999;57:763–74. doi: 10.1016/s0006-2952(98)00354-2. [DOI] [PubMed] [Google Scholar]
- 14.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Lu C, Liu L, et al. Reactivation of methylation-silenced tumor suppressor gene p16INK4a by nordihydroguaiaretic acid and its implication in G1 cell cycle arrest. Life Sci. 2008;82:247–55. doi: 10.1016/j.lfs.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Byun HM, Choi SH, Laird PW, et al. 2’-Deoxy-N4-[2-(4- nitrophenyl)ethoxycarbonyl]-5-azacytidine: a novel inhibitor of DNA methyltransferase that requires activation by human carboxylesterase 1. Cancer Lett. 2008;266:238–48. doi: 10.1016/j.canlet.2008.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–71. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Liu J, Zhao Y. PKM2 inhibitor shikonin suppresses TPA-induced mitochondrial malfunction and proliferation of skin epidermal JB6 cells. Mol Carcinog. 2012 doi: 10.1002/mc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviello G, Romano B, Borrelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl) 2012;90:925–34. doi: 10.1007/s00109-011-0856-x. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Patterson S, Hawk E. Chemoprevention--history and general principles. Best Pract Res Clin Gastroenterol. 2011;25:445–59. doi: 10.1016/j.bpg.2011.10.012. [DOI] [PubMed] [Google Scholar]