Abstract
Background:
Linoleic acid is the most abundant polyunsaturated fatty acid in human nutrition and found in most vegetable oils and certain food products. In the present study, we investigated the effects of linoleic acid on the growth of human epithelial adenocarcinoma AGS cells.
Methods:
MTT assay, flow cytometry, RT-PCR and Western-blot analyses were used to investigate the effects and underlying mechanisms of linoleic acid on AGS cells. The effects of this compound were also tested on prostaglandin E2 (PGE2) production and telomerase activity.
Results:
Our data indicated that growth inhibition of AGS cells by linoleic acid treatment was associated with induction of apoptosis. Linoleic acid treatment decreased the expression levels of the cyclooxygenase (COX)-2 mRNA and protein without causing significant changes in the COX-1 levels, which was correlated with the inhibition of PGE2 synthesis. Linoleic acid treatment also decreased the expression of human telomerase reverse transcriptase (hTERT), a main determinant of the telomerase enzymatic activity, and activity of telomerase, with inhibiting the expression of c-myc in a concentration-dependent manner.
Conclusions:
Taken together, our results indicate that linoleic acid inhibits the production of PGE2 and activity of telomerase by suppressing COX-2 and hTERT expression.
Keywords: Linoleic acid, AGS cells, Prostaglandin E2, Telomerase
INTRODUCTION
Fatty acids are carboxylic acids with long aliphatic tails, which are either saturated or unsaturated. As precursors of lipid-signaling molecules, polyunsaturated fatty acids play key roles in several biological processes for cell signaling and involved in the regulation of gene expression as ligands for transcription factors.1,2 Among them, linoleic acid, an unsaturated omega-6 fatty acid, is the most abundant polyunsaturated fatty acid in human nutrition and obtained from plant based dietary sources.3,4 Many studies claim that a high linoleic acid intake may promote inflammation in humans.5,6 This compound also has been reported to promote cancer cell growth, invasion and metastasis, and enhances angiogenesis.7–9 However, some studies found that linoleic acid not only inhibits cancer cell proliferation and but also selectively kills cancer cells through apoptosis induction without damaging normal cells.10–13 For example, Maggiora et al.14 observed that linoleic acid inhibits the growth of liver and prostate cancer cells, but has no effect on growth of bladder and breast cancer cells. In addition, Lu et al.15 indicated that linoleic acid induced cancer cell apoptosis by enhancing cellular oxidant status and inducing mitochondrial dysfunction. Zhang et al.16 recently reported that linoleic acid promotes cell apoptosis in hepatoma cells through induction of calcium-dependent endoplasmic reticulum stress. We also previously investigated the effects of linoleic acid in gastric adenocarcinoma cells and found that linoleic acid induced apoptotic cell death through activation of Fas/Fas ligand pathway.17
Therefore, in order to further investigate the effect of linoleic acid on the growth inhibition in cancer cells, the effects of this compound were tested on the expression of cyclooxygenases (COXs) and human telomerase reverse transcriptase (hTERT), which are enzymes that catalyzes the rate-limiting step in prostaglandin synthesis from arachidonic acid and the catalytic subunit of telomerase that help to elongate telomere length, respectively in the human gastric carcinoma AGS cell line. The present data indicated that down-regulation of COX-2 and hTERT expression by linoleic acid treatment was associated with an inhibition of prostaglandin E2 (PGE2) release and telomerase activity in AGS cells.
MATERIALS AND METHODS
1. Cell culture and linoleic acid treatment
AGS cells were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in RPMI-1640 medium (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS), 2 μm L-glutamine and penicillin/streptomycin (Gibco-BRL). Linoleic acid was purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA) and prepared as previously described.17
2. Cell viability study
For cell viability analysis, cells were cultured in the presence or absence of linoleic acid. After 96 h of culture, the cells were trypsinized and washed with phosphatebuffered saline (PBS), and the viable cells were scored using a Neubauer hemocytometer with trypan blue exclusion. Each experiment was repeated at least three times.
3. Detection of apoptosis by annexin-V FITC staining
The cells were washed with PBS and re-suspended in an Annexin-V binding buffer containing 10 mM HEPES/ NaOH, pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2. Aliquots of the cells were incubated with Annexin-V fluorescein isothiocyanate (FITC, Sigma-Aldrich), mixed, and incubated for 15 min at room temperature in the dark. Propidium iodide (PI, Sigma-Aldrich) at a concentration of 5 μg/ml was added to distinguish the necrotic cells. The apoptotic cells (V+/PI−) were measured by the fluorescence-activated cell sorter analysis in a FACS analyzer (Becton Dickinson, San Jose, CA, USA).
4. RNA extraction and reverse transcription-PCR
Total RNA was prepared using a TRIzol reagent (Invitrogen, CA, USA) and reverse-transcribed using M-MLV reverse transcriptase (Promega, Madison, WI, USA) to produce complementary DNAs according to the manufacturer’s instructions. Polymerase chain reaction (PCR) was carried out in a Mastercycler (Eppendorf, Hamburg, Germany) with the indicated primers (Table 1). Conditions for PCR reactions were 1×(94°C for 3 min); 35×(94°C for 45 s; 58°C for 45 s; and 72°C for 1 min) and 1×(72°C for 10 min). Amplification products obtained by PCR were electrophoretically separated on 1% agarose gel and visualized by ethidium bromide (EtBr, Sigma-Aldrich) staining.
Table 1.
Oligonucleotides used in reverse transcription-PCR
| Gene name | Sequence of primers | |
|---|---|---|
| COX-1 | Sense | 5’-TGC CCA GCT CCT GGC CCG CCG CTT-3’ |
| Antisense | 5’-GTG CAT CAA CAC AGG CGC CTC TTC-3’ | |
| COX-2 | Sense | 5’-TTC AAA TGA GAT TGT GGG AAA AT-3’ |
| Antisense | 5’-AGA TCA TCT CTG CCT GAG TAT CTT-3’ | |
| hTERT | Sense | 5’-AGC-CAG-TCT-CAC-CTT-CAA-CC-3’ |
| Antisense | 5’-GTT-CTT-CCA-AAC-TTG-CTG-ATG-3’ | |
| TEP-1 | Sense | 5’-TCA-AGC-CAA-ACC-TGA-ATC-TGA-G-3’ |
| Antisense | 5’-CCC-CGA-GTG-AAT-CTT-TCT-ACG-C-3’ | |
| hTR | Sense | 5’-TCT-AAC-CCT-AAC-TGA-GAA-GGG-CGT-AG-3’ |
| Antisense | 5’-GTT-TGC-TCT-AGA-ATG-AAC-GGT-GGA-AG-3’ | |
| Sp-1 | Sense | 5’-ACA GGT GAG VTT GAC CTC AC-3’ |
| Antisense | 5’-GTT GGT TTG CAC CTG GTA TG-3’ | |
| c-myc | Sense | 5’-AAG-ACT-CCA-GCG-CCT-TCT-CTC-3’ |
| Antisense | 5’-GTT-TTC-CAA-CTC-CGG-GAT-CTG-3’ | |
| GAPDH | Sense | 5’-CGG-AGT-CAA-CGG-ATT-TGG-TCG-TAT-3’ |
| Antisense | 5’-AGC-CTT-CTC-CAT-GGT-GGT-GAA-GAC-3’ |
5. Protein extraction and Western blot analysis
For isolation of total protein fractions, cells were collected and lysed with cell lysis buffer [20 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM ethylenediaminetetraacetic acid, 0.5 g/ml leupeptin, 1% Na3CO4, 1 mM phenylmethanesulfonyl fluoride]. Then the protein concentrations were quantified using a BioRad protein assay (BioRad Lab., Hercules, CA, USA) according to the manufacturer’s instructions. For Western blot assay, the proteins were separated by SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, NH, USA) by electroblotting. After being blocked with blocking solution (1% BSA in PBS plus 0.05% Tween-20) at room temperature for 1 h, the blots were then probed with the specific primary antibodies and incubated overnight at 4°C. Following 1 h of incubation with the secondary antibodies, the blots were visualized by enhanced chemiluminescence (ECL, Amersham) solution according to the manufacturer’s procedure.
6. Measurement of PGE2 production
To measure the quantity of PGE2 generated by AGS cells, medium from the cultures under the same conditions was collected and the quantity of PGE2 production was measured using a PGE2 enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical Co., Ann Arbor, MI, USA). The concentration (pg/ml) of PGE2 in the cell culture medium was calculated based on the concentrations of the standard solution according to the recommended procedure.
7. Telomerase activity assay
Telomerase activity was measured using a PCR-based telomeric repeat amplification protocol (TRAP) ELISA kit (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer’s description. For the TRAP reaction, 2 μl of cell extract (containing 2 μg protein) was added to 25 μl of reaction mixture with the appropriate amount of sterile water to make a final volume of 50 ml. PCR was performed as follows: primer elongation (25°C for 30 min), telomerase inactivation (94°C for 5 min), product amplification by the repeat of 30 cycles (94°C for 30 s, 50°C for 30 s, and 72°C for 90 s). Hybridization and the ELISA reaction were carried out following the manufacturer’s instructions.
8. Statistical analysis
The data were expressed as means±SD for triplicate experiments. Statistical analyses were performed using Student’t test. P<0.05 was considered as statistically significantly.
RESULTS
1. Linoleic acid inhibits cell viability and induces apoptosis in AGS cells
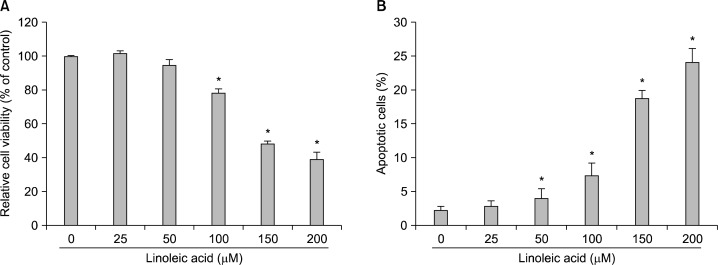
To investigate the potential effects of linoleic acid on cell growth, AGS cells were treated with various concentrations of linoleic acid for 96 h, and the cell numbers were then measured by the tryphan blue exclusion method. As shown in Fig. 1A, linoleic acid induced significant inhibition of AGS cell viability in a concentration-dependent manner. To measure apoptotic cell death upon linoleic acid treatment, we stained cells for annexin V. As can be seen in Fig. 1B, after treatment with 150 μM and 200 μM of linoleic acid for 96 h, the percentages of apoptotic cells increased from approximately 2.2% to 18.7% and 24.1%, respectively. These results suggest that linoleic acid-inhibited AGS cell growth was associated with induction of apoptosis.
Fig. 1.
Inhibition of cell growth and induction of apoptosis by linoleic acid treatment in AGS human gastric adenocarcinoma cells. (A) After cells were seeded, the cells were treated with the indicated concentrations of linoleic acid for 96 h, and then cell viability was measured by hemocytometer counts of trypan blue-excluding cells. (B) The cells were stained with annexin-V and the percentages of apoptotic cells were then analyzed using flow cytometric analysis. Each point represents the mean±SD of three independent experiments. Significance was determined using Student’s t-test (*P<0.05 vs. untreated control).
2. Linoleic acid inhibits the expression of COX-2 and production of PGE2 in AGS cells
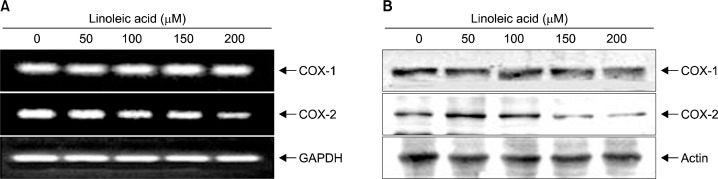
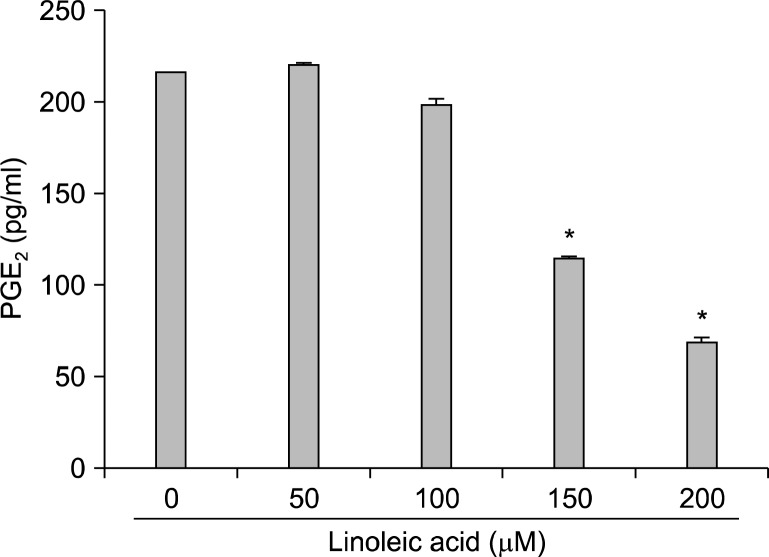
Next, RT-PRC and Western blot analyses were assessed in order to elucidate whether or not linoleic acid-induced growth inhibition was associated with the inhibition of PGE2 synthesis. Our results indicated that the levels of COX-2 mRNA and proteins were down-regulated in linoleic acid-treated AGS cells in a concentration-dependent manner (Fig. 2). However, those of COX-1 were remained unchanged. Therefore, supernatant from cell culture media was collected and PGE2 levels were determined with the ELISA kit. According to the ELISA data, treatment with linoleic acid resulted in a significant declines of PGE2 production (53% and 31% by treatment with 150 μM and 200 μM of linoleic acid, respectively) compared to the untreated control (Fig. 3). Taken together, these data indicate that linoleic acid inhibits the PGE2 production via suppression of CXO-2 expression at the transcription level.
Fig. 2.
Effects of linoleic acid on levels of COXs expression in AGS cells. (A) After treatment with linoleic acid for 96 h, total RNA was isolated, and RT-PCR was performed using the indicated primers. The amplified PCR products were run in a 1% agarose gel and visualized by EtBr staining. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping control gene. (B) Cells grown under the same conditions as (A) were collected, lysed and cellular proteins were separated on SDS-poly-acrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using the ECL detection system. Actin was used as a loading control.
Fig. 3.
Inhibition of PGE2 production in AGS cells after exposure to linoleic acid. After 96 h incubation with linoleic acid, the PGE2 accumulation in the medium was determined by an ELISA kit. Data are expressed as mean±SD of three independent experiments. Significance was determined by Student’s t-test (*P<0.05 vs. untreated control).
3. Linoleic acid suppresses the expression of hTERT and telomerase activity in AGS cells
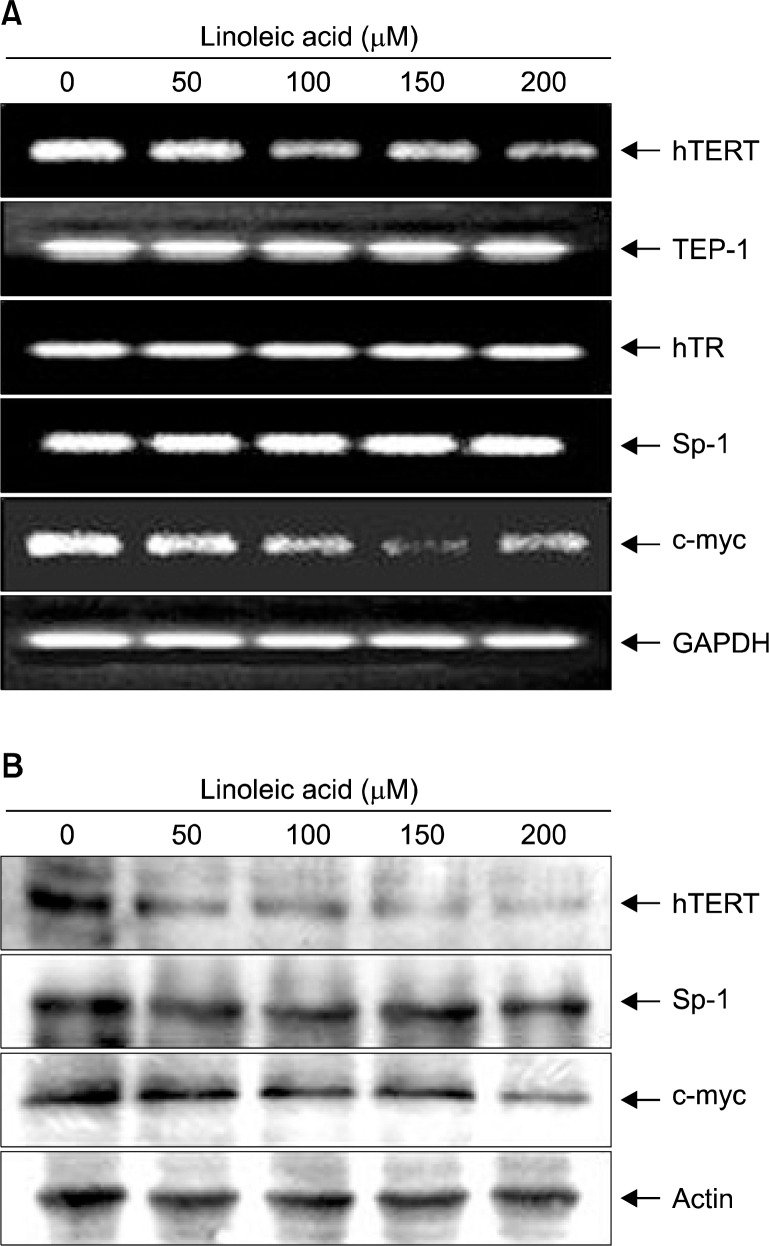
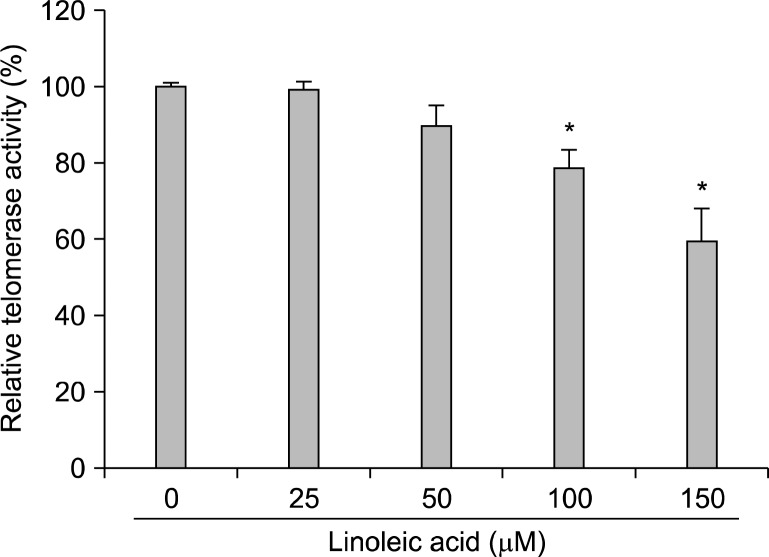
We next tried to reveal whether the linoleic acid-mediated cytotoxic effect on AGS cells is also associated with the inhibition of telomerase activity. As indicated in Fig. 4A, we found that linoleic acid treatment decreased hTERT and c-myc mRNA levels, and had no effect on telomerase associated protein-1 (TEP-1), human telomerase RNA (hTR) and Sp-1 mRNA expression (Fig. 4A). Moreover, Western blot analyses also confirmed the down-regulation of hTERT and c-myc proteins in AGS cells treated with linoleic acid in a concentration-dependent manner (Fig. 4B). Furthermore, linoleic acid treatment resulted in a concentration-dependent reduction of telomerase activity in AGS cells (Fig. 5), indicating that linoleic acid-induced inhibition of telomerase activity may be due to down-regulation of hTERT and c-myc.
Fig. 4.
Effects of LA on levels of telomere regulatory factors expression in AGS cells. (A) After treatment with linoleic acid for 96 h, total RNAs were isolated, and RT-PCR was performed using the indicated primers. GAPDH was used as a housekeeping control gene. (B) Cells grown under the same conditions as (A) were collected, lysed and cellular proteins were separated on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using the ECL detection system. Actin was used as a loading control.
Fig. 5.
Inhibition of telomerase activity by linoleic acid treatment in AGS cells. (A) After 96 h incubation with linoleic acid, telomerase activity of AGS cells were measured using a TRAP-ELISA kit. For one sample, 2×105 cells were lysed, and 1/100 was used in the assay. Data represent the relative mean values±SD of three independent experiments. Significance was determined by Student’s t-test (*P<0.05 vs. untreated control).
DISCUSSION
Prostaglandins are lipid mediators that are involved in many normal physiological processes and are implicated in many pathological processes such as inflammation and cancer.18 COX, referred to as prostaglandin-endoperoxide synthase, is an enzyme that is responsible for formation of important biological mediators called prostanoids, including prostaglandins, prostacyclin and thromboxane, from arachidonic acid. At present, three COX isoenzymes are known: COX-1, COX-2, and COX-3.19 COX-1 is considered to be the constitutively expressed form in most mammalian cells and thought to serve housekeeping functions. COX-3 is a splice variant of COX-1, which retains intron one and has a frameshift mutation.20 On the other hand, COX-2 is undetectable in most normal tissues and rapidly induced by different products, such as tumor promoters, growth factors or inflammatory cytokines. In addition, COX-2 has been shown to be upregulated in various carcinomas and to have a central role in tumorigenesis. Moreover, the tumorigenic potential of COX-2 overexpression has frequently been associated with resistance to apoptosis in certain cell types.21,22 Therefore, the specific inhibition of COX-2 expression and the blockade of the PGs cascade with chemotherapy agents would be an effective approach in the prevention and treatment of cancer. Thus, we investigated here whether linoleic acid-induced anti-proliferative effect of AGS cells was associated with an inhibition of COX-2 expression and its function. As shown in Fig. 2, we observed that linoleic acid markedly inhibited COX-2 mRNA and protein expression, however, the levels of COX-1 remained unaltered. Linoleic acid also inhibited the production of PGE2 in AGS cells (Fig. 3). The data suggested that the inhibition of PGE2 synthesis through down-regulation of COX-2 expression is associated with the results that linoleic acid inhibited the growth and induced apoptosis.
Telomeres are localized in the physical ends of eukaryotic chromosomes and essential units that stabilize the ends of eukaryotic chromosome to prevent the loss of genetic information. Therefore, disruption of the telomere structure, by telomeric DNA cleavage or loss of telomere binding protein functions, is associated with senescence and cell death.23,24 However, malignant cells exhibit pronounced activation of telomerase, which adds telomeric repeats to the ends of replicating chromosomes to prevent telomere shortening, and subsequently leads to immortal cell characteristics and tumorigenesis.25,26 These observations suggests that telomerase activity regulation has been considered as a strategy for control of senescence and cell death. Telomere length in humans is primarily controlled by three major components; hTR, TEP-1 and hTERT. Among them, hTERT is considered a viable cancer therapy target because hTERT is highly expressed in cancer cells, but not in normal cells.27,28 In this study, we observed that application of linoleic acid to AGS cells decreases telomerase activity via down-regulation of hTERT in transcription and translation (Fig. 4, 5).
According to previous studies, expression of hTERT is strictly regulated at the transcriptional level by several transcription factors, particularly, Sp-1 and c-myc.29,30 c-myc directly binds with the E-box at the promoter of hTERT and induces hTERT transcription.31 In addition to c-myc binding sites, the core promoter, which is necessary for hTERT expression, also contains several putative Sp-1/Sp-3 binding sites; Sp-1 works in conjunction with c-myc to activate transcription of hTERT.32,33 However, in some cancer cells, telomerase activity can apparently be regulated independently on Sp-1 and/or c-myc.34,35 In the present study, the levels of c-myc mRNA and protein expressions, but not Sp-1, in AGS cells were concentration-dependently inhibited by linoleic acid treatment (Fig. 5), demonstrating inactivation of telomerase activity by linoleic acid was associated with down-regulation of c-myc.
In conclusion, we demonstrated here that linoleic acid potently suppresses the proliferation of AGS human gastric cancer cells by inducting apoptosis. The growth inhibitory effects of linoleic acid were associated with a specific inhibition of COX-2 expression and concomitant with a loss of PGE2 synthesis. Our results also indicated that linoleic acid potently suppresses the telomerase activity by decreasing the hTERT and c-myc expression. Therefore, the present work suggests that loss of COX-2 expression and telomerase activity may be good surrogate biomarkers for assessing anti-cancer activity of linoleic acid.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2008-0062611).
REFERENCES
- 1.Nakamura MT, Cheon Y, Li Y, Nara TY. Mechanisms of regulation of gene expression by fatty acids. Lipids. 2004;39:1077–83. doi: 10.1007/s11745-004-1333-0. [DOI] [PubMed] [Google Scholar]
- 2.Duplus E, Forest C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem Pharmacol. 2002;64:893–901. doi: 10.1016/s0006-2952(02)01157-7. [DOI] [PubMed] [Google Scholar]
- 3.Choque B, Catheline D, Rioux V, Legrand P. Linoleic acid: Between doubts and certainties. Biochimie. 2014;96:14–21. doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Deckelbaum RJ, Torrejon C. The omega-3 fatty acid nutritional landscape: health benefits and sources. J Nutr. 2012;142:587S–91S. doi: 10.3945/jn.111.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–41. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Bassaganya-Riera J, Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2010;13:569–73. doi: 10.1097/MCO.0b013e32833b648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan J, McEntee MF. Dietary (n-6) PUFA and intestinal tumorigenesis. J Nutr. 2004;134:3421S–6S. doi: 10.1093/jn/134.12.3421S. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka T, Adair JE, Lih FB, Hsi LC, Rubino M, Eling TE, Tomer KB, Yashiro M, Hirakawa K, Olden K, Roberts JD. Elevated dietary linoleic acid increases gastric carcinoma cell invasion and metastasis in mice. Br J Cancer. 2010;103:1182–91. doi: 10.1038/sj.bjc.6605881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishioka N, Matsuoka T, Yashiro M, Hirakawa K, Olden K, Roberts JD. Linoleic acid enhances angiogenesis through suppression of angiostatin induced by plasminogen activator inhibitor 1. Br J Cancer. 2011;105:1750–8. doi: 10.1038/bjc.2011.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mormile R, Vittori G, De Michele M, Squarcia U, Quaini F. Linoleic acid and colorectal cancer cell growth suppression: is the deregulation of mitochondrial survivin the key factor? Int J Colorectal Dis. 2012;27:1383–4. doi: 10.1007/s00384-011-1407-1. [DOI] [PubMed] [Google Scholar]
- 11.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–53. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 12.Andrade LN, de Lima TM, Curi R, Castrucci AM. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol In Vitro. 2005;19:553–60. doi: 10.1016/j.tiv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Begin ME, Dae UN, Ells G, Horrobin DF. Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins Leukot Med. 1985;19:177–86. doi: 10.1016/0262-1746(85)90084-8. [DOI] [PubMed] [Google Scholar]
- 14.Maggiora M, Bologna M, Cerù MP, Possati L, Angelucci A, Cimini A, Miglietta A, Bozzo F, Margiotta C, Muzio G, Canuto RA. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer. 2004;112:909–19. doi: 10.1002/ijc.20519. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Yu H, Ma Q, Shen S, Das UN. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010;9:106. doi: 10.1186/1476-511X-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012;11:1. doi: 10.1186/1476-511X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon JI, Kim GY, Park KY, Ryu CH, Choi YH. Induction of apoptosis by linoleic acid is associated with the modulation of Bcl-2 family and Fas/FasL system and activation of caspases in AGS human gastric adenocarcinoma cells. J Med Food. 2008;11:1–8. doi: 10.1089/jmf.2007.073. [DOI] [PubMed] [Google Scholar]
- 18.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–11. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Willoughby DA, Moore AR, Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet. 2000;355:646–8. doi: 10.1016/S0140-6736(99)12031-2. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by crosstalk between two distinct growth factor receptors. FASEB J. 2003;17:1640–7. doi: 10.1096/fj.02-1011com. [DOI] [PubMed] [Google Scholar]
- 22.Méric JB, Rottey S, Olaussen K, Soria JC, Khayat D, Rixe O, Spano JP. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Gancarcíková M, Zemanová Z, Brezinová J, Berková A, Vcelíková S, Smigová J, Michalová K. The role of telomeres and telomerase complex in haematological neoplasia: the length of telomeres as a marker of carcinogenesis and prognosis of disease. Prague Med Rep. 2010;111:91–105. [PubMed] [Google Scholar]
- 24.Oulton R, Harrington L. Telomeres, telomerase, and cancer: life on the edge of genomic stability. Curr Opin Oncol. 2000;12:74–81. doi: 10.1097/00001622-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Ann Med. 2001;33:123–9. doi: 10.3109/07853890109002067. [DOI] [PubMed] [Google Scholar]
- 26.Kyo S, Takakura M, Tanaka M, Murakami K, Saitoh R, Hirano H, Inoue M. Quantitative differences in telomerase activity among malignant, premalignant, and benign ovarian lesions. Clin Cancer Res. 1998;4:399–405. [PubMed] [Google Scholar]
- 27.Autexier C, Greider CW. Telomerase and cancer: revisiting the telomere hypothesis. Trends Biochem Sci. 1996;21:387–91. [PubMed] [Google Scholar]
- 28.Darimont C, Zbinden I, Avanti O, Leone-Vautravers P, Giusti V, Burckhardt P, Pfeifer AM, Macé K. Reconstitution of telomerase activity combined with HPV-E7 expression allow human preadipocytes to preserve their differentiation capacity after immortalization. Cell Death Differ. 2003;10:1025–31. doi: 10.1038/sj.cdd.4401273. [DOI] [PubMed] [Google Scholar]
- 29.Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8:137–42. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 30.Casillas MA, Brotherton SL, Andrews LG, Ruppert JM, Tollefsbol TO. Induction of endogenous telomerase (hTERT) by c-Myc in WI-38 fibroblasts transformed with specific genetic elements. Gen. 2003;316:57–65. doi: 10.1016/s0378-1119(03)00739-x. [DOI] [PubMed] [Google Scholar]
- 31.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–7. [PubMed] [Google Scholar]
- 32.Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, Ariga H, Inoue M. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT) Nucleic Acids Res. 2000;28:669–77. doi: 10.1093/nar/28.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wooten LG, Ogretmen B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem. 2005;280:28867–76. doi: 10.1074/jbc.M413444200. [DOI] [PubMed] [Google Scholar]
- 34.Drissi R, Zindy F, Roussel MF, Cleveland JL. C-Myc-mediated regulation of telomerase activity is disabled in immortalized cells. J Biol Chem. 2001;276:29994–30001. doi: 10.1074/jbc.M101899200. [DOI] [PubMed] [Google Scholar]
- 35.Xiao X, Sidorov IA, Gee J, Lempicki RA, Dimitrov DS. Retinoic acid-induced downmodulation of telomerase activity in human cancer cells. Exp Mol Pathol. 2005;79:108–17. doi: 10.1016/j.yexmp.2005.06.001. [DOI] [PubMed] [Google Scholar]