Abstract
Background:
The prognosis of H. pylori infection-negative gastric cancer (HPIN-GC) has been rarely investigated. Applying a strict definition of H. pylori status, the prognosis and molecular prognostic markers in HPIN-GC were evaluated.
Methods:
A combination of multiple methods was carried out to strictly evaluate H. pylori infection in gastric cancer (GC) patients between June 2003 and October 2012 at Seoul National University Bundang Hospital. H. pylori infection was defined as negative if histology, a rapid urease test, culturing, serology and history of H. pylori eradication were all negative. Patients with severe gastric atrophy by the serum pepsinogen test or histology were assumed to have had a previous H. pylori infection. Epstein-Barr virus (EBV) in situ hybridization, PCR-based microsatellite instability (MSI) testing, and p53 immunohistochemistry were performed.
Results:
Compared to 509 H. pylori infection-positive gastric cancer (HPIN-PC) patients, 24 HPIN-GC patients showed a significantly higher frequency of cardia location (P=0.013), and the depth of invasion in HPIN-GC was more advanced, although there was no statistical significance (pT3-pT4, 37.5% for HPIN-GC vs. 28.5% for HPIP-GC, P=0.341). In multivariate analysis, depth of invasion and lymph node metastasis were identified as the most important prognostic factors for relapse-free survival and overall survival (P<0.001). However, the status of H. pylori infection was not an independent prognostic factor for relapse-free survival and overall survival. The positivity of EBV in both groups was low, and the survivals according to MSI and p53 status in HPIN-GC patients were not significantly different.
Conclusions:
The status of H. pylori infection was not a prognostic factor for survival in GC patients when applying the strict definition of H. pylori infection. The prognostic implication of MSI and p53 on survival in HPIN-GC patients was not clear.
Keywords: Helicobacter pylori, Gastric cancer, Prognosis, p53, Microsatellite instability
INTRODUCTION
Helicobacter pylori (H. pylori) is strongly associated with gastric cancer (GC) through epidemiologic and clinical studies, and it has been classified as a class I carcinogen by International Agency for Research on Cancer.1 However, the etiology of GC is not limited to H. pylori infection. Dietary factors such as salt and nitrates contribute to the development of GC, and Epstein-Barr virus (EBV) is also responsible for approximately 5% of GC cases.2 Although the definition of H. pylori infection-negative gastric cancer (HPIN-GC) has not been established well, the prevalence of HPIN-GC is considered low, especially in Korea and Japan.3–7 The reported prevalence in both countries ranged from 0.66% to 10.6%.4,6,7 Our group reported that 5.4% cases of GC were negative for H. pylori infection.7
The prognosis of HPIN-GC has been rarely investigated in the literature.3,5,8 In two studies conducted in Western countries,3,5 the positivity of H. pylori infection in GC patients was 24.7% and 14.0%, respectively. The survivals in H. pylori-infection positive gastric cancer (HPIP-GC) patients were better than those in HPIN-GC, and the H. pylori infection status was reported to be an prognostic factor, independently of other well-known prognostic factors.3,5 In term of the diagnostic methods, the H. pylori status in GC patients was assessed by bacterial culture, histological analysis, serology, and/or PCR method. However, the diagnostic criteria were not as strict as in the recent research on the prevalence of HPIN-GC, and the status of H. pylori infection in the two studies was not properly evaluated. In the recent prevalence studies, including our report, strict definitions of H. pylori infection were employed, and precise diagnosis of HPIN-GC was tried with a combination of various diagnostic methods.6,7
With regard to the carcinogenesis of GC, genetic and epigenetic changes in oncogenes and tumor suppressor genes, cell cycle regulators, and DNA repair genes have been reported.9 The microsatellite instability (MSI) is defined as length changes of microsatellites, which are repeating sequences of 1–6 base pairs of DNA. The MSI is caused by an impairment of DNA mismatch repair system.9 The p53 tumor suppressor gene is the most commonly mutated gene in various human cancers, and alteration or inactivation of p53 allows a cell with damaged DNA to escape from normal growth, resulting in cancer development.9,10 The mutated p53 proteins accumulate with a prolonged half-life in amounts and can be detected by immunohistochemical methods.10 In GC patients, the clinicopathologic characteristics and prognostic roles of MSI and p53 expression have been reported in several studies.11–15 However, considering of the majority of HPIP-GC in GC patients, the results of the previous reports could represent the role of MSI and p53 expression in HPIP-GC. To our knowledge, there is no study evaluating the molecular prognostic markers such as MSI and p53 expression, confined to HPIN-GC.
The aim of the present study was to compare the clinicopathological and molecular features of HPIN-GC with those of HPIP-GC, applying a strict definition of H. pylori status, and to analyze the prognosis of GC patients according to the H. pylori status. In addition, the usefulness of the molecular prognostic markers established in HPIP-GC was re-evaluated in HPIN-GC.
MATERIALS AND METHODS
1. Patients
Between June 2003 and October 2012, patients diagnosed as gastric cancer by endoscopic biopsy were prospectively enrolled at Seoul National University Bundang Hospital, South Korea. Patients who had not received endoscopic resection or surgery, or who had been lost from follow-up were excluded from the present study. Patients with complete disappearance of GC after endoscopic biopsy and incomplete medical records were also excluded. All patients were ethnically Koreans and provided informed consent. The study protocol was approved by the Ethics Committee at Seoul National University Bundang Hospital.
2. Initial Determination of H. pylori Infection Status
To determine the current status of H. pylori infection, three biopsy-based tests (histology, rapid urease test, and culture) were employed. The protocols for the three tests were previously described in detail.7 To identify previous H. pylori infection, the sero-positivity and eradication history were investigated. Sero-positivity was assessed by enzyme-linked immunosorbent assay (ELISA) for anti-H. pylori antibody in each patient’s serum (Genedia H. pylori ELISA; Green Cross Medical Science Corp, Eumsung, South Korea). In addition, eradication history was evaluated in each patient by a questionnaire.
3. Evaluation of Gastric Atrophy by Serum Pepsinogen Test and Histologic Findings
In fasting serum collected from each patient, the concentrations of pepsinogen (PG) I and II were measured using a Latex-enhanced Turbidimetric Immunoassay (Shima Laboratories, Tokyo, Japan). Based on the results, patients were categorized as having no, mild to moderate, or severe gastric atrophy as previously described4,7,16; no atrophy, PG I >70 and PG I/II ratio >3.0; severe atrophy, PG I≤30 and PG I/II≤2.0; mild and moderate atrophy, 30< PG I≤70 and 2.0< PG I/II≤3.0. The status of gastric atrophy was also evaluated by histology, using four biopsy specimens obtained from each patient (one each from greater and lesser curvatures of antrum and body). The gastric mucosa was categorized as having no atrophy, non-metaplastic atrophy, metaplastic atrophy, or non-applicable as recommended by international group of pathologists.17
4. EBV In Situ Hybridization
Sections were digested with proteinase K and were hybridized for 2 h at 37°C with a fluorescein-conjugated EBV oligonucleotide probe for EBV-encoded small RNAs (EBERs; Novocastra, Newcastle upon Tyne, United Kingdom). Hybridization products were detected using an alkaline phosphatase-conjugated antibody to FITC [affinity-isolated rabbit F(ab’)]. 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium was used as an enzyme substrate to demonstrate alkaline phosphatase activity, and it were counterstained with Mayer’s hematoxylin. Positive staining was observed under light microscopy as black granules at the site of hybridization. Only those cases with signals within tumor cell nuclei were considered to be positive.18
5. p53 Immunohistochemistry
Sections 4μm thick were cut from each tissue array block and then deparaffinized and dehydrated. Immunohistochemical staining was performed using an automatic immunostainer (BenchMark® XT, Ventana Medical Systems Inc., Tucson, AZ) according to the manufacturer’s instructions. The primary antibody used was mouse monoclonal antibody for p53 (DO7, Dako, Carpinteria, Calif., USA). An antigen retrieval process was performed using microwave. Immunostaining 10% or more nuclear staining of tumor cells was considered positive.19
6. MSI Testing
Tumor DNA was extracted from paraffin-embedded tissues of tumors from individual patients. Normal DNA was extracted from the surrounding normal tissue. Five microsatellite markers (BAT-25, BAT-26, D2S123, D5S346 and D17S250), recommended by a National Cancer Institute workshop on MSI, were used to analyze paired normal and tumor DNA.20 Polymerase chain reaction (PCR) was performed using a DNA auto-sequencer (ABI 3730 genetic analyzer; Applied Biosystems, Foster City, CA). The shift of PCR products from tumor DNA was compared to that of DNA from normal mucosa. The size of each fluorescent PCR product was calculated using GeneMapper software (Applied Biosystems, Foster City, CA). According to the guideline of the National Cancer Institute, cases positive for ≥2 markers were considered as high-frequency MSI (MSI-H), while cases positive for <2 markers as low-frequency MSI (MSI-L) or microsatellite stable (MSS).20,21
7. Follow-Up
The patients who underwent curative resection of GC were included in a follow-up program in the departments of Surgery at Seoul National University Bundang hospital.22,23 The follow-up investigations were scheduled at 3-month intervals for the first 2 years, then every 6 months for the next 3 years, and annually until the patient’s death. The program consisted of physical examination, routine blood tests, endoscopy, and ultrasonography or computed tomography. Clinical outcomes were obtained from medical records until the date of death, loss-to-follow-up or October 2012 (end date of the study). Causes of death were ascertained by medical records and death certificate.
8. Statistical Analysis
To compare the clinicopathologic and molecular characteristics, Student’s t-test or Chi-square test (Fisher’s exact test) was used for continuous variables and categorical variables, respectively. The log-rank test was used in Kaplan-Meier survival analyses to investigate the effects of variables on survival. The effect of H. pylori infection on survival was assessed with the Cox proportional hazards regression, including all significant variables from the univariate analysis and age as covariates. All analyses were carried out using the SPSS for Windows (version 20.0; SPSS, Chicago, IL, USA). The results were considered statistically significant when p-values were less than 0.05.
RESULTS
1. Determination of the H. pylori Infection Status
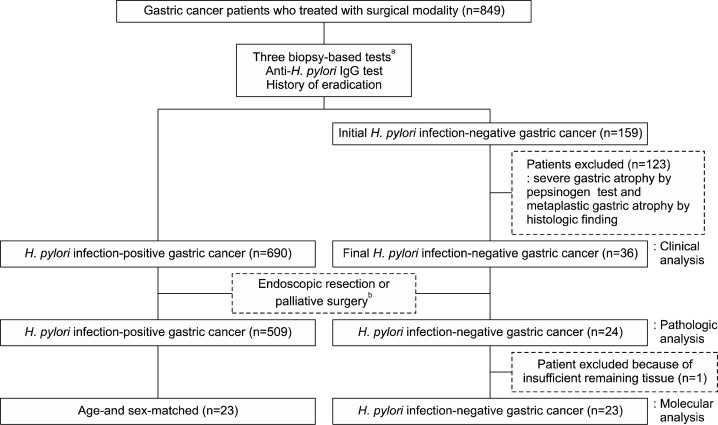
In the 849 GC patients, the H. pylori infection status was determined according to the strict definition, and these selection processes are summarized in Fig. 1. We determined strict definition of H. pylori infection-negative as followed; 1) absent metaplastic atrophy both in the antrum and body, PG I/II ratio >3; 2) non-applicable for atrophy either in the antrum or body but absent intestinal metaplasia both in the antrum and body, PG I/II ratio >3; 3) absent atrophy both in the antrum and body, PG I/II ratio >3 but present intestinal metaplasia either in the antrum or body; 4) present mild atrophy either in the antrum or body but absent intestinal metaplasia both in the antrum and body, PG I/II ratio >3. By the three biopsy-based tests, 529 patients were diagnosed with current H. pylori infection, and 161 patients were diagnosed with previous infection by anti-H. pylori IgG positivity or eradication history. Altogether, 690 patients were categorized as HPIP-GC. In the 159 patients initially classified as HPIN-GC, patients with serologic and histologic diagnoses of severe gastric atrophy according to our definition, were excluded, because there is a high possibility of eliminating H. pylori infection during the process of gastric atrophy.4,7,24 Finally, 36 patients were categorized as HPIN-GC.
Fig. 1.
Flow chart applying the strict definition of H. pylori infection for the comparison of clinicopathologic and molecular characteristics between H. pylori-infection positive and negative gastric cancer patients. aHistology, rapid urease test, and culture for H. pylori. bPatients who had undergone endoscopic resection or palliative surgery were excluded in the pathologic evaluation.
2. Clinicopathologic and Molecular Characteristics between H. pylori-Infection Positive and Negative Gastric Cancers
The clinicopathologic features between HPIP-GC and HPIN-GC were compared (Table 1). The clinical features such as age and sex were similar in both groups, except PG II and I/II ratio used for the diagnosis of HPIN-GC. Curative surgery was performed in 518 HPIP-GC and 24 HPIN-GC patients. Among 518 HPIP-GC patients who underwent curative surgery, nine patients were excluded from further analysis because of incomplete record or insufficient remaining tissue. In the pathologic analysis, there was no difference in tumor size between two groups, but HPIN-GC group showed a significantly higher frequency of cardia location than HPIP-GC (20.8% vs. 7.1%, P=0.013). The depth of invasion in HPIN-GC group was more advanced than that in HPIP-GC group, although there was no statistical significance (pT3-pT4, 37.5% for HPIN-GC vs. 28.5% for HPIP-GC, P=0.341). The proportion of lymph node metastasis in HPIN-GC group was more than that in HPIP-GC group, but there was no statistical significance (45.8% for HPIN-GC and 36.9% for HPIP-GC, P=0.378).
Table 1.
Clinicopathologic and molecular characteristics of gastric cancer patients by H. pylori infection status
| Clinical analyses, total | H. pylori positive | H. pylori negative | P-value |
|
|
|
||
| 690 | 36 | ||
|
| |||
| M/F, n (%) | 464/226 (67.2/32.8) | 24/12 (66.7/33.3) | 0.942 |
| Age (mean±SD), years | 59.1±11.9 | 55.9±13.1 | 0.120 |
| Serum Pepsinogen (PG) test | |||
| PG I (ng/mL) | 63.4±44.8 | 62.3±35.8 | 0.891 |
| PG II (ng/mL) | 25.0±7.4 | 11.4±7.1 | <0.001 |
| PG I/II ratio | 3.1±2.1 | 5.8±2.0 | <0.001 |
| Histologic Type* | |||
| Tubular ADC, W/D, n (%) | 156 (22.6) | 7 (19.4) | 0.961 |
| Tubular ADC, M/D, n (%) | 222 (32.2) | 11 (30.6) | |
| Tubular ADC, P/D, n (%) | 196 (28.4) | 11 (30.6) | |
| Signet ring cell carcinoma, n (%) | 91 (13.2) | 6 (6.2) | |
| Others, n (%)† | 25 (3.6) | 1 (2.8) | |
| Treatment modalities | |||
| Endoscopic resection | 142 (20.6) | 9 (25.0) | |
| Curative surgery | 518 (75.1) | 23 (63.9) | 0.120 |
| Palliative surgery | 30 (4.3) | 4 (11.1) | |
|
| |||
| Pathologic analyses, total‡ | H. pylori positive | H. pylori negative | P-value |
|
|
|
||
| 509 | 24 | ||
|
| |||
| Tumor size (mean±SD), cm | 4.1±2.7 | 3.9±1.8 | 0.877 |
| Tumor location | |||
| Cardia, n (%)§ | 36 (7.1) | 5 (20.8) | 0.013 |
| Noncardia, n (%) | 473 (92.9) | 19 (79.2) | |
| Lauren classification | |||
| Intestinal, n (%) | 253 (49.7) | 10 (41.7) | 0.441 |
| Diffuse or mixed, n (%) | 256 (50.3) | 14 (58.3) | |
| Depth of invasion, n (%) | |||
| pT1–pT2 | 364 (71.5) | 15 (62.5) | 0.341 |
| pT3–pT4 | 145 (28.5) | 9 (37.5) | |
| Lymph node metastasis, n (%) | |||
| pN0 | 321 (63.1) | 13 (54.2) | 0.378 |
| pN1–pN3 | 188 (36.9) | 11 (45.8) | |
| TNM stage, n (%) | |||
| I | 309 (60.7) | 11 (45.8) | 0.146 |
| II, III, IV | 200 (39.9) | 13 (54.2) | |
|
| |||
| Molecular analyses, total | H. pylori positive | H. pylori negative | P-value |
|
|
|
||
| 23∥ | 23 | ||
|
| |||
| EBV, n (%) | |||
| Positive | 1 (7.7)¶ | 1 (6.7)¶ | 1.000 |
| Negative | 12 (92.3) | 14 (93.3) | |
| MSI, n (%) | |||
| MSI-High | 2 (8.7) | 3 (13.6)¶ | 0.665 |
| MSI-Low/MSS# | 21 (91.3) | 19 (86.4) | |
| p53, n (%) | |||
| Positive | 10 (43.5) | 11 (47.8) | 0.767 |
| Negative | 13 (56.5) | 12 (52.2) | |
ADC, adenocarcinoma; W/D, well-differentiated; M/D, moderately differentiated; P/D, poorly differentiated.
Includes mucinous adenocarcinoma, adenosquamous carcinoma, and undifferentiated carcinoma.
Patients who underwent endoscopic resection or palliative surgery were excluded in the pathologic analysis. Patients with insufficient tissue or incomplete record were also excluded.
Located within 2 cm below the gastroesophageal junction.
Age- and sex-matched patients randomly selected from H. pylori-infection positive gastric cancer patients.
EBV or MSI were not performed due to insufficient remaining tissue.
Microsatellite instability-low or microsatellite stable.
To investigate causative and prognostic factors in HPIN-GC, EBV in situ hybridization, p53 immunohistochemistry, and PCR-based MSI testing were performed in the surgical specimens (Table 1). Among 24 HPIN-GC who underwent curative surgery, one patient was excluded from further analysis because of insufficient remaining tissue. Age- and sex-matched 23 patients were randomly selected from HPIP-GC patients to properly compare with HPIN-GC patients (Fig. 1). The positivity of EBV in both groups was low (6.7% vs. 7.7%), which was not significantly different. Similarly, the distributions of MSI-H and p53 positivity in HPIN-GC were not significantly different from those in HPIP-GC.
3. Influence of H. pylori Status on Survival
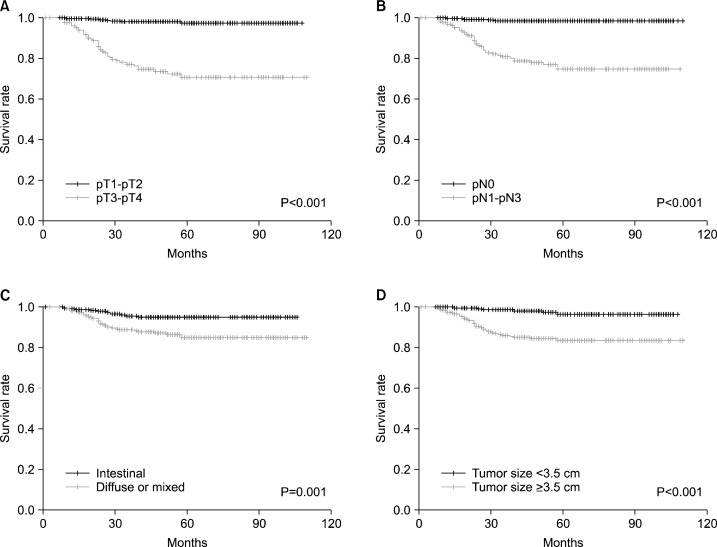
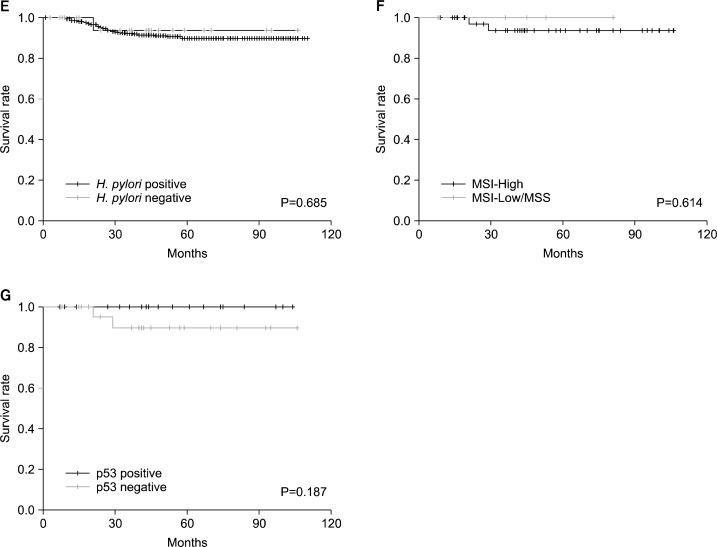
In 509 HPIP-GC and 24 HPIN-GC patients, the influence of H. pylori status on long-term survival was evaluated. In the entire patients, 44 patients had died of tumor recurrence; the 5-year survival rate was 89.8% (standard error (SE), 1.5%). Only one of the HPIN-GC patients had died of tumor recurrence. Univariate analyses showed a significant association between relapse-free survival (RFS) and tumor size (≥3.5cm), Lauren classification, depth of invasion (pT1-pT2/pT3-pT4), and lymph node metastasis (pN0/pN1-pN3) (data not shown). With regard to overall survival (OS), there is an association with age, tumor size, Lauren classification, depth of invasion, and lymph node metastasis (Fig. 2A–D). The 5-year survival rate were as follows; 99.1% (SE, 0.5%) in pT1-pT2 versus 70.9% (SE, 4.2%) in pT3-pT4, 99.4% (SE, 0.5%) in pN0 versus 86.6% (SE, 2.6%) in pN1-pN3, 96.5% (SE, 1.2%) in intestinal type versus 91.5% (SE, 1.8%) in diffused or mixed type, 98.6% (SE, 0.8%) in small tumor size (<3.5cm) versus 83.6% (SE, 2.5%) in large size (≥3.5cm). In multivariate analyses, depth of invasion, lymph node metastasis, and Lauren classification were identified as independent prognostic factors for both RFS and OS (Table 2). However, the status of H. pylori infection was not a significant factor in both univariate and multivariate analyses (Fig. 2E, Table 2). The 5-year rate of OS was 96.9% (SE, 0.8%) in H. pylori-positive and 93.8% (SE, 6.1%) in H. pylori-negative subjects, respectively. In addition, no significant effect of H. pylori status on survival was found in the subgroup analysis (such as tumor location, pT and pN stages) (data not shown).
Fig. 2.
Effects of H. pylori status and molecular markers on overall survival. Overall survival in 533 gastric cancer patients according to (A) T stage, (B) N stage, (C) Lauren classification, (D) tumor size, and (E) H. pylori status. Overall survival in 24 H. pylori-infection negative gastric cancer patients according to (F) microsatellite instability (MSI) and (G) p53 expression.
Table 2.
Prognostic factors for relapse-free survival and overall survival in multivariate Cox proportional hazard analysis
| Relapse-free survival | Overall survival | |||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Depth of invasion† | 6.58 (2.92–14.81) | <0.001 | 5.30 (2.20–12.74) | <0.001 |
| Lymph node metastasis‡ | 6.67 (2.47–18.04) | <0.001 | 6.70 (2.20–20.44) | 0.001 |
| Lauren classification | 1.96 (1.06–3.64) | 0.033 | 2.24 (1.10–4.53) | 0.026 |
| Age (years) | 1.02 (1.00–1.04) | 0.063 | 1.03 (1.01–1.06) | 0.007 |
| H. pylori infection status | 0.780 (0.19–3.21) | 0.731 | 0.51 (0.07–3.70) | 0.504 |
pT1-pT2 vs. pT3-pT4,
pN0 vs. pN1-pN3.
4. Molecular prognostic markers in H. pylori-Infection Negative Gastric Cancers
For the evaluation of usefulness as a prognostic factor in HPIN-GC, the clinicopathologic features were stratified by MSI and p53 status (Table 3). In MSI-H, both HPIP-GC and HPIN-GC showed a tendency of older age, and intestinal classification, compared to MSI-L/MSS. HPIN-GC with MSI-L/MSS showed a significantly higher frequency of cardia location, compared to HPIP-GC with MSI-L/MSS (P=0.042), but the frequency of cardia location was still lower than that of noncardia location (21.1% vs. 78.9%). In p53 positive group, HPIN-GC had significantly higher frequencies of cardia location compared to HPIP-GC, but there was no statistical significance. The RFS and OS were assessed by MSI and p53 status in 23 HPIN-GC patients (Fig. 2F and 2G), but there was no significant difference.
Table 3.
Clinicopathologic characteristics of H. pylori-infection positive and negative gastric cancer patients by MSI and p53 status
| MSI-High
|
MSI-Low/MSS
|
|||||||
| H. pylori positive (n=2) | H. pylori negative (n=3) | H. pylori positive (n=21) | H. pylori negative (n=19) | |||||
|
|
|
|
|
|||||
| n | % | n | % | n | % | n | % | |
|
| ||||||||
| M/F, n (%) | 1/1 | 50/50 | 2/1 | 66.7/33.3 | 13/8 | 61.9/38.1 | 11/8 | 57.9/42.1 |
| Age (mean±SD), years | 65.0±11.3 | 64.7±8.0 | 53.6±11.5 | 53.4±12.0 | ||||
| Size (mean±SD), cm | 2.0±0.0 | 3.6±1.5 | 3.6±2.3 | 3.9±1.8 | ||||
| Location | ||||||||
| Cardia, n (%)* | 0 | 0 | 1 | 33.3 | 0 | 0 | 4 | 21.1† |
| Noncardia, n (%) | 2 | 100 | 2 | 66.7 | 21 | 100 | 15 | 78.9 |
| Lauren classification | ||||||||
| Intestinal, n (%) | 2 | 100 | 3 | 100.0 | 8 | 38.1 | 6 | 31.6 |
| Diffuse or mixed, n (%) | 0 | 0 | 0 | 0.0 | 13 | 61.9 | 13 | 68.4 |
| Depth of invasion, n (%) | ||||||||
| pT1–pT2 | 2 | 100 | 1 | 33.3 | 14 | 66.7 | 13 | 68.4 |
| pT3–pT4 | 0 | 0 | 2 | 66.7 | 7 | 33.3 | 6 | 31.6 |
| Lymph node metastasis, n (%) | ||||||||
| pN0 | 2 | 100 | 2 | 66.7 | 14 | 66.7 | 10 | 52.6 |
| pN1–pN3 | 0 | 0 | 1 | 33.3 | 7 | 33.3 | 9 | 47.4 |
|
| ||||||||
| p53 positive | p53 negative | |||||||
|
|
|
|||||||
| H. pylori positive (n=10) | H. pylori negative (n=11) | H. pylori positive (n=13) | H. pylori negative (n=12) | |||||
|
|
|
|
|
|||||
| n | % | n | % | n | % | n | % | |
|
| ||||||||
| M/F, n (%) | 7/3 | 70.0/30.0 | 7/4 | 63.6/36.4 | 7/6 | 53.8/46.2 | 7/5 | 58.3/41.7 |
| Age (mean±SD), years | 57.2±10.9 | 55.5±12.5 | 52.5±12.3 | 53.4±12.3 | ||||
| Size (mean±SD), cm | 4.2±2.5 | 3.8±2.2 | 3.0±1.9 | 4.0±1.7 | ||||
| Location | ||||||||
| Cardia, n (%)* | 0 | 0 | 4 | 36.4 | 0 | 0 | 1 | 8.3 |
| Noncardia, n (%) | 10 | 100 | 7 | 63.6 | 13 | 100 | 11 | 91.7 |
| Lauren classification | ||||||||
| Intestinal, n (%) | 6 | 60.0 | 6 | 54.5 | 4 | 30.8 | 4 | 33.3 |
| Diffuse or mixed, n (%) | 4 | 40.0 | 5 | 45.5 | 9 | 69.2 | 8 | 66.7 |
| Depth of invasion, n (%) | ||||||||
| pT1–pT2 | 8 | 80.0 | 8 | 72.7 | 8 | 61.5 | 7 | 58.3 |
| pT3–pT4 | 2 | 20.0 | 3 | 27.3 | 5 | 38.5 | 5 | 41.7 |
| Lymph node metastasis, n (%) | ||||||||
| pN0 | 7 | 70.0 | 6 | 54.5 | 9 | 69.2 | 6 | 50.0 |
| pN1-pN3 | 3 | 30.0 | 5 | 45.5 | 4 | 30.8 | 6 | 50.0 |
Located within 2 cm below the gastroesophageal junction.
P=0.042, compared to H. pylori infection-positive gastric cancer with MSI-Low/MSS.
DISCUSSION
During the past decade, the definition of HPIN-GC has been continuously changed in the literature.3–7,25 Kato et al. reported HPIN-GC prevalence of 2.0% to 10.6% using anti-H. pylori antibody and PG tests,4 but the diagnostic tests have been challenged because of the spontaneous seroconversion of antibody and the false negative rate of PG tests.26,27 In other studies using serology and PCR test, the negative rates of H. pylori infection in GC were 14% and 18.8%.5,25 However, PCR test for H. pylori is not usually used in the clinical setting. The limited number (three or less) of diagnostic methods was considered as insufficient for detecting H. pylori infection, and more methods were needed to precisely diagnose the current and previous infection.7 Matsuo et al. employed a combination of multiple methods such as serologic, endoscopic, and histologic evaluations to define H. pylori status as strictly as possible.6 Regarding natural clearance or unexpected eradication of H. pylori, the absence of histologic gastritis and atrophic change was included in the definition of HPIN-GC. However, the definition of H. pylori status was extremely strict, in which even active gastritis in the absence of H. pylori detection was considered as previous H. pylori infection. Consequently, the prevalence of HPIN-GC was estimated 0.66%, which was much lower than those of the previous studies.3–5,8 The interobserver variability of histologic and endoscopic observation of atrophic change could be also problematic.7 In our previous research, severe gastric atrophy was assessed by serum PG test and histologic evaluation of metaplastic gastric atrophy to exclude the GC patients with possible past infection, considering that H. pylori causes atrophy/intestinal metaplasia and can be eliminated after long-time colonization.7,24 The prevalence of HPIN-GC was calculated as 5.4%,7 and the combination of multiple diagnostic methods was also employed in the present study.
The clinicopathologic features of the present study were compatible with the previous studies (Table 1).3–7,25 In the literature, the higher distributions of cardia location and diffuse type in HPIN-GC were frequently reported,3,5,6,25 and female predominance was also found.3,25 In the present study, the significantly higher frequency of cardia location was oserved (Table 1). With regard to the pathologic T and N staging, the advanced T and N stages in HPIN-GC were previously reported, but the results were not consistent.3,5,28
H. pylori infection as an independent prognostic factor in GC was recently demonstrated in the two studies conducted in Western countries.3,5 Meimarakis et al. reported that H. pylori infection was an independent prognostic factor for GC in addition to depth of invasion, lymph node metastasis, and age, defining H. pylori infection status by anti-H. pylori antibody, histologic analyses, and bacterial culture.3 Thus, the author recommended careful follow-up and more aggressive treatment for HPIN-GC patients. In another study, in which H. pylori status was determined by serology and PCR for vacA, H. pylori infection, depth of invasion, lymph node metastasis, and tumor location were reported to be independent prognostic factors.5 From the results of the two studies, the clinical implication of H. pylori infection was expanded to influence GC prognosis.
Similarly, in earlier research by our group,7 the poor prognosis of HPIN-GC could be inferred from the clinicopathologic features of HPIN-GC such as advanced pT stage. However, the survival analysis of the present study revealed that there is no association between H. pylori status and GC prognosis (Fig. 2E and Table 2). The pT and pN stages were identified as the most important prognostic factors for and RFS and OS (Fig. 2A–B and Table 2). These findings are supported by the studies conducted in Taiwan and China, in which H. pylori status was not correlated with survival, although the definition of H. pylori infection was not strict in the studies.8,28 This discordance could be explained mainly by two factors. First, the two studies showing the prognostic value of H. pylori infection were conducted in Western GC patients,3,5 so the biologic and genetic aspects of H. pylori and GC could be different from those of Asian patients.29 The better prognosis of GC in Asian countries could influence the prognostic results.29–31 The good prognosis in Korean GC patients has been repeatedly reported previously.31,32 Second, in the Western studies,3,5 the definition of HPIN-GC was not strict as in Asian studies,6,7 and past infection, which can be ruled out by the presence of atrophic change, was overlooked, although IgG antibody test can detect a part of past infection. Long standing infection of H. pylori might lead to the clearance of H. pylori itself,4,7,24 but the remaining atrophy and intestinal metaplasia still have a potential of carcinogenesis.
EBV-associated GC has distinct clinicopathologic characteristics, such as male predominance, young age, cardia location, and lymphoepithelial carcinoma.33,34 In contrast, EBV-associated GC was reported to not be associated with H. pylori infection, depth of invasion, lymph node metastasis, and the clinical stage in a meta-analysis.33 Several studies demonstrated the differences in survival between EBV-positive and EBV-negative GC, but there has been still considerable controversy.33 In the present study, the frequency of EBV positivity in both HPIP-GC and HPIN-GC was approximately 5%, and there was no association between EBV and HPIN-GC (Table 1). Given the limited number of EBV-positive patients, further analysis was not performed.
The frequency of MSI in GC has been reported from 9.5 to 44%,9 and distinctive clinicopathologic features such as older age, distal location, larger size, intestinal classification, lower lymph node involvement, and improved survival have been suggested.9,12–14 The tendencies of older age and intestinal classification in MSI-H were also demonstrated in both HPIP-GC and HPIN-GC of the present study (Table 3). Regarding p53 expression in GC, the positivity rate has been reported to range from 4% to 71%, and the association with intestinal classification has been reported.11,15,19 The prognostic value of p53 on survival is not conclusive, but several studies have demonstrated a poor prognosis.15,19 In the present study, the tendency of intestinal classification is observed in HPIP-GC (60.0% vs. 30.8%) and in HPIN-GC (54.5% vs. 33.3%) (Table 3). Interestingly, among all the 5 cardia cancer patients of HPIN-GC, four patients (80%) were p53 positive whereas only on patient was MSI-H, and the all the 5 cardia patients in HPIN-GC showed advanced pT (pT3-pT4, n=3) or pN (pN1-pN3, n=4) stages. The tendency of cardia location and advanced stage in p53 positive HPIN-GC patients could be due to the features of cardia cancer (Table 3). In GC patients with cardia location, the lack of association with H. pylori and the high expression of p53 were often noted in the previous studies.35–37 With respect to prognosis, the interpretation of the clinicopathologic features according to MSI and p53 expression should be cautious, because the influence of MSI and p53 on survival was not clear in the present study (Fig. 2F, 2G).
With the strict definition of H. pylori infection, we found 4.2% (36/849) cases of gastric cancer were HPIN-GC in the present study. The prevalence of HPIN-GC is regarded as low.6,7 In other words, recruiting an adequate sample size might take too long time. Thus, the attempt testing the molecular markers in HPIN-GC, like the present study, is needed for clinicians, although the number of cases was limited. To our knowledge, this is the first trial evaluating the prognostic molecular markers in HPIN-GC. The application of the prognostic markers, MSI and p53, to the patients with NPIN-GC should be deferred until the prognostic role of MSI and p53 becomes clear in HPIN-GC.
In conclusion, it was found that when applying the strict definition of H. pylori infection, the status of H. pylori infection was not a prognostic factor for survival in GC patients. Depth of invasion and lymph node metastasis were confirmed as the most important prognostic factors. The prognostic implication of MSI and p53 on survival in HPIN-GC patients was not clear.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korea government (MSIP) (No. 2011-0030001).
REFERENCES
- 1.International Agency for Research on Cancer Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton JP, Meltzer SJ. A review of the genomics of gastric cancer. Clin Gastroenterol Hepatol. 2006;4:416–25. doi: 10.1016/j.cgh.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Meimarakis G, Winter H, Assmann I, Kopp R, Lehn N, Kist M, et al. Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol. 2006;7:211–22. doi: 10.1016/S1470-2045(06)70586-1. [DOI] [PubMed] [Google Scholar]
- 4.Kato S, Matsukura N, Tsukada K, Matsuda N, Mizoshita T, Tsukamoto T, et al. Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci. 2007;98:790–4. doi: 10.1111/j.1349-7006.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrelli D, Pedrazzani C, Berardi A, Corso G, Neri A, Garosi L, et al. Negative Helicobacter pylori status is associated with poor prognosis in patients with gastric cancer. Cancer. 2009;115:2071–80. doi: 10.1002/cncr.24253. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16:415–9. doi: 10.1111/j.1523-5378.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, et al. Helicobacter pylori-negative gastric cancer in South Korea: incidence and clinicopathologic characteristics. Helicobacter. 2011;16:382–8. doi: 10.1111/j.1523-5378.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee WJ, Lin JT, Shun CT, Lee WC, Yu SC, Lee PH, et al. Comparison between resectable gastric adenocarcinomas seropositive and seronegative for Helicobacter pylori. Br J Surg. 1995;82:802–5. doi: 10.1002/bjs.1800820627. [DOI] [PubMed] [Google Scholar]
- 9.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–10. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 10.Zafirellis K, Karameris A, Milingos N, Androulakis G. Molecular markers in gastric cancer: can p53 and bcl-2 protein expressions be used as prognostic factors? Anticancer Res. 2005;25:3629–36. [PubMed] [Google Scholar]
- 11.Deveci MS, Deveci G. Prognostic value of p53 protein and MK-1 (a tumor-associated antigen) expression in gastric carcinoma. Gastric Cancer. 2007;10:112–6. doi: 10.1007/s10120-007-0418-7. [DOI] [PubMed] [Google Scholar]
- 12.Falchetti M, Saieva C, Lupi R, Masala G, Rizzolo P, Zanna I, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–32. doi: 10.1016/j.humpath.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Corso G, Pedrazzani C, Marrelli D, Pascale V, Pinto E, Roviello F. Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch Surg. 2009;144:722–7. doi: 10.1001/archsurg.2009.42. [DOI] [PubMed] [Google Scholar]
- 14.Seo HM, Chang YS, Joo SH, Kim YW, Park YK, Hong SW, et al. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99:143–7. doi: 10.1002/jso.21220. [DOI] [PubMed] [Google Scholar]
- 15.Goncalves AR, Carneiro AJ, Martins I, de Faria PA, Ferreira MA, de Mello EL, et al. Prognostic significance of p53 protein expression in early gastric cancer. Pathol Oncol Res. 2011;17:349–55. doi: 10.1007/s12253-010-9333-z. [DOI] [PubMed] [Google Scholar]
- 16.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–53. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 17.Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249–59. doi: 10.1046/j.1365-2036.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee HS, Chang MS, Yang HK, Lee BL, Kim WH. Epstein- barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epstein-barr virus- negative carcinoma. Clin Cancer Res. 2004;10:1698–705. doi: 10.1158/1078-0432.ccr-1122-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee HK, Lee HS, Yang HK, Kim WH, Lee KU, Choe KJ, et al. Prognostic significance of Bcl-2 and p53 expression in gastric cancer. Int J Colorectal Dis. 2003;18:518–25. doi: 10.1007/s00384-003-0491-2. [DOI] [PubMed] [Google Scholar]
- 20.Oh JR, Kim DW, Lee HS, Lee HE, Lee SM, Jang JH, et al. Microsatellite instability testing in Korean patients with colorectal cancer. Fam Cancer. 2012;11:459–66. doi: 10.1007/s10689-012-9536-4. [DOI] [PubMed] [Google Scholar]
- 21.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 22.Hwang SH, Park do J, Jee YS, Kim MC, Kim HH, Lee HJ, et al. Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg. 2009;144:559–64. doi: 10.1001/archsurg.2009.110. discussion 565. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Ahn SH, Park do J, Kim HH, Lee HJ, Yang HK. Laparoscopic total gastrectomy with d2 lymphadenectomy for advanced gastric cancer. World J Surg. 2012;36:2394–9. doi: 10.1007/s00268-012-1669-y. [DOI] [PubMed] [Google Scholar]
- 24.Kang HY, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51:2310–5. doi: 10.1007/s10620-006-9276-0. [DOI] [PubMed] [Google Scholar]
- 25.Wu MS, Hung HW, Wang JT, Tseng CC, Shun CT, Wang HP, et al. Helicobacter pylori-seronegative gastric carcinoma: a subset of gastric carcinoma with distinct clinico-pathologic features. Hepatogastroenterology. 1998;45:2432–6. [PubMed] [Google Scholar]
- 26.Kokkola A, Kosunen TU, Puolakkainen P, Sipponen P, Harkonen M, Laxen F, et al. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619–24. doi: 10.1034/j.1600-0463.2003.1110604.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiyohira K, Yoshihara M, Ito M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentration as a marker of Helicobacter pylori infection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol. 2003;38:332–8. doi: 10.1007/s005350300060. [DOI] [PubMed] [Google Scholar]
- 28.Qiu HB, Zhang LY, Keshari RP, Wang GQ, Zhou ZW, Xu DZ, et al. Relationship between H. Pylori infection and clinicopathological features and prognosis of gastric cancer. BMC Cancer. 2010;10:374. doi: 10.1186/1471-2407-10-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5:177–82. doi: 10.1007/s101200200031. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Mailey B, Senthil M, Artinyan A, Sun CL, Bhatia S. Disparities in gastric cancer outcomes among Asian ethnicities in the USA. Ann Surg Oncol. 2009;16:2433–41. doi: 10.1245/s10434-009-0584-4. [DOI] [PubMed] [Google Scholar]
- 31.Park do J, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multi-center retrospective study. Surg Endosc. 2012;26:1548–53. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 32.Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592–8. doi: 10.1002/cncr.25550. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein- Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354–65. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 34.Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol Int. 2010;60:337–50. doi: 10.1111/j.1440-1827.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 35.Gonda G, Bajtai A, Nagy P, Szanto I, Kiss J. Quantitative analysis of p53 expression and cell proliferation in gastric carcinomas. An immunohistochemical study. Hepatogastroenterology. 2004;51:273–6. [PubMed] [Google Scholar]
- 36.Figueroa JD, Terry MB, Gammon MD, Vaughan TL, Risch HA, Zhang FF, et al. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control. 2009;20:361–8. doi: 10.1007/s10552-008-9250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JY, Lee HS, Kim N, Shin CM, Lee SH, Park YS, et al. Prevalence and clinicopathologic characteristics of gastric cardia cancer in South Korea. Helicobacter. 2012;17:358–68. doi: 10.1111/j.1523-5378.2012.00958.x. [DOI] [PubMed] [Google Scholar]