Abstract
Ginsenoside Rg3, one of the major ingredients of heat-processed ginseng, has been reported to inhibit the growth of various cancer cells. We previously reported that Rg3 inhibited the proliferation and induced apoptosis of breast cancer (MDA-MB-231) cells. In the present study, we have explored the mechanism underlying the anti-proliferative and proapoptotic effects of Rg3 in MDA-MB-231 cells, which have constitutively activated NF-κB and the mutant form of p53. Rg3 inhibited DNA binding and transcriptional activity of NF-κB and these effects were attributable to its suppression of IKKβ activity, degradation of IκBα and subsequent nuclear translocation of the p65 subunit of NF-κB. Similarly, the constitutive activation of ERK and Akt through phosphorylation was gradually reduced in MDA-MB-231 cells treated with Rg3. The pharmacological inhibitors of these kinases both U0126 (MEK1/2 inhibitor) and LY294002 (PI3K inhibitor) abrogated the NF-κB DNA binding activity in MDA-MB-231 cells. In addition, Rg3 treatment lowered the levels of the mutant p53 in concentration- and time-dependent manners. Rg3 also increased the association between p53 and its negative regulator Mdm2 in MDA-MB-231 cells. These findings suggest that Rg3 induced apoptosis in MDA-MB-231 cells, which is mediated by blocking NF-κB signaling via inactivation of ERK and Akt as well as destabilization of mutant p53.
Keywords: Ginsenoside Rg3, NF-κB, p53, Apoptosis
INTRODUCTION
The roots of rhizome of Panax plants have been used for the treatment of many diseases and for enhancing physical strength and immunity. Among them, Panax ginseng C.A. Meyer has a wide array of pharmacological and physiological effects.1,2 Rg3, one of ginsenosides derived from heat-processed ginseng, was found to be a potent inhibitor of tumor cell growth.3,4 Rg3 induces apoptosis through generation of reactive oxygen species (ROS) in some cancer cells.5,6 In our previous study, Rg3 induced the proteolytic cleavage of caspase-3 and poly(ADP-ribose) polymerase (PARP) in human breast cancer MDA-MD231 cells. Moreover, a ROS scavenger attenuated the proteolytic cleavage of caspase-3 and down-regulation of Bcl-2 induced by Rg3.6 In addition, it was reported that Rg3 inhibited the growth of colon and prostate cancer cells and enhanced their susceptibility to anti-cancer agents, which was associated with suppression of NF-κB activation.7,8
In many malignant tumors, constitutive NF-κB activation is observed, which is causally linked to inflammation, proliferation, resistant to apoptosis, invasion, etc.9 Therefore, inhibition of abnormally elevated NF-κB activation in precancerous or malignant cells is considered to be an important strategy for cancer chemoprevention as well as therapy.10 The mammalian NF-κB family includes p65/RelA, RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2). NF-κB proteins are characterized by the presence of a conserved 300-amino acid Rel homology domain located toward the N-terminus that is responsible for dimerization, interaction with IκBs, and binding to DNA.11 These Rel family members can exist as homo- or heterodimers, the most abundant form of intracellular NF-κB is p50/p65 heterodimer. This heterodimeric NF-κB protein remains sequestered in the cytoplasm as an inactive complex with one of its inhibitory counterpart IκB subunits, including IκBα, IκBβ, IκBɛ, IκBγ, Bcl-3 and the precursor Rel proteins p100 and p150. Most of the stimuli that activate NF-κB signaling cause phosphorylation of IκB, which triggers ubiquitination and subsequent degradation of this inhibitory subunit. Phosphorylation of IκB is catalyzed by a complex enzyme consisting of two IκB kinases (IKKs), IKKα and IKKβ, together with a modifying subunit called NEMO or IKK.12 Upon phosphorylation-mediated degradation of IκBα, active NF-κB is translocated into the nucleus, and regulates the expression of inducible genes encoding proteins involved in anti-apoptosis (e.g., IAPs, XIAP, and Bcl-2), cell proliferation (e.g., c-Myc, cyclooxygenase-2, and cyclin D1), and metastasis (e.g., MMP-9 and VEGF).11,13–15
Stabilization and overexpression of mutant p53 are hallmarks found in nearly 50% of human tumors.16 There are also myriad of clinical studies correlating high levels of mutant p53 with more aggressive behavior of tumors and poor prognosis. Mutant p53 can inhibit the function of wild-type p53, possibly through oligomerization.17 Ectopic expression of mutant p53 in p53-null cell lines can increase the oncogenic potential and drug resistance.16,18,19 Thus, while the wild-type form of p53 is a tumor suppressor, mutant forms of p53 may function as oncoproteins. In this context, mutant p53 represents one of the most important clinical targets for anti-cancer drug intervention.20 Wild-type p53 is destroyed when the proto-oncogene Mdm2 is overexpressed. Mdm2, by acting as an E3 ubiquitin ligase, down-regulates p53 through proteasome-mediated degradation.21–23
In the present study, we have investigated effects of Rg3 on NF-κB and p53 signaling pathways in MDA-MB-231 cells.
MATERIALS AND METHODS
1. Materials
Rg3 was prepared and purified as described previously.24 Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY, USA). Bicinchoninic acid (BCA) protein assay reagent was a product of Pierce Biotechnology (Rockfold, IL, USA). Polyvinylidene difluoride membranes were supplied from Gelman Laboratory (Ann Arbor, MI, USA). Antibodies against extracellular signal-regulated kinase (ERK)1/2, phospho-EKR1/2, p65, p53 and α- tubulin were products of SantaCruz Biotechnologies Co. (Victoria, BC, Canada). Antibodies against Akt, phospho-Akt, IκBα, and lamin B were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody of MDM2 was a product of Calbiochem (Darmstadt, Germany). NF-κB consensus oligonucleotide was purchased from Promega (Madison, WI, USA).
2. Cell culture
MDA-MB-231 cells were grown at 37°C in DMEM supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere of 5% CO2 and 95% air. Cells were plated at an appropriate density according to the scale of each experiment, and the medium was changed at 2-day intervals.
3. Western blot analysis
MDA-MB-231 cells were lysed in RIPA lysis buffer [150 mM NaCl, 0.5% Triton X100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 20 mM EDTA, 1 mM dithiothreitol (DTT), 1 mM Na3VO4, and protease inhibitor] for 30 min at 0°C followed by centrifugation at 12,000×g for 15 min. The protein concentration of the supernatant was measured by using the BCA reagent (Pierce, Rockfold, IL, USA). Protein (40 μg) was separated by running through 10% SDS-PAGE gel and transferred to the PVDF membrane (Gelman Laboratory, Ann Arbor, MI, USA). The blots were blocked with 5% non-fat dry milk-PBST buffer [PBS containing 0.1% Tween-20] for 1 h at room temperature. The membranes were incubated for 2 h at room temperature with 1:1000 dilution of the antibodies. Equal lane loading was ensured by using actin (Sigma Chemical Co., St, Louis, MO, USA). The blots were rinsed three times with PBST buffer for 10 min each. Washed blots were treated with 1:5000 dilution of the horseradish peroxidase conjugated-secondary antibody for 1 h and washed again three times with PBST buffer. The transferred proteins were visualized with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK).
4. Immunoprecipitation
Cell lysates (300 μg) were incubated with 2 μg of anti-p53 overnight at 4°C and Protein A-agarose beads (Amersham) for 1 h at 4°C. Lysates-bead complexes were washed three times with lysis buffer and resolved by 10% SDS-polyacrylamide gel electrophoresis. Association of mutant p53 with Mdm2 was detected by incubating the blots with anti-Mdm2 antibody.
5. Electrophoretic mobility shift assay (EMSA)
Confluent cells in 100 mm dishes were treated with Rg3. Cells were gently washed twice with ice-cold PBS, scraped in 1 ml of PBS, and centrifuged at 5,000×g for 8 min at 4°C. Pellets were suspended in 100 μl of hypotonic buffer (10 mM HEPES, pH 7.9; 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA and 0.1 mM PMSF) for 15 min on ice, and 1 μl of 10% Nonidet P-40 solution was added for 5 min. The mixture was then centrifuged at 14,000×g for 15 min. The supernatant (i.e., the cytosolic fraction) was kept for Western blot analysis. The nuclei fraction were suspended in 80 μl of buffer (50 mM HEPES, pH 7.9; 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 10% glycerol) for 30 min on ice and centrifuged at 12,000×g for 15 min. The supernatant containing nuclear proteins was collected and stored at −70°C after determination of the protein concentration. EMSA was performed using a DNA-protein-binding detection kit (Gibco BRL) according to the manufacturer’s protocol. Briefly, the NF-κB oligo-nucleotide probe (5’-AGT TGA GGG GAC TTT CCC AGG C-3’) was labeled with [γ-32P]ATP by T4 polynucleotide kinase and purified on a Nick column. The binding reaction was performed in 25 μl of the mixture containing 5 μl of incubation buffer (10 mM Tris-HCl, pH 7.5; 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 4% (v/v) glycerol, and 0.1 mg/ml sonicated salmon sperm DNA), 10 μg of nuclear extracts, and the labeled probe at 100,000 cpm. After 30-min incubation at room temperature, 2 μl of 0.1% bromophenol blue was added, and samples were electrophoresed on a 6% nondenaturing polyacrylamide gel at 150 V for 1.5 h. Finally, the gel was dried and exposed to an X-ray film.
6. Luciferase assay
A day before transfection, cells were subcultured at a density of 1×105/ml in a 6-well plate to maintain 50% confluency. Cells were transiently transfected with NF-κB- luciferase vector using Fugene 6 reagents according to the instructions supplied by the manufacturer (Roche Molecular Biochemicals, Mannheim, Germany). After 48 h, the medium was exchanged, and cells were treated with Rg3. Cells were then washed twice with PBS, lysed with reporter lysis buffer (Promega) and centrifuged at 12,000×g for 1 min at 4°C. The supernatant was stored at −70°C for the luciferase assay. Cell lysates (20 μl) were mixed with 20 μl of the luciferase assay reagent at room temperature, and the luciferase reporter activity was measured by a luminometer (AutoLumat LB953, EG and G Berthold. Bad Widbad, Germany).
7. IKK kinase assay
Whole cell lysates was immunoprecipitated with an antibody against IKKβ, followed by treatment with protein A-Sepharose bead (Zymed Laboratories Inc.). After incubation overnight at 4°C, the beads were washed with lysis buffer and kinase assay buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2, 2 mM MnCl2, 2 mM DTT, 50 mM β- glycerophosphate, 10 mM p-nitrophenyl phosphate, and 0.1 mM sodium orthovandate). Kinase reactions were initiated by the addition of 30 μl of kinase assay buffer containing 2 μCi of substrate full-length IκBα (amino acids 1–317). After incubation at 30°C for 30 min, the reaction was terminated by boiling with SDS-PAGE loading buffer for 5 min. Finally, the protein was resolved on 10% SDS-PAGE, the gel was stained and dried, and the phosphorylated form of IκBα was visualized by exposure to an X-ray film.
RESULTS
1. Effect of Rg3 on NF-κB DNA binding activity
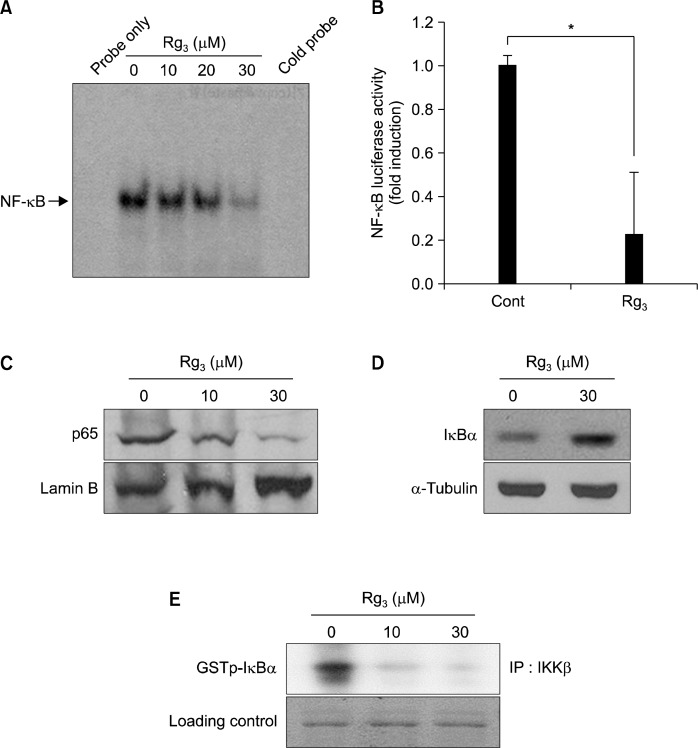
NF-κB is a transcription factor that regulates the expression of anti-apoptotic proteins. To investigate the effects of Rg3 on the activation of this transcription factor, the nuclear extract from MDA-MB-231 cells treated with Rg3 was analyzed by EMSA. Rg3 inhibited the DNA binding of NF-κB contained in the nuclear extract obtained from MDA-MB-231 cells (Fig. 1A). Rg3 also reduced the transcriptional activity of NF-κB as determined by the luciferase reporter gene assay in the same cells (Fig. 1B). In addition, Rg3 treatment inhibited the nuclear translocation of the p65 subunit of NF-κB (Fig. 1C). IKKβ is a protein kinase responsible for phosphorylation of IκBα which sequesters NF-κB in the cytoplasm.12 Phosphorylated IκBα is rapidly polyubiquitinated and degraded by proteasomes, thereby liberating NF-κB for translocation into the nucleus and subsequent modulation of target gene expression. We also observed that Rg3 inhibited the degradation of IκBα (Fig. 1D) and the catalytic activity of IKKβ (Fig. 1E) as determined by Western blot analysis and the kinase assay, respectively.
Fig. 1.
Effects of Rg3 on NF-κB DNA-binding and IKK activity in MDA-MB-231 cells. (A) The NF-κ B DNA-binding activity was assessed by EMSA after treatment of MDA-MB-231 cells with Rg3 (10, 20, and 30 μM) for 24 h. (B) The NF-κB transcriptional activity was measured by the luciferase assay at 24 h following Rg3 (30 μM) treatment. MDA-MB-231 cells were transiently transfected with the plasmid containing the NF-κB- driven luciferase gene construct. The results are presented as means±S.D. (n=3). *P<0.05 (C, D) MDA-MB-231 cells treated with Rg3 (30 μM) for 24 h, and then the cytosolic and nuclear extracts were prepared. The accumulation of p65 in nucleus and Iκ B in the cytosolic fraction was measured by Western blot analysis. (E) MDA-MB-231 cells treated with Rg3 (30 μM) for 24 h. The Whole cell lysates were immu-noprecipitated with an antibody against IKKβ, and subjected to the IKKβ kinase assay.
2. Effects of Rg3 on activation of ERK and Akt
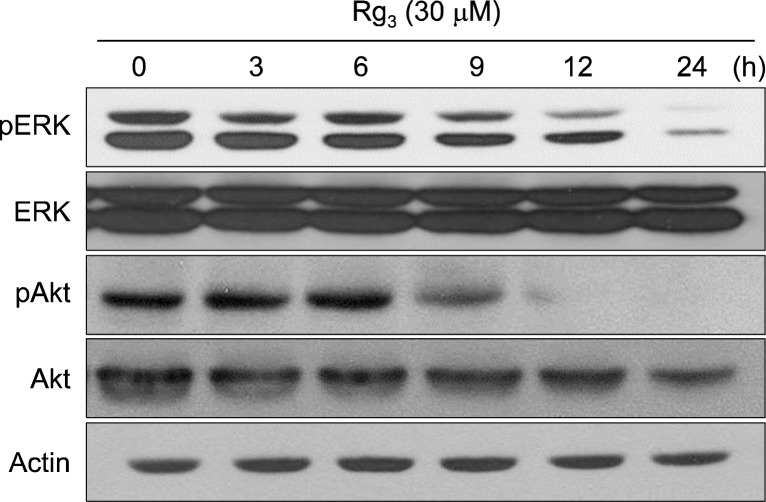
To further elucidate the molecular mechanism underlying suppression of NF-κB signaling by Rg3, we examined its effects on the activation of ERK and Akt, two major kinases involved in cell survival, in MDA-MD-231 cells after Rg3 treatment for 24 h. As shown in Fig. 2, Rg3 significantly inhibited the constitutive activation of ERK through phosphorylation, whereas the level of total-ERK remained unchanged. Suppression of Akt phosphorylation is closely linked to the cell death in response to a broad spectrum of apoptotic stimuli. Akt/PKB, a downstream target of phosphatidylinositol 3-kinase (PI3K), is activated through phosphorylation at serine 473 and threonine 308 residues. As illustrated in Fig. 2, there was a significant decline in the levels of phosphorylated Akt 12 h after the Rg3 treatment in MDA-MB-231 cells.
Fig. 2.
Rg3 inhibited Phosphorylation of ERK and Akt MDA-MB-231 cells were treated with Rg3 (30 μM) at various time intervals, and the phosphorylated as well as the basal level of Akt and ERK were examined by Western blot analysis.
3. Effect of Rg3 on the stability of mutant p53
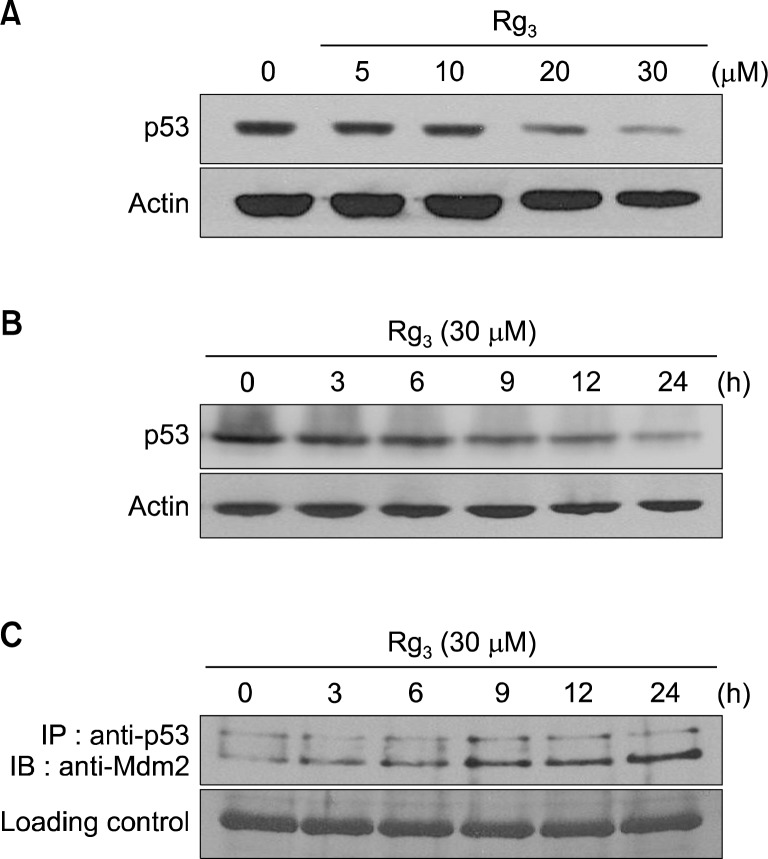
In normal cells, p53 is present at very low levels due to rapid degradation mediated by Mdm2. Mdm2 binds to p53 and promotes its ubiquitination by acting as an ubiquitin E3 ligase.21–23 p53 tumor suppressor gene is mutated in nearly 50% of human cancers.16 In general, tumor cells with mutant p53 accumulate p53 at relatively high levels. To determine whether Rg3 treatment could down-regulate expression of mutant p53, a level of mutant p53 was determined by Western blot analysis in MDA-MB-231 cells treated with Rg3. As shown in Fig. 3A and B, Rg3 treatment suppressed the expression of mutant p53 in concentration- and time-dependent manners. Moreover, Rg3 treatment increased the interaction between p53 and its negative regulator Mdm2 (Fig. 3C) as determined by the immunoprecipitation assay. These findings suggest that Rg3 induces destabilization of mutant p53 through enhancement of Mdm2 binding to mutant p53 protein.
Fig. 3.
Effects of Rg3 on expression level of mutant p53 and its association with Mdm2 in MDA-MB-231 cells. (A, B) MDA-MB-231 cells were treated with Rg3, and the level of mutant p53 was assessed by Western blot analysis. (C) Rg3 increased the interaction between mutant p53 and Mdm2. MDA-MB-231 cells were harvested following treatment with Rg3 (30 μM). p53 was then immunoprecipitated with p53 antibody and analyzed by Western blot analysis with Mdm2 antibody.
DISCUSSION
In our previous study, we have demonstrated that Rg3 induces the apoptosis in MDA-MB-231 cells, which is mediated through down-regulation of Bcl-2 and generation of ROS.6 Activation of NF-κB has been observed in various malignant tumors. The NF-κB pathway confers resistance to apoptosis in cancer cells through induction of Bcl-2 expression.25 Therefore, we speculated that Rg3 could inhibit NF-κB signaling. Rg3 treatment did suppress DNA-binding and transcriptional activities of NF-κB in MDA-MB 231 cells. Suppression of NF-κB activation by Rg3 was associated with suppression of IKKβ activity and subsequently IκBα degradation and nuclear translocation of p65. Consistent with our data, Kim and Hong have reported that Rg3 inhibits the DNA binding ability as well as transcriptional activity of NF-κB in colon and prostate cancer cells, which rendered these cells susceptible to docetaxel.7,8 In our previous study, Rg3 pretreatment suppressed the NF-κB activation and expression of its major target protein cyclooxygenase-2 in mouse skin stimulated with the tumor promoter phorbol ester.26
NF-κB activation has been known to be regulated by protein kinases such as Akt and ERK.27,28 Numerous intra-cellular signal transduction pathways converge with the activation of the MAPK family proteins, which act independently or coordinately to regulate expression of genes involved in cell proliferation and differentiation.29 Akt and ERK are important components of intracellular signaling cascades mediating cell survival. The ERK signaling cascade starts with the phosphorylation and concurrent activation of MEK by Raf, which is followed by phosphorylation and activation of ERK.30 We observed that Rg3 inhibited the phosphorylation of ERK and Akt. These findings suggest that the inhibitory effect of Rg3 on constitutive NF-κB activation are likely to be attributable to its suppression of ERK and Akt activation as upstream targets in MDA-MB231 cells.
ROS is known to play a crucial role in cell apoptosis. We observed that Rg3-induced apoptosis in MDA-MB-231 cells is associated with generation of ROS as the prototypic antioxidant N-acetyl cysteine attenuated the down-regulation of Bcl-2 expression as well as proteolytic cleavage of caspase-3.6 Some studies have reported that ROS can induce cytotoxicity through suppression of NF-κB activity and NF-κB dependent gene expression.31,32 IKK suppression results in increased ROS production and secondary loss of mitochondrial transmembrane potential (ΔΨm)33. Moreover, inhibitors of NF-κB have been shown to induce apoptosis through generation of ROS. For instance, andrographolide, an NF-κB inhibitor derived from the leaves of Andrographis paniculata, induces apoptosis through generation of ROS.34,35 Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is an in-tracellular ROS producer. NOX is a complex composed of membrane-bound (p22phox and NOX 1-4) and cytoplasmic (Rac, p47phox, and p67phox) subunits. When it is activated, cytoplasmic subunits interact with their membrane-bound counterparts and generate an active complex that oxidizes NADPH, leading to the production of ROS.36 Activation of the NOX subunit p47phox is required for ROS production in vascular cells. Andrographolide induced phosphorylation of p47phox.34
One of the molecules that negatively regulates Bcl-2 is the p53 tumor suppressor protein. The wild type p53 molecule is often upregulated during apoptosis induced by some chemotherapeutic and chemopreventive agents.37,38 In contrast, mutation in the p53 gene and alteration of its pathways are associated with augmented invasiveness, metastasis, and recurrence of cancer.39 It is increasingly evident that overexpression of mutant p53 in cultured cells interferes with apoptosis, enhances proliferation, and increases resistance to chemotherapy.18,40,41 Mutant p53 prolongs TNF-α-induced NF-κB activation in cultured cells and intestinal organoid cultures.42 Mice harboring a germline p53 mutation develop severe chronic inflammation and persistent tissue damage upon treatment with dextran sulfate sodium, and are highly prone to inflammation-associated colon cancer.42 We observed that Rg3 decreased the expression of mutant p53 time dependently in MDA-MB-231 cells, which may account for its inhibitory effect on the growth of these cells harboring a p53 mutant R280K. In contrast to our observations, Yuan et al. have reported that Rg3 induces apoptosis through up-regulation of p53 in HT-29 colon cancer cells harboring a mutant form of p53 (R273H).3 However, knockdown of the R273H p53 mutant form by small interfering RNA in A431 cells increased the level of procaspase-3 and sensitized the cells to drug-induced apoptosis.43 Moreover, histone deacetylase (HDAC) inhibitors suppress transcription of both wild-type and mutant p53, which reduces the levels of p53 proteins.44 Targeted disruption of HDAC8 expression significantly triggers anti-proliferative effects in cells harboring with mutant p53, which is accompanied by decreased p53 expression.44
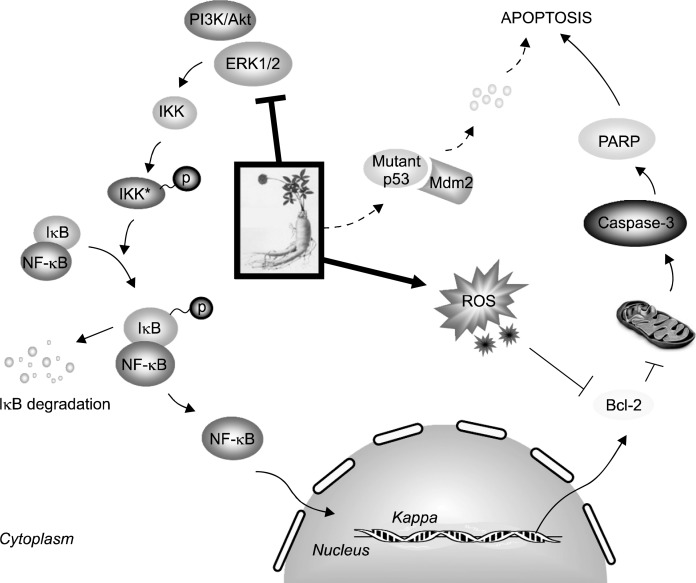
In conclusion, Rg3 inhibited NF-κB signaling via possibly inactivation of ERK and Akt as well as destabilization of mutant p53, which may contribute to suppression of Bcl-2 expression and subsequently induction of apoptosis in MDA-MB-231 cells (Fig. 4).
Fig. 4.
A proposed molecular mechanisms underlying suppression of NF-κB signaling and induction of apoptosis by Rg3 in MDA- MB-231 cells.
Acknowledgments
This work was supported by grants from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2009-0077736).
REFERENCES
- 1.Surh YJ, Na HK, Lee JY, Keum YS.Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer J Korean Med Sci 200116Suppl:S38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun TK. Panax ginseng--a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 3.Yuan HD, Quan HY, Zhang Y, Kim SH, Chung SH. 20(S)- Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep. 2010;3:825–31. doi: 10.3892/mmr.2010.328. [DOI] [PubMed] [Google Scholar]
- 4.Min JK, Kim JH, Cho YL, Maeng YS, Lee SJ, Pyun BJ, et al. 20(S)-Ginsenoside Rg3 prevents endothelial cell apoptosis via inhibition of a mitochondrial caspase pathway. Biochem Biophys Res Commun. 2006;349:987–94. doi: 10.1016/j.bbrc.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 5.Choi YJ, Lee HJ, Kang DW, Han IH, Choi BK, Cho WH. Ginsenoside Rg3 induces apoptosis in the U87MG human glioblastoma cell line through the MEK signaling pathway and reactive oxygen species. Oncol Rep. 2013;30:1362–70. doi: 10.3892/or.2013.2555. [DOI] [PubMed] [Google Scholar]
- 6.Kim BM, Kim DH, Park JH, Na HK, Surh YJ. Ginsenoside Rg3 induces apoptosis of human breast cancer (MDA-MB- 231) cells. J Cancer Prev. 2013;18:177–85. doi: 10.15430/JCP.2013.18.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SM, Lee SY, Cho JS, Son SM, Choi SS, Yun YP, et al. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-kB. Eur J Pharmacol. 2010;631:1–9. doi: 10.1016/j.ejphar.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS, Kim Y, et al. Inhibition of NF-kB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res. 2009;32:755–65. doi: 10.1007/s12272-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 9.Shishodia S, Aggarwal BB. Nuclear factor-kB activation: a question of life or death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 10.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkB p65 protein expression. Oncogene. 1997;15:1987–94. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MS, Ghosh S. Signaling to NF-kB. Genes & development. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 12.Karin M, Delhase M. The Iκ B kinase (IKK) and NF-kB: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 13.Chen LF, Greene WC. Shaping the nuclear action of NF-kB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 14.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kB and IkB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Gaynor RB. IkB kinases: key regulators of the NF-kB pathway. Trends Biochem Sci. 2004;29:72–9. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Peng Y, Chen L, Li C, Lu W, Chen J. Inhibition of MDM2 by hsp90 contributes to mutant p53 stabilization. J Biol Chem. 2001;276:40583–90. doi: 10.1074/jbc.M102817200. [DOI] [PubMed] [Google Scholar]
- 17.Kern SE, Pietenpol JA, Thiagalingam S, Seymour A, Kinzler KW, Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992;256:827–30. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- 18.Blandino G, Levine AJ, Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene. 1999;18:477–85. doi: 10.1038/sj.onc.1202314. [DOI] [PubMed] [Google Scholar]
- 19.Dittmer D, Pati S, Zambetti G, Chu S, Teresky AK, Moore M, et al. Gain of function mutations in p53. Nat Genet. 1993;4:42–6. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 20.Brachova P, Thiel KW, Leslie KK. The consequence of oncomorphic TP53 mutations in ovarian cancer. Int J Mol Sci. 2013;14:19257–75. doi: 10.3390/ijms140919257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 22.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–7. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 23.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 24.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, et al. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–4. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 25.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kB and its significance in prostate cancer. Oncogene. 2001;20:7342–51. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 26.Keum YS, Han SS, Chun KS, Park KK, Park JH, Lee SK, et al. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kB activation and tumor promotion. Mutat Res. 2003:523–524. 75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 27.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kB by the Akt/PKB kinase. Curr Biol. 1999;9:601–4. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 28.Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–63. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 29.Hu R, Kong AN. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutrition. 2004;20:83–8. doi: 10.1016/j.nut.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 31.Ginis I, Hallenbeck JM, Liu J, Spatz M, Jaiswal R, Shohami E. Tumor necrosis factor and reactive oxygen species cooperative cytotoxicity is mediated via inhibition of NF-kB. Mol Med. 2000;6:1028–41. [PMC free article] [PubMed] [Google Scholar]
- 32.Moon DO, Kim MO, Kang SH, Choi YH, Kim GY. Sulforaphane suppresses TNF-a-mediated activation of NF-kB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009;274:132–42. doi: 10.1016/j.canlet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Tilstra JS, Gaddy DF, Zhao J, Dave SH, Niedernhofer LJ, Plevy SE, et al. Pharmacologic IKK/NF-kB inhibition causes antigen presenting cells to undergo TNFa dependent ROS- mediated programmed cell death. Sci Rep. 2014;4:3631. doi: 10.1038/srep03631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YY, Hsu MJ, Sheu JR, Lee LW, Hsieh CY. Andrographolide, a Novel NF-kB Inhibitor, Induces Vascular Smooth Muscle Cell Apoptosis via a Ceramide-p47phox- ROS Signaling Cascade. Evid Based Complement Alternat Med. 2013;2013:821813. doi: 10.1155/2013/821813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Evens AM, Prachand S, Singh AT, Bhalla S, David K, et al. Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin Cancer Res. 2010;16:4755–68. doi: 10.1158/1078-0432.CCR-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–7. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 37.He H, Zang LH, Feng YS, Chen LX, Kang N, Tashiro S, et al. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-kB survival pathway in A375-S2 cells. J Ethnopharmacol. 2013;148:544–55. doi: 10.1016/j.jep.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 38.Singh M, Bhui K, Singh R, Shukla Y. Tea polyphenols enhance cisplatin chemosensitivity in cervical cancer cells via induction of apoptosis. Life Sci. 2013;93:7–16. doi: 10.1016/j.lfs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–64. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 40.Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402. doi: 10.1016/s1535-6108(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 41.Li R, Sutphin PD, Schwartz D, Matas D, Almog N, Wolkowicz R, et al. Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene. 1998;16:3269–77. doi: 10.1038/sj.onc.1201867. [DOI] [PubMed] [Google Scholar]
- 42.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, et al. Mutant p53 prolongs NF-kB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–46. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong RP, Tsang WP, Chau PY, Co NN, Tsang TY, Kwok TT. p53-R273H gains new function in induction of drug resistance through down-regulation of procaspase-3. Mol Cancer Ther. 2007;6:1054–61. doi: 10.1158/1535-7163.MCT-06-0336. [DOI] [PubMed] [Google Scholar]
- 44.Yan W, Liu S, Xu E, Zhang J, Zhang Y, Chen X, et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene. 2013;32:599–609. doi: 10.1038/onc.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]