Abstract
Background:
The extract of Allium cepa Linn is commonly used as adjuvant food for cancer therapy. We assumed that it includes a potential source of anti-cancer properties.
Methods:
We investigated anti-cancer effects of polyphenols extracted from lyophilized A. cepa Linn (PEAL) in AGS human cancer cells.
Results:
PEAL inhibited cell growth in a dose-dependent manner. It was related to caspase-dependent apoptosis. We confirmed this finding with annexin V staining. PEAL up-regulated p53 expression, and subsequent Bax induction, down regulated Bcl-2 protein, anti-apoptotic protein. In addition, PEAL suppressed Akt activity and PEAL-induced apoptosis were significantly accentuated with Akt inhibitor (LY294002).
Conclusions:
Our data suggested that PEAL induce caspase-dependent apoptosis through mitochondrial pathway by up-regulating p53 protein, and subsequent Bax protein as well as by modulating Bcl-2 protein, and that PEAL induces caspase-dependent apoptosis at least in part through the inhibition of phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. This study provides evidence that PEAL might be useful for the treatment of cancer.
Keywords: Allium cepa, polyphenols, Apoptosis, Bcl-2, Akt
INTRODUCTION
Allium cepa Linn (onion, A. cepa), a member of the family Liliaceae, has been used as a supplementary folk remedy for cancer treatment. A. cepa has plentiful flavonoids and organosulfur compounds,1 which have exhibited anti- cancer properties. A French epidemiological study showed that high intake of A. cepa was correlated with lower risk of cancer.2 A. cepa extract has been demonstrated to have inhibitory effects on carcinogenesis. In addition, A. cepa and its constituents have anti-cancer potential.3 However, the molecular mechanisms of the anti-cancer effects of polyphenols extracted from lyophilized A. cepa Linn (PEAL) are poorly understood in human cancer cells.
As advances in medical science, our lifespan has been extended. The elderly cancer patients and their cancer- related mortality are expected to increase because the elderly have a high risk for cancer development. Therefore, it is essential to find out less toxic agents in controlling cancer for this population. To meet the demands for finding out less toxic agents, dietary agents has arisen as one of the candidates for the possibility of controlling cancer with minimal toxicity because these dietary agents are known to have or enhance anti-inflammatory and/or anti-cancer activity without showing any toxicities.4 Moreover, many studies suggested that the most dominant mechanism of anti-cancer effects of the dietary agents be apoptosis triggered by modulating numerous molecular targets. Apoptosis is a type I programmed cell death requiring active-energy consuming process. It features cytoplasmic shrinkage, blebbing of the plasma membrane, chromatin condensation, and DNA degradation.5 Apoptosis usually occurs through intrinsic pathway, which is mediated by mitochondria.
In this study, we investigated the mechanisms of anti-cancer effects of PEAL on human AGS cells.
MATERIALS AND METHODS
1. Preparation of PEAL
PEAL isolated from the plants of A. cepa Linn which we bought from the market and 100 mg/mL concentration stock solution was made by dissolving the PEAL in distilled water. For the isolation of PEAL, the lyophilized plant material (100 g) of flowers was ground into powder and extracted in aqueous 70% methanol (500 mL) at 50°C for 12 h. The extract was filtered through a Buchner funnel and concentrated to 100 mL at 50°C with evaporator. The concentrated solution was washed with n-hexane (100 mL ×3), extracted with ethyl acetate (100 mL×3), dried, and kept at 4°C with light shield. PEAL contains 8 flavonoids; (1): quercetin 3,7,4’-triglucoside; (2): quercetin 7,4’- diglucoside (3): quercetin 3,4’-diglucoside (4): Isorhamnetin 3,4’-diglucoside (5): quercetin 3-glucoside (6): quercetin 4’-glucoside (7): isorhamnetin 4’-galactoside (8): isorhamnetin 4’-glucoside
2. Cells and reagents
Human AGS cells from the American type culture collection (Rockville, MD, USA) were cultured in RPMI 1640 medium (Invitrogen Corp, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (GIBCO BRL, Grand Island, NY, USA), 1 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. Antibodies against Bcl-2, Bid, Bcl-xL, phospho p53, p53, BAX, c-IAP-2, X-linked IAP (XIAP), Akt, phospho Akt, ERK, phospho ERK, and procaspase 3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against poly (ADP-ribose) polymerase (PARP) was purchased from PharMingen (San Diego, CA, USA). Antibody against β-actin was from Sigma (Beverly, MA, USA). Peroxidase-labeled donkey anti-rabbit and sheep anti- mouse immunoglobulin, and an enhanced chemiluminescence (ECL) kit were purchased from Amersham (Arlington Heights, IL). All other chemicals not specifically cited here were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
3. Cell viability
For the cell viability assay, the cells were seeded onto 24-well plates at a concentration of 1×105 cells/ml, and then treated with the indicated concentration of PEAL for 24 h. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetra-zolium bromide (MTT, 0.5 mg/ml) was subsequently added to each well. After 3 h of additional incubation, 100 μL of a solution containing 10% SDS (pH 4.8) plus 0.01 N HCl was added to dissolve the crystals. The absorption values at 570 nm were determined with an enzyme-linked immunosorbent assay (ELISA) plate reader.
4. Flow cytometry assay analysis for the sub-G1 phase
The cells were plated at a concentration of 2×105 cells/well in six-well plates. Reduced (sub-G1) DNA content was measured by propidium iodide (PI) staining. The DNA content in each cell nucleus was determined with a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA).6
5. Nuclear staining
The cells were harvested after treatment with the indicated concentration of PEAL, washed with phosphate- buffered saline (PBS) and fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature. Fixed cells were washed with PBS and stained with 2.5 μg/ml of 4,6-diamidino-2-phenylindole (DAPI) solution for 10 min at room temperature. The cells were washed two times with PBS, and analyzed by a fluorescent microscope.
6. Detection of apoptosis by annexin-V fluorescein isothiocyanate (FITC) staining
The cells were washed with PBS and re-suspended in an annexin-V binding buffer containing 10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2. Aliquots of the cells were incubated with annexin-V FITC, mixed, and incubated for15 min at room temperature in the dark. Propidium Iodide (PI) at a concentration of 5 mg/ml was added to distinguish the necrotic cells. The apoptotic cells (Annexin V+/PI−) were measured with a flow cytometer.
7. Western blot analysis
The cells were harvested and lysed with lysis buffer (20 mM sucrose, 1 mM EDTA, 20 μM Tris-Cl, pH 7.2, 1 mM DTT, 10 mM KCl, 1.5 mM mgCl2, 5 μg/mL pepstatin A, 10 μg/mL leupeptin, and 2 μg/mL aprotinin) to prepare total protein. Protein concentrations were determined using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA). For Western blot analysis, an equal amount of protein was subjected to electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA) by electroblotting. Blots were probed with the indicated antibodies. The membranes were then incubated with diluted enzyme-linked secondary antibodies for 1 h at room temperature. After washing, the membranes were developed by enhanced chemiluminescence.
8. Measurement of mitochondrial membrane potential (MMP, Δ Ψ m)
The MMP (ΔΨ m) in living cells was measured by flow cytometry with the lipophilic cationic probe JC-1, which is a ratiometric, dual-emission fluorescent dye. There are two excitation wavelengths, 527 nm for the monomer form and 590 nm for the J-aggregate form. Quantitation of green fluorescent signals reflects the amount of damaged mitochondria. The cells were trypsinized and the cell pellets were re-suspended in 500 μl of PBS, incubated with 10 μM JC-1 for 20 min at 37°C. The cells were subsequently washed once with cold PBS, suspended in a total volume of 500 μl and analyzed using flow cytometry.
9. Statistical analysis
Data represent means±standard deviations. Statistical significance was determined using the one-way analysis of variance (ANOVA) with post-test Neuman-Keuls for more than two groups and Studen’s t test for two groups. P< 0.05 was accepted as statistically significant.
RESULTS
1. PEAL induces apoptosis in human AGS cancer cells
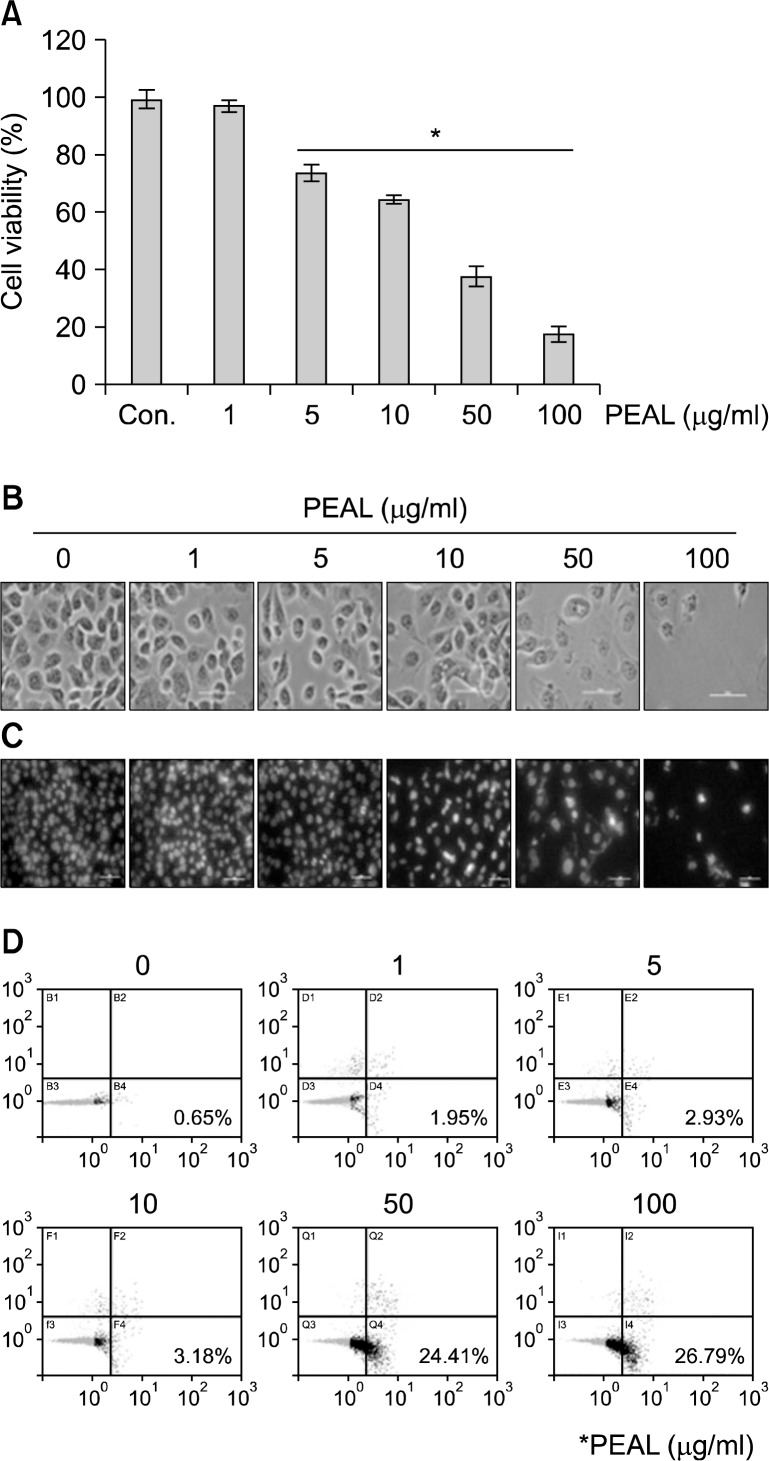
To investigate the anti-cancer activity of PEAL, AGS cells were treated with various concentrations of PEAL for 48 h. The cell growth was assessed by MTT assay. The MTT assay revealed that the growth of AGS cells were inhibited by PEAL treatment in a dose-dependent manner, and the 50% inhibition of cell growth (IC50) was less than 50 μg/mL (Fig. 1A). To determine whether the cause of the decrease in cell viability was apoptotic cell death, we assessed the changes in cellular and nuclear morphology of PEAL- treated cells under microscopy with or without DAPI staining. The light microscope finding showed that the cells with shrinking cells and cytoplasmic blebs were observed at a concentration of 50 μg/mL (Fig. 1B). As shown in Fig. 1C, PEAL-induced nuclear condensation and fragmentation increased in a dose-dependent manner. Finally we also measured the early apoptotic cells (Annexin V+/PI−) by flow cytometry. The early apoptotic cells were increased in a dose-dependent manner (Fig. 1D).
Fig. 1.
Effects of PEAL on the cell viability of AGS cells. The cells were seeded at the density of 1×105 cells per ml and incubated with the indicated concentrations of PEAL for 24 h at the indicated concentrations of PEAL. (A) Cell viability was assessed by the MTT assay. Data are expressed as mean±SD of three independent experiments (*P<0.05 versus control). (B) Cell morphology was assessed under the light microscopy (magnification ×200). (C) Effects of PEAL on the morphology of the nuclei of AGS cells. The nuclei stained with DAPI solution were observed under fluorescent microscope using a blue filter (magnification ×200). (D) The apoptotic cells (Annexin V+/PI−) were analyzed by a flow cytometer.
2. PEAL-induced apoptosis is caspase-dependent in human AGS cancer cells
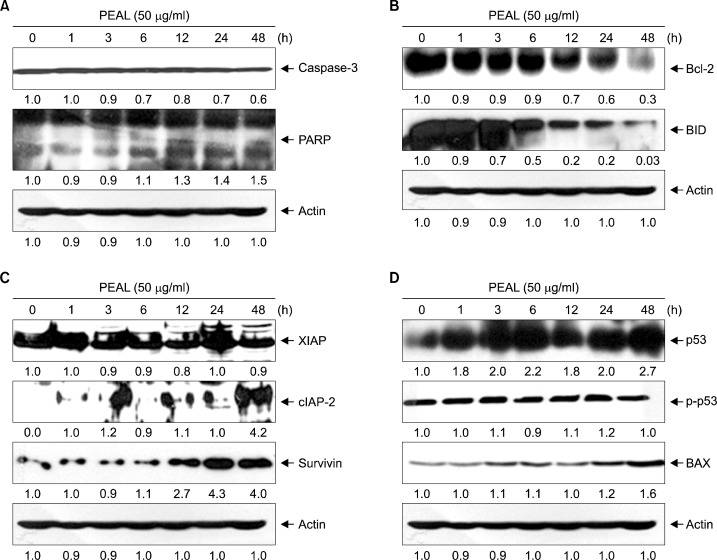
We then assessed the effects of PEAL at a concentration of 50 μg/mL on caspases and their substrates (PARP). PEAL decreased the expression levels of procaspase-3 in a time- dependent manner. With the decrease of procaspases, the cleavages of PARP were increased in a time-dependent manner (Fig. 2A). This finding suggests that PEAL induce caspase-dependent apoptosis.
Fig. 2.
PEAL-induced apoptosis in AGS cells. AGS cells were incubated at indicated concentrations of PEAL for 48 h. (A–D) Equal amounts of cell lysate (30 μg) were resolved by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. The proteins were visualized using an ECL detection system. β-actin was used as an internal control. The expression of the indicated proteins were measured by densitometry and expressed as relative ratio. The results were representatives of two independent experiments.
3. PEAL down-regulates Bcl-2 in human AGS cancer cells
To elucidate further underlying mechanisms of PEAL- induced apoptosis, we investigated the levels of Bcl-2 and inhibitor of apoptosis (IAP) family members, which play a crucial role in apoptosis and conferring cancer cell drug resistance.7 Western blotting revealed that PEAL induced cleavage of Bid (activation of Bid protein) in a time- dependent manner and significantly suppressed the expression of Bcl-2 (anti-apoptotic proteins) (Fig. 2B) whereas the expression of c-IAP-2, X-IAP, and survivin was not suppressed (Fig. 2C). These findings suggest that activation of Bid protein, and down-regulation of Bcl-2 are associated with PEAL-induced apoptosis in AGS cells.
4. PEAL induces apoptosis through up-regulation of p53 as well as Bax induction
p53 is a well-known tumor suppressor protein which regulates the cell cycle and, plays its role in conserving stability by preventing genome mutation. In cancer cells, p53 also plays its role in initiating apoptosis. Hence, we investigated the effects of PEAL on p53 and a down-stream molecule Bax protein. Western blotting revealed that PEAL induced the expression of p53 and a down-stream molecule Bax protein in a time-dependent manner (Fig. 2D). These findings suggest that activation of p53 protein might be the important mechanisms of PEAL-induced apoptosis in AGS cells.
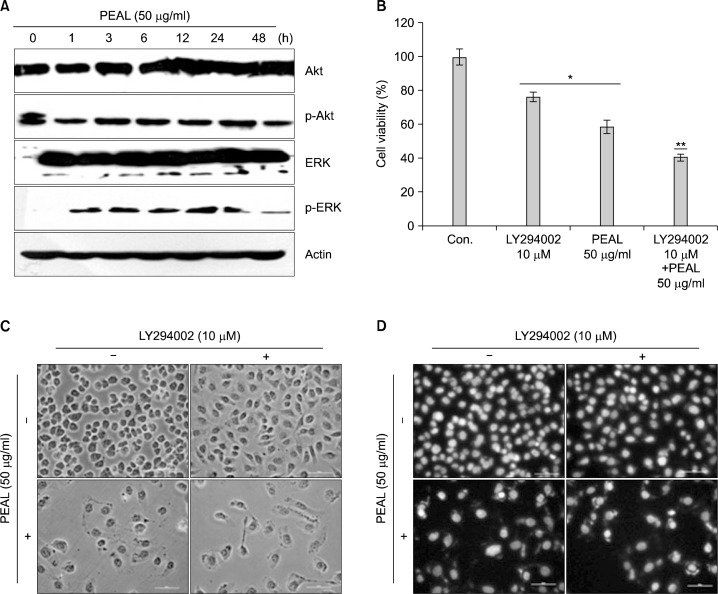
5. PEAL induces apoptosis at least in part by inhibiting Akt activity
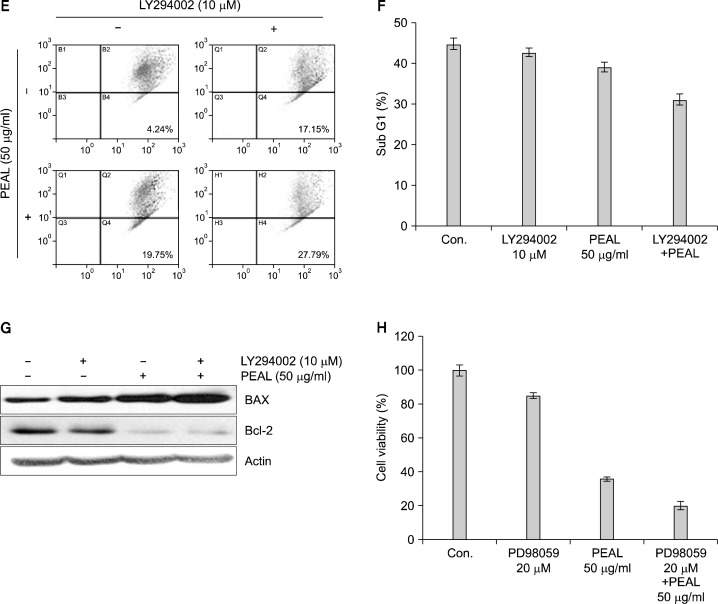
PI3K/Akt pathway plays an important role in regulating apoptosis and cell death. In addition, the expression of anti-apoptotic Bcl-2 protein is regulated by Akt. Hence, we investigated the effects of PEAL on Akt in AGS cells. Since the activity of Akt is regulated by phosphorylation, we assessed the levels of phosphorylated Akt in PEAL- treated AGS cells. The Western blot analysis revealed that PEAL suppressed the phosphorylation of Akt in a time-dependent manner (Fig. 3A). To confirm this finding, we evaluated the effects of PEAL with and without treatment of Akt inhibitor (LY294002). MTT test, light microscope finding and DAPI staining revealed that LY294002 augmented the effect of PEAL on cell viability and apoptosis (Fig. 3B–D). We also confirmed the findings by measuring mitochondrial membrane potential (MMP, ΔΨ m). PEAL and/or LY294002 induced loss of MMP (Δ Ψm) (Fig. 3E). Next, we investigated levels of Bcl-2 and Bax proteins. Western blot revealed that the addition of LY294002 to PEAL significantly augmented Bax Induction with near complete inhibition of Bcl-2. PEAL also induced ERK phosphorylation (Fig. 3A), but an ERK inhibitor augmented PEAL-induced cytotoxicity in AGS cells (Fig. 3H), indicating ERK activation was associated with survival pathway to escape PEAL-induced apoptosis. Taken together, these findings suggest that PEAL induce apoptosis at least in part by inhibiting phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway.
Fig. 3.
The role of Akt/PI3K signaling in PEAL-induced apoptosis. The cells were seeded at the density of 1×105 cells per ml and incubated at the indicated concentrations of PEAL for the indicated time duration. (A and G) Western blot analysis for Akt, ERK, Bax and Bcl-2 proteins. Equal amounts of cell lysate (30 μg) were resolved by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The results are from at least two independent experiments that showed similar patterns (B and F). Cell viability was assessed by the MTT assay. Data are expressed as mean±SD of three independent experiments (*P<0.05 versus control, **P<0.05 versus PEAL treatment). (C) The cell morphology was assessed under the light microscopy (magnification ×200). (D) The morphology of the nuclei. The nuclei stained with DAPI solution were observed under fluorescent microscope using a blue filter (magnification ×200). (E) The cells were stained with JC-1 and incubated at 37°C for 20 min. The mean JC-1 fluorescence intensity was detected using a flow cytometer. The results are from at least two independent experiments that showed similar patterns.
DISCUSSION
This study was designed to determine whether PEAL has anti-cancer properties in human cancer cells, and to further investigate the underlying mechanisms of the anti-cancer effect of PEAL. Indeed, we demonstrated that PEAL induced apoptosis in a dose-dependent manner in human AGS cells. This finding was confirmed by annexin V staining. During the early stages of apoptosis, phosphati-dylserine (PS) on the inner leaflet of the plasma membrane is translocated to the outer layer. PS is exposed on the external surface of the cell. Annexin V forms a shield around negatively-charged phospholipid molecules, competing for phosphatidylserine (PS) binding sites. Hence, annexin V is a sensitive probe for cell surface exposure of PS for quantification of apoptotic cells; Propidium iodide (PI) is a probe for discriminating between apoptotic and necrotic cells. Therefore, we double stained with PI and annexin V. We found that PEAL produced the early apoptotic cells (Annexin V+/PI−) in a dose-dependent manner. It was caspase dependent. Most of caspase-dependent apoptosis was related with mitochondrial pathway. Consistent with this finding, our study demonstrated that PEAL down-regulated Bcl-2 expression which plays a crucial role in apoptosis (Fig. 4B).7 The major component of PEAL was quercetin.8,9 It has been reported that quercetin induces caspase-dependent apoptosis through down-regulation of Bcl-2,10 and mitochondrial-mediated apoptosis pathway,11 which is consistent with our results. In addition, quercetin enhances anti-cancer activity by up-regulating p53 protein.12 Quercetin-induced apoptosis was associated with Akt pathway.10 These findings support our findings, and we confirmed with an Akt inhibitor (LY294002). However, in a certain cell line, PEAL-induced apoptosis was associated with anti-apoptotic factor c-FLIP, Bcl-xL, and cIAP-1 rather than Bcl-2.13 Even in some leukemic cell lines showing the different results,13 Akt pathway was an important pathway in PEAL-induced p53 up-regulation, because the up-regulated p53 can help suppress the Akt signaling.14 PEAL and/or LY294002 also reduced the mitochondrial membrane potential (MMP, Δ Ψm), which indicates that PEAL-induced apoptosis was via the mitochondrial-mediated apoptosis pathway. This study also demonstrated that PEAL induced ERK phosphorylation, which was survival pathway to survive PEAL treatment condition because an ERK inhibitor augmented PEAL-induced cytotoxicity in AGS cells (Fig. 3H). From these findings, our study suggests that PEAL-induced apoptosis is caspase-dependent apoptosis via Akt pathway.
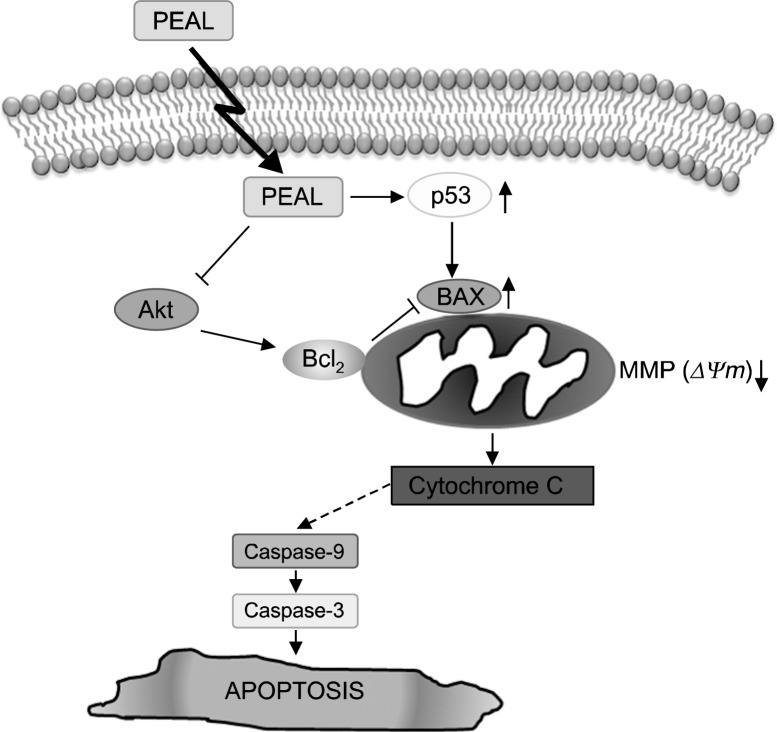
Fig. 4.
Schematic representation of apoptotic effects of PEAL on AGS human gastric cancer cells. PEAL activated p53 and subsequent Bax induction as well as by modulating Bcl-2 protein. The reduce MMP (ΔΨm) triggered by PEAL activates caspase 3, then the activated caspases activate Bid, and thus the Bid augments the loss of MMP (ΔΨm) through activation Bax. Through the process, PEAL induced apoptosis through mitochondrial pathway. In addition, PEAL suppressed phosphorylation of Akt that regulate the anti-apoptotic proteins. This PEAL-induced suppression of Akt may be associated with Bcl-2 suppression. Taken together, this study suggests that PEAL induced caspase-dependent apoptosis by up-regulating p53, and subsequent Bax induction as well as by modulating Bcl-2 protein, and that Akt is the critical upstream signaling that regulate the apoptotic effect of PEAL in AGS human gastric cancer cells.
The merit of PEAL when compared to the quercetin aglycone is that the bioavailability of quercetin in PEAL is much better that that of quercetin aglycone15,16 because quercetin in PEAL is in the form of quercetin glucosides, which are easily absorbed from the intestine.17 PEAL also contains isorhamnetin glucosides, which also have the same merit in the bioavailability. Isorhamnetin is a phytochemical present in many plants and is also a metabolite of quercetin. isorhamnetin is reported to have anti-cancer effects.18,19
In summary, this study demonstrated that PEAL induced caspase-dependent apoptosis in AGS human gastric cancer cells. The apoptosis was triggered through mitochondrial pathway by up-regulating p53, and subsequent Bax induction as well as by modulating Bcl-2 protein (Fig. 4). In addition, PEAL induced caspase-dependent apoptosis at least in part through the inhibition of phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway indicating that PI3K/Akt is the upstream signaling that regulate the apoptotic effect of PEAL. This study provides evidence that PEAL might have anticancer property on human gastric cancer cells.
Acknowledgments
This work was supported by the grants from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0820050).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–9. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 2.Challier B, Perarnau JM, Viel JF. Garlic, onion and cereal fibre as protective factors for breast cancer: a French case-control study. Eur J Epidemiol. 1998;14:737–47. doi: 10.1023/a:1007512825851. [DOI] [PubMed] [Google Scholar]
- 3.Le Bon AM, Siess MH. Organosulfur compounds from Allium and the chemoprevention of cancer. Drug Metabol Drug Interact. 2000;17:51–79. doi: 10.1515/dmdi.2000.17.1-4.51. [DOI] [PubMed] [Google Scholar]
- 4.Surh YJ. Transcription factors in the cellular signaling network as prime targets of chemopreventive phytochemicals. Cancer Res Treat. 2004;36:275–86. doi: 10.4143/crt.2004.36.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker PR, Sikorska M. New aspects of the mechanism of DNA fragmentation in apoptosis. Biochem Cell Biol. 1997;75:287–99. [PubMed] [Google Scholar]
- 6.Cui ZG, Hong NY, Guan J, Kang HK, Lee DH, Lee YK, et al. cAMP antagonizes ERK-dependent antiapoptotic action of insulin BMB Rep 44205–10. [DOI] [PubMed] [Google Scholar]
- 7.Kim R, Tanabe K, Emi M, Uchida Y, Toge T. Modulation of tamoxifen sensitivity by antisense Bcl-2 and trastuzumab in breast carcinoma cells. Cancer. 2005;103:2199–207. doi: 10.1002/cncr.21029. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Mitchell AE. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J Agric Food Chem. 2011;59:857–63. doi: 10.1021/jf1033587. [DOI] [PubMed] [Google Scholar]
- 9.Wiczkowski W, Romaszko J, Bucinski A, Szawara-Nowak D, Honke J, Zielinski H, et al. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr. 2008;138:885–8. doi: 10.1093/jn/138.5.885. [DOI] [PubMed] [Google Scholar]
- 10.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J Nutr. 2006;136:2715–21. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Moon JY, Ahn KS, Cho SK. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev. 2013;2013:596496. doi: 10.1155/2013/596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan ST, Yang NC, Huang CS, Liao JW, Yeh SL. Quercetin enhances the antitumor activity of trichostatin A through upregulation of p53 protein expression in vitro and in vivo. PLoS One. 2013;8:e54255. doi: 10.1371/journal.pone.0054255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han MH, Lee WS, Jung JH, Jeong JH, Park C, Kim HJ, et al. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem Toxicol. 2013;62:382–9. doi: 10.1016/j.fct.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 14.Kim CW, Lu JN, Go SI, Jung JH, Yi SM, Jeong JH, et al. p53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of Bax. Int J Oncol. 2013;43:1495–502. doi: 10.3892/ijo.2013.2070. [DOI] [PubMed] [Google Scholar]
- 15.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 16.Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Leary KA, Day AJ, Needs PW, Mellon FA, O’Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O- methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65:479–91. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Lee EO, Ko SG, Bae HS, Kim CH, Ahn KS, et al. Mitochondria-cytochrome C-caspase-9 cascade mediates isorhamnetin-induced apoptosis. Cancer Lett. 2008;270:342–53. doi: 10.1016/j.canlet.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Ma G, Yang C, Qu Y, Wei H, Zhang T, Zhang N. The flavonoid component isorhamnetin in vitro inhibits proliferation and induces apoptosis in Eca-109 cells. Chem Biol Interact. 2007;167:153–60. doi: 10.1016/j.cbi.2007.02.006. [DOI] [PubMed] [Google Scholar]