Abstract
The epithelium that lines the conducting airways is composed of several distinct cell types that differentiate from common progenitor cells. The signals that control fate selection and differentiation of ciliated cells, a major component of the epithelium, are not completely understood. Ciliated cell differentiation can be accomplished in vitro when primary normal human bronchial epithelial (NHBE) cells are cultured at an air–liquid interface, but is inhibited when NHBE cells are cultured under submerged conditions. The mechanism by which submersion prevents ciliogenesis is not understood, but may provide clues to in vivo regulation of ciliated cell differentiation. We hypothesized that submersion creates a hypoxic environment that prevents ciliated cell differentiation by blocking the gene expression program required for ciliogenesis. This was confirmed by showing that expression of multicilin and Forkhead box J1, key factors needed for ciliated cell differentiation, was inhibited when NHBE cells were cultured in submerged and hypoxic conditions. Multicilin and Forkhead box J1 expression and ciliated cell differentiation were restored in submerged and hypoxic cells upon treatment with the γ-secretase inhibitor, N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester (DAPT), which suggested that Notch signaling was involved. Overexpression of Notch intracellular domain inhibited differentiation in the presence of DAPT, confirming the role of Notch signaling. These results indicate that submersion and hypoxia prevent ciliated cell differentiation by maintaining Notch signaling, which represses genes necessary for ciliogenesis. These data provide new insights into the molecular mechanisms that control human bronchial differentiation.
Keywords: multiciliogenesis, hypoxia, Notch, Forkhead box J1, multicilin
Clinical Relevance
A balance in the cellular composition of the airway epithelium is critical for mucociliary clearance to protect the lungs from damage by inhaled noxious agents, and cellular homeostasis is maintained by the controlled regulation of cell fate choice and differentiation of basal cells to replace cells lost due to injury or disease. The molecular signals that control these processes are not completely understood, and disruption of these signals can lead to epithelial remodeling. Regions of hypoxia have been identified in diseased airways, and this study illustrates that hypoxia may cause epithelial remodeling by influencing Notch signaling.
The epithelium lining the conducting airways acts as the front-line defense to protect the lungs from inhaled pathogens and noxious agents by trapping and expelling them from the airway by the process of mucociliary clearance (MCC). MCC is accomplished by the coordinated efforts of ciliated and goblet cells, two major airway epithelial cell types. Goblet cells secrete mucus, which forms a viscoelastic gel that floats on the epithelial surface and traps inhaled harmful particles and pathogens. The coordinated beating of cilia on the apical surface of the ciliated cells propels this noxious mucus out of the airway before it can damage the lung. Efficient MCC depends upon a proper balance between ciliated and goblet cells. It is important to have a sufficient number of ciliated cells to be able to propel the mucus out of the airway. Too few ciliated cells and/or too much mucus production can lead to toxin-laden mucus accumulation in the airway that can lead to respiratory disease (1).
During embryonic lung development, ciliated and goblet cells differentiate from common progenitor cells in the embryonic foregut endoderm. Ciliated cells begin differentiating after lung bud branching during the late pseudoglandular stage (2, 3) and continue differentiation after birth. In the adult, basal cells residing in the epithelium act as precursor stem cells that can differentiate into different epithelial cell types to replace cells lost due to injury or cell death (4, 5). It is not fully understood how cell fate determination and differentiation of these cells is controlled, but it is likely that cell–cell interactions and multiple signaling cascades modulate the expression of cell-specific transcription factors to control the expression of genes required for unique, differentiated, cell-specific functions (6–8). In respiratory illnesses, such as asthma and chronic obstructive pulmonary disease, the airway epithelial cell composition is altered (5, 9–11). This epithelial remodeling includes ciliated cell loss and goblet cell hyperplasia and metaplasia. It is likely that changes in cell composition are due to environmental conditions that affect normal basal cell differentiation signals during repair after injury or disease to promote goblet cell differentiation and inhibit ciliated cell differentiation, leading to an imbalance in the ratio of these cells that ultimately reduces MCC efficiency. Therefore, a greater understanding of the signaling mechanisms that regulate bronchial cell differentiation would be beneficial for the maintenance of MCC and a healthy respiratory system.
Several transcription factors have been shown to be important for differentiation of multiciliated cells. Forkhead box J1 (FOXJ1) is required for ciliogenesis in the airway, as demonstrated by the observation that FOXJ1 knockout mice do not make motile multiciliated cells (12). During ciliated cell differentiation, FOXJ1 expression is induced in cells that have already committed to the ciliated cell fate at a step before ciliogenesis, and is believed to be important for the expression of genes that are required for later steps in ciliogenesis, including basal body localization and docking (13). More recently, the transcriptional regulator, multicilin (MCI; previously designated IDAS [14]), was found to be regulated by Notch and to be necessary for ciliated cell differentiation in Xenopus and mouse. In addition, MCI was determined to act upstream of FOXJ1 in the pathway of ciliated cell differentiation (15). Another transcription factor, myeloblastosis proto-oncogene, was also shown to be involved in ciliated cell differentiation, and acts downstream of MCI, but upstream of FOXJ1 (16). Thus, the pathway to multiciliated cell differentiation is complex and involves multiple transcription factors. Understanding the molecular mechanisms that control the expression of these factors is necessary for elucidating the pathway of bronchial ciliated cell differentiation.
Human bronchial epithelial cell differentiation can be recapitulated in vitro using air–liquid interface (ALI) culture techniques. Primary normal human bronchial epithelial (NHBE) cells, harvested from donor organs, can be grown as undifferentiated cells using standard submerged culture conditions. Cells can then be transferred to a porous membrane and the apical media removed while the basal media remain, creating an ALI. Over the next few weeks, the cells differentiate into a pseudostratified epithelium containing goblet and ciliated cells with transcriptional profiles similar to in vivo epithelial cells (17, 18). Ciliated cell differentiation is inhibited if the cells are kept submerged, indicating that establishment of the ALI is necessary for ciliated cell differentiation (19, 20). The molecular basis for this inhibition is not understood, and might provide important clues toward understanding in vivo differentiation of ciliated cells during development, because ciliated cells develop in the embryonic lung, which is a submerged environment (21), and during replacement and repair in the adult airway.
Using the NHBE cells cultured in vitro, we investigated the molecular events that are involved in the inhibition of ciliated cell differentiation in submerged conditions. The results suggest that submersion creates a hypoxic environment that potentiates Notch signaling and represses the expression of genes essential for ciliated cell differentiation. In addition, we show that ciliated cell differentiation and production of motile cilia do not require an ALI, but can be accomplished by interruption of Notch signaling while culturing NHBE cells in submerged conditions on plastic dishes.
Materials and Methods
NHBE Cell Culture
NHBE cells were isolated from human lungs from organ donors that were rejected for transplant, provided by the Life Alliance Organ Recovery Agency of the University of Miami with institutional review board consent. Cells were cultured as previously described (22). Briefly, NHBE cells were cultured for 21 days on human collagen IV–coated 0.33-cm2 or 1-cm2 Transwell permeable membranes (3421 or 3460; Corning, Corning, NY). NHBE cells were grown submerged (0.5 ml) until confluent, and then cultured either at an ALI in air (21% O2), submerged (with ALI media) at apical liquid volumes ranging from 0.125 to 0.5 ml, or at an ALI in hypoxia (0.5% O2). Hypoxic conditions were created using a Coy O2 control glove box (Coy Laboratory Products, Grass Lake, MI) using nitrogen gas to control oxygen levels. For Notch signaling inhibition, N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester (DAPT; D5942; Sigma, St. Louis, MO) was used. DAPT was dissolved in DMSO at a concentration of 23 mM and diluted 1:2,300 into media for a final concentration of 10 μM. For 96-well plate experiments, NHBE cells were plated on tissue culture–treated 96-well plates (353219; BD Biosciences, San Jose, CA), coated with human collagen IV, and grown in a volume of 150 μl for 21 days with either 10 μM DAPT or DMSO (vehicle control). Passage 1 cells from a minimum of three different donors were used for all experiments unless otherwise stated.
Immunofluorescence
For immunofluorescence staining of FOXJ1, influenza hemagglutinin epitope (HA), and cilia (acetylated α-tubulin) on Transwell permeable membranes (3421 or 3460; Corning) or FOXJ1 and cilia on a 96-well plate (353219; BD Biosciences), NHBE cells were fixed with 4% paraformaldehyde for 20 minutes at room temperature (RT), and then permeabilized with 1% Triton X-100 for 15 minutes at RT. After being fixed and permeabilized, cells were washed with PBS and blocked with 3% BSA for 1.5 hours at RT. After blocking, primary antibodies for anti-FOXJ1 (AF3619; R&D Systems, Minneapolis, MN), anti-HA (2367, 1:500; Cell Signaling, Danvers, MA), and anti–acetylated α-tubulin (T6793, 1:2000; Sigma) were added apically in 3% BSA and incubated overnight at 4°C. After washing off the primary antibody with PBS, species-specific, fluorophore-conjugated secondary antibodies were added in 3% BSA for 1 hour at RT. Secondary antibodies were washed with PBS and then nuclei were stained with Hoechst in PBS for 20 minutes at RT. All secondary antibodies (488, 555, 647) were Alexa Fluor coupled (Life Technologies, Carlsbad, CA). Transwell filters were mounted on slides using Fluoro-Gel (17985-10; Electron Microscopy Sciences, Hatfield, PA), and fluorescent images were taken on a Leica DM6000 microscope with a SP5 confocal module (Leica, Wetzlar, Germany). For 96-well plates, NHBE cells were submerged in PBS, and fluorescent images were also taken on a Leica DMI6000 microscope with a SP5 confocal module. Confocal images were generated using Volocity version 6.1.1 software (Perkin-Elmer, Waltham, MA).
For additional methods, see the online supplement.
Results
Apical Volume–Dependent Inhibition of Ciliated NHBE Cell Differentiation in Submerged Culture
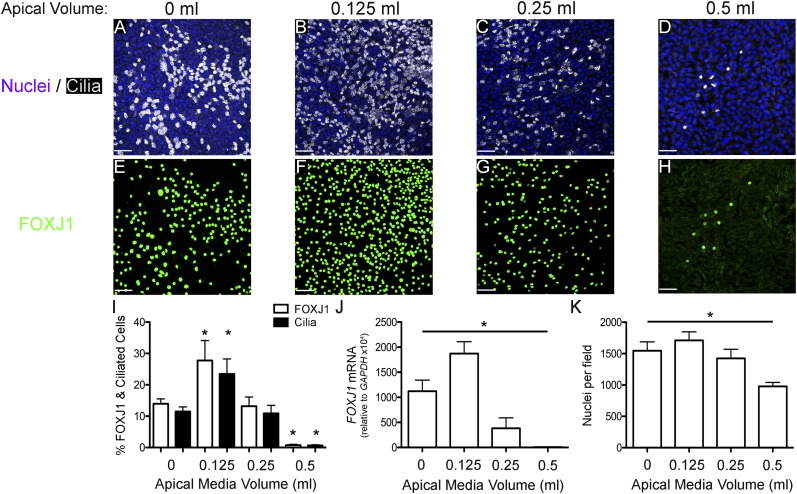
Human ciliated airway epithelial cell differentiation in vitro is inhibited when cultured submerged, but not in ALI (20, 23). In addition, rat tracheal epithelial ciliated cell differentiation decreased as the volume of apical fluid increased (19). The mechanism for the inhibition of ciliated cell differentiation by submersion is unclear, but may be due to inhibition of expression of a gene or genes necessary for ciliated cell determination or differentiation. Therefore, we sought to determine whether submersion inhibits FOXJ1 expression and, if so, is it volume dependent. To answer these questions, undifferentiated NHBE cells were cultured submerged under various apical media volumes on 1.2-cm-diameter Transwell filters for 21 days and assessed for FOXJ1 expression and ciliated cell differentiation by immunofluorescence staining. Qualitative visual analysis indicated that ciliated cells and FOXJ1-positive (FOXJ1+) cells were increased over ALI (0 ml) control (Figures 1A and 1E) when submerged under 0.125 ml of apical media (Figures 1B and 1F); cells in 0.25 ml apical media had similar numbers of FOXJ1+ and ciliated cells as ALI control (Figures 1C and 1G), but cells in 0.5 ml apical media showed a large decrease in ciliated and FOXJ1+ cells (Figures 1D and 1H). Quantification of ciliated and FOXJ1+ cells (Figure 1I) confirmed that the percentage of ciliated cells significantly decreased roughly 10-fold, from approximately 13% in ALI to approximately 0.8% when submerged with 0.5 ml media. FOXJ1 messenger RNA (mRNA) levels, measured by quantitative RT-PCR (Figure 1J), showed results consistent with the patterns of FOXJ1 expression observed by immunofluorescent staining, except that there was a decrease in the FOXJ1 mRNA in cells submerged under 0.25 ml. The effect of submersion on cell density was determined by counting the number of nuclei per microscopic field. This showed that submersion in 0.125 and 0.25 ml had no significant effect on cell density, but submersion in 0.5 ml significantly decreased cell density by approximately 33% (Figure 1K). These results indicate that submersion with 0.5 ml, but not with 0.25 ml, reduces ciliated cell differentiation by preventing FOXJ1 expression. Interestingly, submersion with 0.125 ml, the lowest apical medium volume tested, increases FOXJ1 expression and ciliogenesis over ALI, suggesting that a small amount of surface liquid stimulates ciliogenesis.
Figure 1.
Forkhead box J1 (FOXJ1) expression and ciliated cell differentiation are reduced by submersion in an apical volume–dependent manner. (A–H) Representative extended focus confocal immunofluorescent images of NHBE cells cultured for 21 days with apical media volumes of 0 ml (A and E), 0.125 ml (B and F), 0.25 ml (C and G), and 0.5 ml (D and H) stained for nuclei (Hoechst, blue) and cilia (acetylated α-tubulin, white; A–D) or FOXJ1 (green; E–H). (I) Percent FOXJ1-positive (FOXJ1+; white bars) and ciliated (black bars) cells at the apical media volumes, indicated on the x axis, showing a significant increase at 0.125 ml and a significant decrease at 0.5 ml. (J) FOXJ1 messenger RNA (mRNA) levels after 21 days of differentiation at the apical media volumes, indicated on the x axis, normalized to glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA, showing that the amount of FOXJ1 mRNA increases at 0.125 ml and decreases significantly at higher apical volumes, which is similar to changes in FOXJ1+ cells. (K) Quantification of nuclear density after 21 days submerged with different volumes of media showing a significant reduction in cells in 0.5 ml. Scale bar, 50 μm. Data shown are means (± SEM). One-way ANOVA (*P < 0.05; n = 3).
Hypoxia Inhibits Ciliated Cell Differentiation
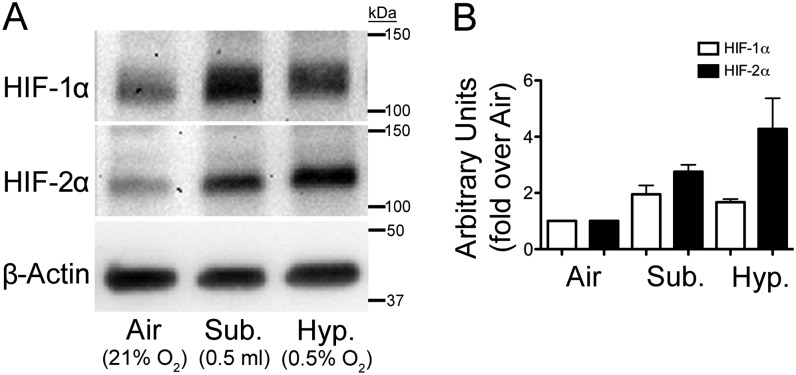
The mechanism for the inhibition of FOXJ1 expression and ciliogenesis by submersion with greater apical volumes is not understood. It has been shown that O2 concentration at the level of the cells decreases as the apical media volume is increased (24, 25). In addition, hypoxic conditions inhibit differentiation of different cell types (26–29). Therefore, we hypothesized that submersion at greater apical media volumes creates a hypoxic environment that inhibits ciliated cell differentiation. To determine if submersion causes NHBE cells to be hypoxic, undifferentiated NHBE cells were cultured at an ALI in air for 72 hours, then submerged with 0.5 ml media or placed at 0.5% O2 in a hypoxic chamber for 5 hours, and the levels of hypoxia-inducible factor (HIF) 1α and HIF-2α proteins were measured by Western blot. HIF-1α and HIF-2α are the primary intracellular signal transducers in response to hypoxia, and are degraded in normoxia (21% O2), but are stabilized and accumulate rapidly when placed in hypoxic conditions (30). Therefore, an increase in these proteins is indicative of hypoxia. The results show that both HIF-1α and HIF-2α increase when cells are switched to hypoxic or submerged conditions compared with air (Figures 2A and 2B), indicating that submersion does create a hypoxic environment.
Figure 2.
Submersion and hypoxia induce hypoxia-inducible factor (HIF) 1α and HIF-2α proteins. NHBE cells cultured 72 hours at an air–liquid interface (ALI) in air (21% O2) then switched to an ALI in hypoxia (0.5%; Hyp.) or submerged (0.5 ml; Sub.) for 5 hours. (A) Western blots of whole-cell protein lysates with antibodies to HIF-1α, HIF-2α, and β-actin showing both HIF-1α and HIF-2α induction in submerged and hypoxic conditions compared with ALI (Air) control. (B) Densitometry quantification of the Western blot data from two lungs. The data show increases in HIF proteins, indicating that submersion causes a hypoxic environment. β-actin was used as a loading control. Data shown are means (± SEM).
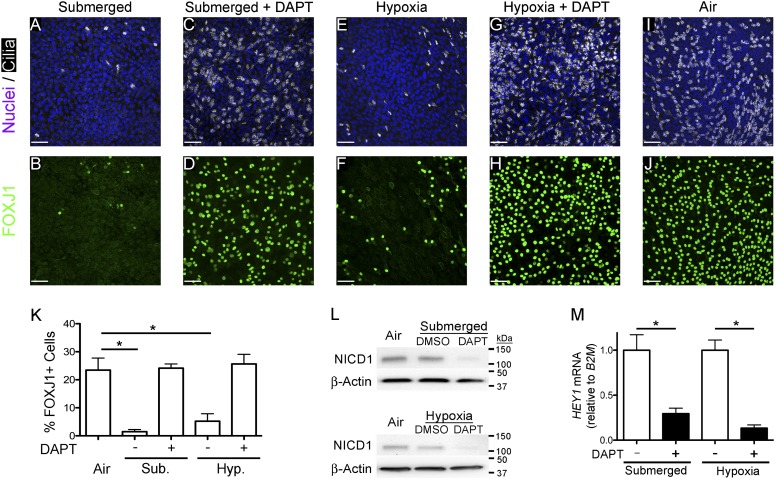
To test whether hypoxia prevents ciliated cell differentiation and FOXJ1 expression, undifferentiated NHBE cells were cultured using ALI conditions in a hypoxic chamber at 0.5% O2, ALI in air, or submerged in 0.5 ml media for 21 days, and the number of FOXJ1+ ciliated cells was determined by immunofluorescence staining. The results show that both submerged (Figures 3A and 3F) and hypoxic cells (Figures 3C and 3H) have fewer FOXJ1-expressing ciliated cells than cells cultured at an ALI in air (Figures 3E and 3J). Quantification of FOXJ1-expressing cells showed the inhibition to be significant (Figure 3K). Therefore, hypoxia, like submersion, reduces FOXJ1 expression and ciliogenesis, which is consistent with the hypothesis that submersion prevents FOXJ1 expression and cilia formation by creating a low-oxygen environment.
Figure 3.
γ-secretase inhibition restores FOXJ1 expression and ciliated cell differentiation in submerged and hypoxic conditions. (A–J) Representative extended-focus confocal immunofluorescent images of NHBE cells after 21 days of culture (A and B) submerged (0.5 ml), (C and D) submerged (0.5 ml) with 10 μM N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester (DAPT), (E and F) ALI in hypoxia (0.5% O2), (G and H) ALI in hypoxia (0.5% O2) with 10 μM DAPT, or (I and J) ALI in air, and stained for nuclei (Hoechst, blue) and cilia (acetylated α-tubulin, white) or FOXJ1 (green). Scale bars, 50 μm. (K) Graph displaying quantification of the percent FOXJ1+ in each condition showing a significant decrease in submerged and hypoxic conditions compared with the air control, and showing that DAPT restores ciliogenesis to control ALI levels. One-way ANOVA (*P < 0.05; n = 3). (L) Western blots of whole-cell lysates (40 μg) from NHBE cells cultured 24 hours at an ALI in air, submerged (0.5 ml) with and without 10 μM DAPT (top panel), or 48 hours at an ALI in air, and ALI in hypoxia (0.5% O2) with and without 10 μM DAPT (bottom panel) probed for activated-Notch1 (Notch intracellular domain [NICD] 1). The data show that NICD1 levels do not increase over ALI (Air), and that DAPT decreases NICD1 protein levels in submersion and hypoxia. β-actin was used as the loading control. (M) Quantitative RT-PCR (qRT-PCR) Notch target gene, hairy/enhancer-of-split related with YRPW motif 1 (HEY1), mRNA from NHBE cells cultured for 21 days in submerged and ALI in hypoxia (0.5% O2) (Hyp.) with and without 10 μM DAPT. HEY1 mRNA was normalized to the housekeeping gene, β2-microglobin (B2M), and shows that DAPT inhibits Notch signaling. Data shown are means (± SEM). Student’s t test (*P < 0.05; n = 3).
Inhibition of γ-Secretase Restores Ciliated Cell Differentiation in Submerged and Hypoxic Conditions
Previous studies showed that hypoxic inhibition of neuronal and myogenic (29) and pancreatic cell differentiation (31) requires Notch signaling. In addition, Notch signaling has been shown to prevent ciliogenesis (32, 33); suggesting that inhibition of FOXJ1 expression and ciliated cell differentiation in submerged and hypoxic conditions may require Notch signaling. Notch signaling requires cleavage of the Notch receptor by γ-secretase to release the Notch intracellular domain (NICD), which enters the nucleus to affect gene expression (34). To test whether Notch signaling may be involved in the prevention of ciliated cell differentiation in submerged and hypoxic conditions, NHBE cells were differentiated using ALI conditions in a hypoxic environment (0.5% O2) or submerged (0.5 ml) with the γ-secretase inhibitor, DAPT (35), or vehicle control, and the effect on ciliated cell differentiation was measured by immunofluorescence staining. These results indicate that DAPT treatment restored FOXJ1 expression and ciliated cell differentiation in submerged (Figures 3B and 3G) and hypoxic (Figures 3D and 3I) conditions to ALI control levels (Figure 3K). Previously, a roughly 33% decrease in cell density was observed in submerged conditions (0.5 ml) compared with ALI. To determine whether hypoxia also reduced cell density, and if DAPT had any effect on cell density, the cell density in these conditions was quantified. These results show that there was a similar reduction in cell density in hypoxia compared with ALI, and that DAPT did not change cell densities in submerged or hypoxic environments, indicating that DAPT increased ciliated cell differentiation without changing cell density in submersion or hypoxia (see Figure E1 in the online supplement). To ensure that Notch signaling was inhibited by DAPT treatment, activated Notch1 (NICD1) protein levels were measured in ALI, submersion, and hypoxia with or without DAPT. These data show that NICD1 levels were reduced by DAPT in submerged and hypoxic conditions. In addition, submersion or hypoxia did not induce NICD1 protein in 24 or 48 hours, respectively (Figure 3L). As an additional test for Notch signaling, the expression of the Notch target gene, hairy/enhancer-of-split related with YRPW motif 1 (HEY1), was measured in submerged and hypoxic cells with and without DAPT, and found to be significantly reduced by DAPT treatment (Figure 3M). These data indicate that submersion and hypoxia prevention of ciliated cell differentiation requires γ-secretase activity, consistent with the hypothesis that Notch signaling may mediate the reduction of ciliated cell differentiation in submerged and hypoxic conditions.
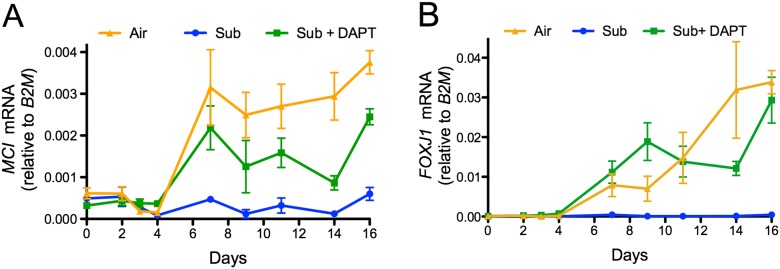
MCI Is Transcriptionally Repressed in Submerged Cultures and Induced by DAPT
MCI is a transcriptional regulator that is repressed by Notch signaling, necessary for multiciliated cell differentiation, and induces FOXJ1 expression in Xenopus models of ciliogenesis (15). Because FOXJ1 expression is inhibited in submerged NHBE cells in a γ-secretase–dependent manner, we asked whether MCI expression is also inhibited in submerged cells and, if so, could it be restored by DAPT treatment. The levels of MCI and FOXJ1 mRNAs were measured at different time points during differentiation of NHBE cells cultured submerged with and without DAPT and in control ALI conditions. Figures 4A and 4B show that MCI and FOXJ1 mRNAs were not expressed after 16 days in submerged cells, whereas both mRNAs were induced beginning around Day 7 in submerged conditions with DAPT and ALI, demonstrating that submersion represses the expression of MCI in a γ-secretase–dependent manner.
Figure 4.
Multicilin (MCI) expression is inhibited by submersion in a γ-secretase–dependent manner. Expression of (A) MCI and (B) FOXJ1 mRNAs during NHBE differentiation at an ALI in air (21% O2; orange), submerged (0.5 ml; blue), or submerged (0.5 ml) with 10 μM DAPT (green). MCI and FOXJ1 mRNAs are normalized to the housekeeping gene, B2M. The graphs show that expression of MCI and FOXJ1 mRNAs is inhibited by submersion, but that inhibition is overcome by adding DAPT (n = 3). Data shown are means (± SEM).
Overexpression of NICD Prevents Ciliated Cell Differentiation in Submerged and Hypoxic Conditions with DAPT
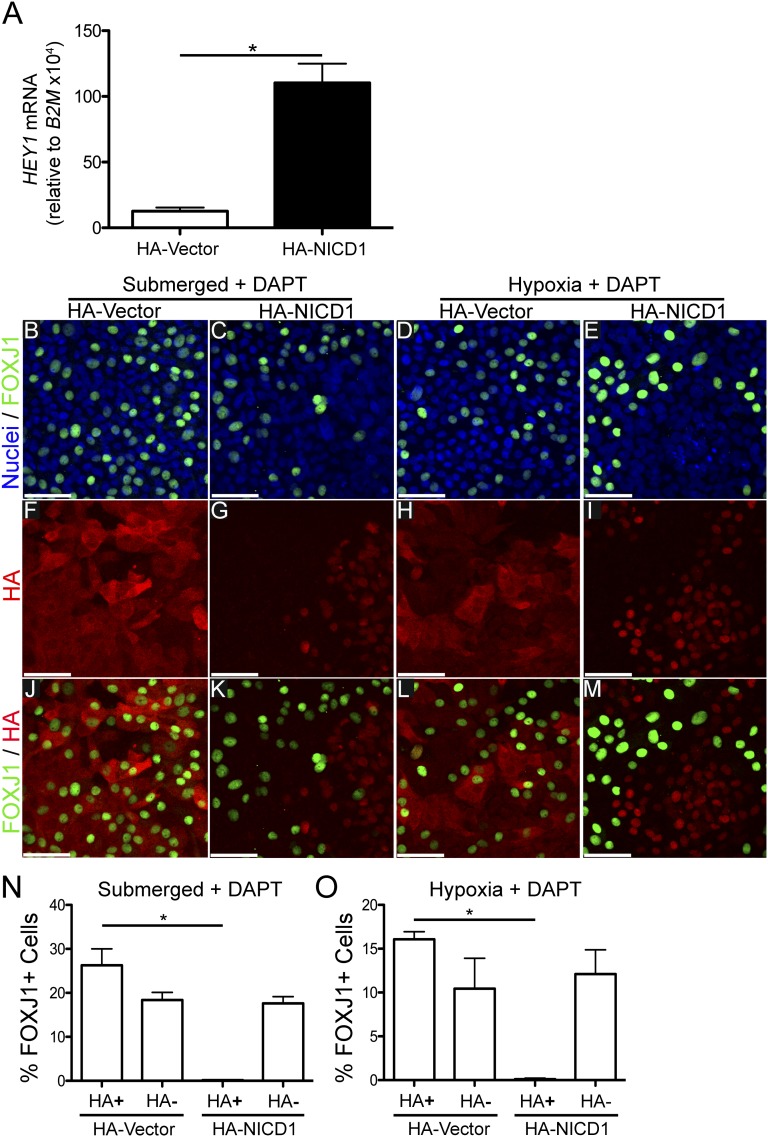
γ-secretase is not specific for Notch, and cleaves other proteins that may affect differentiation, (e.g., E-cadherin [36, 37]). This raised the possibility that inhibiting the cleavage of a protein other than Notch is necessary for ciliated cell differentiation in submerged and hypoxic conditions. To test this, NHBE cells were transduced with lentivirus to express an N-terminally HA-tagged NICD1 (HA-NICD1) to determine whether constitutive expression of NICD1 is sufficient to prevent FOXJ1 expression and ciliated cell differentiation in DAPT-treated submerged and hypoxic NHBE cells. If NICD1 expression is sufficient, it will suggest that γ-secretase cleavage of Notch, and not another protein, is responsible for reducing ciliated cell differentiation in submerged and hypoxic conditions. However, if cells expressing constitutively active NICD1 in DAPT-treated submerged and hypoxic cells do express FOXJ1, it will suggest that cleavage of another γ-secretase substrate protein is required to prevent differentiation. To ensure that expression of HA-NICD1 in NHBE cells activates Notch signaling, the level of the HEY1 mRNA was measured in cells transduced with HA-NICD1 or a control HA-expressing vector (HA-vector). Figure 5A shows a significant increase in HEY1 mRNA expression in the HA-NICD1 transduced cells compared with HA-vector, indicating that HA-NICD1 induces Notch signaling. To determine the effect of HA-NICD1 expression on ciliated cell differentiation, NHBE cells were cultured for 3 weeks submerged or at an ALI in 0.5% O2 in the presence of DAPT, fixed, and stained for HA to identify HA-NICD1 transduced cells, and for FOXJ1 to identify ciliated cells (Figures 5B–5M). Cells transduced with the HA-NICD1 lentivirus showed nuclear HA staining (Figures 5G and 5I), indicating that HA-NICD1 was being properly localized to the nucleus. Cells transduced with the HA-vector lentivirus showed cytoplasmic HA staining (Figures 5F and 5H). To quantify the effect of HA-NICD1 expression on FOXJ1 expression, the percentage of HA-positive (HA+) cells expressing FOXJ1 was quantified and compared with the nontransduced (i.e., HA-negative [HA−]) cells expressing FOXJ1 in the same culture, and to HA-vector transduced cells. Quantification of the HA-NICD1 transduced (HA+) cells in DAPT-treated submerged cells showed that 0.15 (± 0.03)% were FOXJ1+, whereas 17.6 (± 1.5)% of the nontransduced (HA−) cells expressed FOXJ1. In HA-vector control transduced (HA+) cells, 26.3 (± 3.7)% of the cells were FOXJ1+ and 18.4 (± 1.7)% of the nontransduced cells expressed FOXJ1 when cultured submerged with DAPT (Figure 5N). Similar results were obtained when the cells were cultured in hypoxia plus DAPT conditions (Figure 5O), with 0.11 (± 0.11)% of HA-NICD1 transduced (HA+) cells and 12.1 (± 2.8)% of nontransduced (HA−) cells expressing FOXJ1. In hypoxia, 16.1 (± 0.9)% of the HA-vector control transduced cells were FOXJ1+, and 10.4 (± 3.5)% of the nontransduced cells expressed FOXJ1. Similar results were obtained using lentivirus to express HA-NICD3 in NHBE in submersion and hypoxia with DAPT (Figure E2). These results are similar to previously published results using a transgenic mouse model expressing NICD1 (33). These data indicate that reducing NICD1 or NICD3 levels by inhibiting γ-secretase with DAPT in NHBE cells cultured submerged or in hypoxia is necessary for FOXJ1 expression, and strongly suggest that Notch signaling mediates the reduction of ciliated cell differentiation in submersion or hypoxic conditions.
Figure 5.
Expression of influenza hemagglutinin epitope–tagged (HA)-NICD1 increases Notch signaling and inhibits FOXJ1 in DAPT-treated submerged and hypoxic cells. (A) qRT-PCR of HEY1 mRNA from NHBE cells transduced with lentiviruses expressing either HA-tagged NICD1 (HA-NICD1) or vector control (HA-vector), showing a significant increase in the Notch target gene, HEY1 mRNA, in HA-NICD1 transduced cells, indicating that HA-NICD1 induces Notch signaling. HEY1 mRNA was normalized to the housekeeping gene, B2M mRNA. Student’s t test (*P < 0.05; n = 3). (B–M) Representative extended-focus confocal immunofluorescent images of NHBE cells transduced with HA-NICD1 or HA-vector lentiviruses. Cells were grown for 3 weeks in submerged conditions with 10 μM DAPT or in hypoxia (0.5% O2) with 10 μM DAPT, and then stained for nuclei (Hoechst, blue), FOXJ1 (green), or HA (red). (B–E) Hoechst and FOXJ1 merged images, (F–I) images of HA alone, and (J–M) HA and FOXJ1 merged images. The images show cytoplasmic HA staining colocalizing with FOXJ1 in HA-vector transduced cells, whereas HA-NICD1 transduced cells have nuclear HA staining and significantly less colocalization with FOXJ1 staining. Quantification of percent FOXJ1+ HA-NICD1 or HA-vector transduced (HA+) and nontransduced (HA−) NHBE cells in submerged plus DAPT (N) and hypoxia plus DAPT (O) conditions showing significant reduction of FOXJ1+ cells in HA-NICD1 transduced cells compared with nontransduced cells, and to HA-vector control transduced cells in DAPT-treated submerged and hypoxic conditions. These results indicate that expression of HA-NICD1 inhibits ciliated cell differentiation in the presence of DAPT, and suggest that DAPT promotes ciliogenesis by blocking Notch signaling. A minimum of 500 cells from 3 different lung donors was counted for each group. Scale bar, 50 μm. Data shown are means (± SEM). One-way ANOVA (*P < 0.05; n = 3).
Motile Ciliated Cell Differentiation Using Submerged Culture Conditions on Plastic
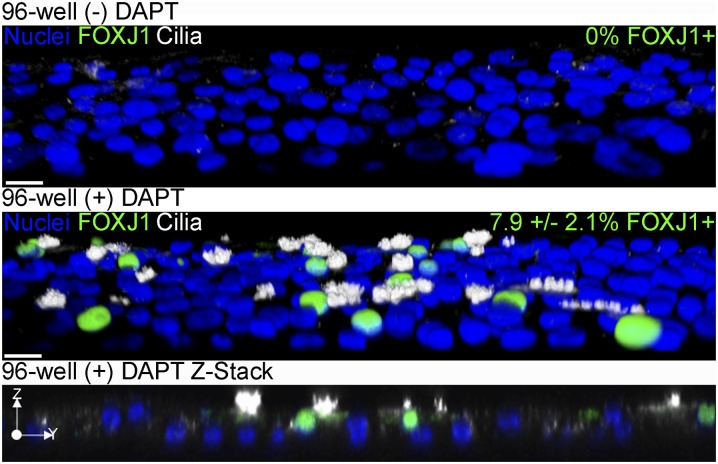
The observation that inhibiting γ-secretase with DAPT promotes ciliated cell differentiation in submerged culture conditions shows that an ALI was not required for ciliogenesis. This suggests that previous attempts to differentiate ciliated cells using standard submerged culture in plastic dishes were unsuccessful because of prolonged Notch signaling under these conditions. Therefore, it should be possible to differentiate ciliated cells in submerged conditions in the presence of DAPT in plastic cell culture plates rather than submerged on porous membranes. To test this, undifferentiated NHBE cells were cultured submerged in 150 μl of media on collagen-coated wells of a 96-well plate for 21 days with and without DAPT. Cells were then fixed and immunostained for FOXJ1 and cilia. In the absence of DAPT, no FOXJ1+ or ciliated cells were detected, but cells treated with DAPT expressed FOXJ1 and were ciliated (Figure 6). In addition, these cilia were motile (Figure E3), and their beat frequency was measured to be 10.18 (± 2.84) Hz, which is within the range of published ciliary beat frequency observed in NHBE cells differentiate at an ALI (38). In addition, the length of cilia on NHBE cells cultured either at an ALI on Transwell inserts or submerged on plastic were similar, measuring 8.82 (± 1.04) μm and 9.15 (± 1.56) μm, respectively. Thus, neither an ALI nor porous filters are necessary for the differentiation of motile ciliated cells in the presence of DAPT.
Figure 6.
DAPT enables FOXJ1 expression and ciliated cell differentiation of NHBE cells in submerged culture on plastic in 96-well plates. Representative three-dimensional (3D) opacity renderings of 40× confocal immunofluorescent images of NHBE cells cultured in wells of a 96-well plate submerged for 21 days (top) or submerged with 10 μM DAPT (middle); a Z-stack of submerged conditions with 10 μM DAPT is also shown (bottom). Cells were stained for cilia (acetylated α-tubulin, white), FOXJ1 (green), and nuclei (Hoechst, blue). The percent FOXJ1+ cells are indicated in the upper right corner of the upper two panels. The 3D images were cropped and rotated along the z axis to view cells from above the apical surface. Scale bar, 20 μm (n = 3).
Discussion
Ostrowski and Nettesheim (19) showed that differentiation of rat tracheal ciliated cells decreased when the cells were submerged in greater volumes of apical fluid. Our data confirmed a similar response to increased apical fluid volumes for human bronchial ciliated cell differentiation. However, smaller-diameter Transwell membranes were used in this study, so, although similar apical volumes were used, the calculated depth of the apical fluid is greater for the human cells. Specifically, the rat study showed a 90% reduction of rat cell ciliogenesis at calculated depths of 1 mm or greater, whereas human cell differentiation was not significantly reduced until the calculated apical media was depth roughly 4 mm. In addition, submersion at a depth of approximately 1 mm enhanced human cell differentiation. Because increasing apical liquid depth decreases O2 concentration at the level of the cells, it suggests that there may be differences in the O2 concentration threshold for ciliated cell differentiation between rat and human cells. Alternatively, there may be other differences that affect O2 concentration (e.g., menisci height, as smaller-diameter filters were used in the present study). In any case, the correlation between greater depth and inhibition of ciliated cell differentiation is consistent in both studies.
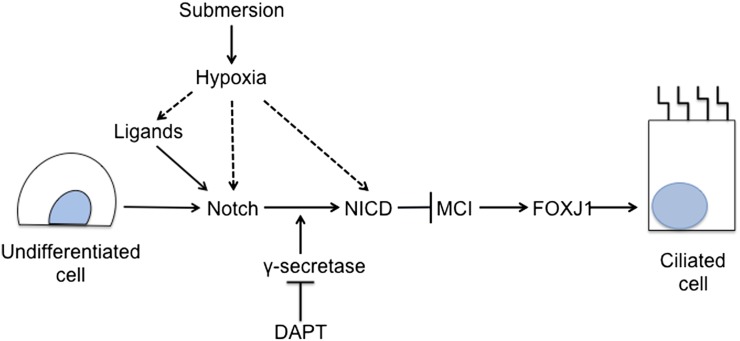
The hypothesis that submersion prevents ciliated cell differentiation by making the cells hypoxic was supported by the observations that HIF-1α and HIF-2α accumulate rapidly after cells were switched from an ALI in air to submerged culture or to hypoxia, and ciliated cell differentiation is reduced in ALI at 0.5% O2. The transcription factors, HIF-1α and HIF-2α, are the major mediators of hypoxic response. These proteins bind to HIF-1β to directly regulate many hypoxia response genes, including genes involved with angiogenesis (39) and embryogenesis (40). In addition, hypoxia has been shown to inhibit differentiation of pancreatic β cells (26) and myoblasts (29) via interaction with the Notch signaling pathway. Taken together, these data suggest that submersion and hypoxia potentiate Notch signaling to repress the induction of genes necessary for ciliated cell development as diagrammed in Figure 7. However, the mechanism of potentiation of Notch signaling by hypoxia is not completely understood. Several possible mechanisms have been proposed. Hypoxia has been shown to potentiate Notch signaling by increasing the levels of Notch receptor expression (41), or by increasing expression of Notch ligands (42). HIF-1α can also bind to NICD and potentiate Notch signaling and alter gene expression (29). In addition, NICD activity can be enhanced directly in hypoxia by reduced asparaginyl hydroxylation (43). Further studies are needed to determine which, if any, of these mechanisms might prolong Notch activation in hypoxic or submerged culture.
Figure 7.
The proposed model for the regulation of ciliated cell differentiation by oxygen and Notch signaling. Submersion and/or hypoxia cause a low-oxygen environment that potentiates Notch signaling and prevents MCI and FOXJ1 expression, factors required for ciliated cell differentiation. Possible mechanisms for hypoxia potentiation of Notch signaling are indicated by the dashed arrow, and include induction of ligand expression, induction of Notch receptor expression, and direct interaction with NICD. Inhibition of γ-secretase with DAPT prevents Notch cleavage into the activated NICD form, and inhibits Notch signaling, which permits MCI and FOXJ1 expression and ciliated cell differentiation.
Oxygen concentration may play a role in human fetal lung development. Cilia are not seen until about the 12th week of gestation (21). This correlates with the time during embryogenesis when there is an increase of maternal blood flow into the intervillous region of the placenta and increases in the oxygen content from a Po2 of around 20 mm Hg to between 40 and 80 mm Hg, thereby increasing the O2 concentration of the blood delivered to the fetus (44, 45). This is consistent with a model (Figure 7) in which low Po2 in fetal circulation maintains Notch signaling that prevents ciliated cell differentiation in the foregut endoderm until the O2 concentration increases after placental maturation.
Notch signaling has been shown to be a key regulator of differentiation of several airway epithelial cell types. Notch signaling has been reported to promote secretory cell and prevent ciliated cell differentiation (33, 46) and reduce neuroendocrine cells (47) during mouse embryogenesis. Notch signaling has been shown to influence cell fate decisions of basal cells, which act as epithelial stem cells in the adult lung (9). The observation that hypoxia reduces ciliated cell differentiation in NHBE cells in vitro by potentiating Notch signaling suggests that hypoxia may also influence differentiation of other epithelial cell types in the adult airway. Consistent with this idea, hypoxia was shown to increase the number of MUC5AC-positive cells during NHBE cell differentiation in vitro (48). In addition, regions of hypoxia have been observed in the airways of patients with respiratory diseases, such as cystic fibrosis and chronic obstructive pulmonary disease (48, 49), diseases that also exhibit goblet cell hyperplasia, mucus hypersecretion (5, 50), and reduced numbers of ciliated cells (8, 10). Thus, the interplay between oxygen concentration and Notch signaling may contribute to alterations in the cellular composition of the airway epithelium observed in respiratory disease, and suggests a new potential therapeutic target.
To date, the only in vitro method for the differentiation of multiciliated airway epithelial cells has been to culture primary precursor cells using ALI conditions. Our observations show that motile multiciliated cells will differentiate in submerged culture conditions on plastic in the presence of the Notch signaling inhibitor, DAPT. This suggests that exposure to apical air is not necessary for ciliogenesis, and that conventional cell culture approaches are sufficient for ciliated cell differentiation.
Acknowledgments
Acknowledgments
The authors thank Lisa Novak and Gabriel Gaidosh for their help with these experiments.
Footnotes
This work was supported by Flight Attendants’ Medical Research Institute grants (N.L.F., G.E.C., and M.S.), and by National Institutes of Health grants HL-060644 and HL-089399 (M.S.).
Author Contributions: B.J.G. and N.L.F. designed the experiments and B.J.G., M.V., and N.B. executed the experiments. All authors participated in data analysis and writing the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0237OC on April 22, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Salathe M, O’Riordan TG, Wanner A. Treatment of mucociliary dysfunction. Chest. 1996;110:1048–1057. doi: 10.1378/chest.110.4.1048. [DOI] [PubMed] [Google Scholar]
- 2.Toskala E, Smiley-Jewell SM, Wong VJ, King D, Plopper CG. Temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L454–L459. doi: 10.1152/ajplung.00036.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatt EN, Yan XH, Wuerffel MK, Hamilos DL, Brody SL. Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am J Respir Cell Mol Biol. 1999;21:168–176. doi: 10.1165/ajrcmb.21.2.3691. [DOI] [PubMed] [Google Scholar]
- 4.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 5.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:718–725. doi: 10.1513/pats.200605-117SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 7.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas J, Morlé L, Soulavie F, Laurençon A, Sagnol S, Durand B. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol Cell. 2010;102:499–513. doi: 10.1042/BC20100035. [DOI] [PubMed] [Google Scholar]
- 9.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riise GC, Larsson S, Andersson BA. A bronchoscopic brush biopsy study of large airway mucosal pathology in smokers with chronic bronchitis and in healthy nonsmokers. Eur Respir J. 1992;5:382–386. [PubMed] [Google Scholar]
- 11.Thomas B, Rutman A, Hirst RA, Haldar P, Wardlaw AJ, Bankart J, Brightling CE, O’Callaghan C. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol. 2010;126:722–729. doi: 10.1016/j.jaci.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left–right asymmetry. J Clin Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left–right axis defects in forkhead factor HFH-4–null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- 14.Pefani DE, Dimaki M, Spella M, Karantzelis N, Mitsiki E, Kyrousi C, Symeonidou IE, Perrakis A, Taraviras S, Lygerou Z. IDAS, a novel phylogenetically conserved geminin-related protein, binds to geminin and is required for cell cycle progression. J Biol Chem. 2011;286:23234–23246. doi: 10.1074/jbc.M110.207688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol. 2012;14:140–147. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan FE, Vladar EK, Ma L, Fuentealba LC, Hoh R, Espinoza FH, Axelrod JD, Alvarez-Buylla A, Stearns T, Kintner C, et al. MYB promotes centriole amplification and later steps of the multiciliogenesis program. Development. 2013;140:4277–4286. doi: 10.1242/dev.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezzulo AA, Starner TD, Scheetz TE, Traver GL, Tilley AE, Harvey BG, Crystal RG, McCray PB, Jr, Zabner J. The air–liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L25–L31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvorak A, Tilley AE, Shaykhiev R, Wang R, Crystal RG. Do airway epithelium air–liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol. 2011;44:465–473. doi: 10.1165/rcmb.2009-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrowski LE, Nettesheim P. Inhibition of ciliated cell differentiation by fluid submersion. Exp Lung Res. 1995;21:957–970. doi: 10.3109/01902149509031773. [DOI] [PubMed] [Google Scholar]
- 20.de Jong PM, van Sterkenburg MA, Hesseling SC, Kempenaar JA, Mulder AA, Mommaas AM, Dijkman JH, Ponec M. Ciliogenesis in human bronchial epithelial cells cultured at the air–liquid interface. Am J Respir Cell Mol Biol. 1994;10:271–277. doi: 10.1165/ajrcmb.10.3.8117445. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard DA, Lallement AV, Petit AF, Puchelle ES. In vivo ciliogenesis in human fetal tracheal epithelium. Am J Anat. 1989;185:415–428. doi: 10.1002/aja.1001850405. [DOI] [PubMed] [Google Scholar]
- 22.Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski LE, Conner GE, Fregien N, Salathe M. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci. 2006;119:4176–4186. doi: 10.1242/jcs.03181. [DOI] [PubMed] [Google Scholar]
- 23.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- 24.Mamchaoui K, Saumon G. A method for measuring the oxygen consumption of intact cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2000;278:L858–L863. doi: 10.1152/ajplung.2000.278.4.L858. [DOI] [PubMed] [Google Scholar]
- 25.Oze H, Hirao M, Ebina K, Shi K, Kawato Y, Kaneshiro S, Yoshikawa H, Hashimoto J. Impact of medium volume and oxygen concentration in the incubator on pericellular oxygen concentration and differentiation of murine chondrogenic cell culture. In Vitro Cell Dev Biol Anim. 2012;48:123–130. doi: 10.1007/s11626-011-9479-3. [DOI] [PubMed] [Google Scholar]
- 26.Fraker CA, Alvarez S, Papadopoulos P, Giraldo J, Gu W, Ricordi C, Inverardi L, Domínguez-Bendala J. Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem Cells. 2007;25:3155–3164. doi: 10.1634/stemcells.2007-0445. [DOI] [PubMed] [Google Scholar]
- 27.Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 28.Vieira HL, Alves PM, Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol. 2011;93:444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells. 2010;29:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 31.Fraker CA, Ricordi C, Inverardi L, Domínguez-Bendala J. Oxygen: a master regulator of pancreatic development? Biol Cell. 2009;101:431–440. doi: 10.1042/BC20080178. [DOI] [PubMed] [Google Scholar]
- 32.Stubbs JL, Oishi I, Izpisúa Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 35.Liao YF, Wang BJ, Hsu WM, Lee H, Liao CY, Wu SY, Cheng HT, Hu MK. Unnatural amino acid-substituted (hydroxyethyl)urea peptidomimetics inhibit gamma-secretase and promote the neuronal differentiation of neuroblastoma cells. Mol Pharmacol. 2007;71:588–601. doi: 10.1124/mol.106.024299. [DOI] [PubMed] [Google Scholar]
- 36.Haapasalo A, Kovacs DM. The many substrates of presenilin/γ-secretase. J Alzheimers Dis. 2011;25:3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 39.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishida T, Hijioka H, Kume K, Miyawaki A, Nakamura N. Notch signaling induces EMT in OSCC cell lines in a hypoxic environment. Oncol Lett. 2013;6:1201–1206. doi: 10.3892/ol.2013.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, Breier G, Gessler M. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Coleman ML, McDonough MA, Hewitson KS, Coles C, Mecinovic J, Edelmann M, Cook KM, Cockman ME, Lancaster DE, Kessler BM, et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J Biol Chem. 2007;282:24027–24038. doi: 10.1074/jbc.M704102200. [DOI] [PubMed] [Google Scholar]
- 44.Tuuli MG, Longtine MS, Nelson DM. Review: oxygen and trophoblast biology—a source of controversy. Placenta. 2011;32:S109–S118. doi: 10.1016/j.placenta.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid–base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 46.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139:4365–4373. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polosukhin VV, Cates JM, Lawson WE, Milstone AP, Matafonov AG, Massion PP, Lee JW, Randell SH, Blackwell TS. Hypoxia-inducible factor-1 signalling promotes goblet cell hyperplasia in airway epithelium. J Pathol. 2011;224:203–211. doi: 10.1002/path.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ordoñez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]