Abstract
Diacetyl (DA), a component of artificial butter flavoring, has been linked to the development of bronchiolitis obliterans (BO), a disease of airway epithelial injury and airway fibrosis. The epidermal growth factor receptor ligand, amphiregulin (AREG), has been implicated in other types of epithelial injury and lung fibrosis. We investigated the effects of DA directly on the pulmonary epithelium, and we hypothesized that DA exposure would result in epithelial cell shedding of AREG. Consistent with this hypothesis, we demonstrate that DA increases AREG by the pulmonary epithelial cell line NCI-H292 and by multiple independent primary human airway epithelial donors grown under physiologically relevant conditions at the air–liquid interface. Furthermore, we demonstrate that AREG shedding occurs through a TNF-α–converting enzyme (TACE)-dependent mechanism via inhibition of TACE activity in epithelial cells using the small molecule inhibitor, TNF-α protease inhibitor-1, as well as TACE-specific small inhibitor RNA. Finally, we demonstrate supportive in vivo results showing increased AREG transcript and protein levels in the lungs of rodents with DA-induced BO. In summary, our novel in vitro and in vivo observations suggest that further study of AREG is warranted in the pathogenesis of DA-induced BO.
Keywords: amphiregulin, TNF-α–converting enzyme, epidermal growth factor receptor, diacetyl, bronchiolitis obliterans
Clinical Relevance
Bronchiolitis obliterans (BO) is an untreatable disease of airway fibrosis that can occur in the setting of inhalational toxin exposures, including the artificial butter flavoring diacetyl (DA). This study demonstrates, for the first time, using in vivo and in vitro models, that increased shedding of amphiregulin occurs in the setting of DA exposure, suggesting a potential role in the development BO.
Bronchiolitis obliterans (BO) is a fibrotic disease of the small airways that has been linked to select occupational exposures (1, 2). In particular, diacetyl (DA), a volatile ketone used in artificial butter flavoring, has been strongly associated with the development of BO in microwave popcorn and flavor manufacturing workers (2). DA has been shown to cause severe epithelial injury (3, 4) and development of airway fibrosis in our rodent model of DA-induced BO (5). Despite the growing clinical relevance of BO, little is known regarding mechanisms that lead to its development.
Amphiregulin (AREG) is an epidermal growth factor receptor (EGFR) ligand that is involved in the growth and development of normal tissues and cancer cells (6, 7). AREG is expressed as a transmembrane precursor, and is proteolytically cleaved into a soluble form (7) by the TNF-α–converting enzyme (TACE) (8, 9). AREG shedding has been reported after exposure of human bronchial epithelial cells to several toxic insults, such as diesel exhaust and cigarette smoke extract (CSE) (10–12). In addition, in vivo studies have demonstrated that AREG transcript is increased in models of bleomycin-induced lung fibrosis and naphthalene-induced epithelial Clara cell injury (13, 14).
We hypothesized that DA induces TACE-dependent AREG shedding by pulmonary epithelial cells. We sought to prove our hypothesis in vitro and show similar increases in AREG in vivo in the setting of an established animal model of DA-induced BO. We demonstrate, for the first time, that DA exposure, both by liquid application or vapor, cause AREG shedding in a time-dependent manner by NCI-H292 pulmonary epithelial cell line and by primary human airway epithelial cells grown under physiological conditions. Furthermore, using a small molecule inhibitor of TACE (TNF-α protease inhibitor [TAPI]-1) and TACE-specific small inhibitor (si)RNA, we demonstrate that DA-induced AREG shedding by pulmonary epithelial cells occurs through a TACE-dependent mechanism. Finally we translate the in vivo relevance of these in vitro observations and demonstrate elevated AREG transcript in lung tissue, protein in lung fluid, and protein localized to the bronchial epithelial cells in rodents with DA-induced BO.
Materials and Methods
Materials
DA was purchased from Fluka (CAS no. 431-03-8, 99% pure; Fluka Chemical Co., Milwaukee, WI). The TACE inhibitor, TAPI-1, was purchased from EMD Chemicals (Billerica, MA). Materials for RNA isolation, Taqman assays, and siRNA knockdown were purchased from Invitrogen (Grand Island, NY).
NCI-H292 Cell Line
NCI-H292 cells (10, 12) were rinsed twice with Hanks’ balanced salt solution (HBSS) and exposed to DA (5–20 mM) or vehicle control (HBSS) for 30 minutes, given a postexposure HBSS rinse, and finally replenished with serum-free medium in the presence or absence of TAPI-1 (5 or 10 mM) for 6 or 24 hours. Supernatants were then collected for analysis. See the supplemental Materials and Methods for additional details.
Normal Human Bronchial Epithelial Cells
Primary normal human bronchial epithelial (NHBE) cells, cultured at air–liquid interface (ALI) for at least 14 days (15), were washed twice with HBSS, followed by apical surface exposure to 100 μl of DA (10–40 mM) or vehicle control (HBSS) for 30 minutes. After two additional post-exposure apical and basolateral washes, NHBE cells were cultured at ALI with fresh complete basolateral media for 6 or 24 hours. Basolateral and apical supernatants were collected and assayed separately. See the supplemental Materials and Methods for additional details.
EpiAirway TissueTM Culture and Vapor DA Exposure
Human EpiAirway ALI culture inserts (EpiAirway, Ashland, MD) were placed into 1 ml of culture medium (MatTek, Ashland, MA) upon receipt in six-well culture plates for 16–18 hours to equilibrate. After PBS washes, the apical surface was exposed to 25 mM, approximately 1,000 ppm, DA or vehicle control (PBS) vapor for 1 hour on Days 0, 2, and 4. For vapor exposures, 50 μl of DA or PBS was added to a disc in the vapor cup and applied to the culture plate for 1 hour. The basolateral culture medium was collected and replaced each day with 1 ml of fresh medium. See the supplemental Materials and Methods for additional details.
TACE siRNA Knockdown Studies
NCI-H292 cells were treated with DA 18–24 hours after overnight reverse transfection with 10 nM TACE-specific siRNA (s13718). AREG was measured 6 or 24 hours after DA exposure. See the supplemental Materials and Methods for additional details.
In Vivo Experiments
Rats were exposed to 125 mg/kg DA by intratracheal instillation on Day 0. At 1 and 7 days after DA exposure, bronchoalveolar lavage fluid (BALF), lung tissue RNA, and lung pathology were obtained. BALF AREG protein levels and lung tissue AREG transcript levels were measured. In addition, rat lung tissue was evaluated for AREG expression by immunofluorescence, costained with either smooth muscle actin (SMA) or β-catenin, and nuclear counterstain with 4′,6-diamidino-2-phenylindole. See the supplemental Materials and Methods for additional details.
Statistical Analysis
For analysis of AREG shedding dose responses, one-way ANOVA was used to compare all doses among the experimental groups. In the case of two group experiments, nonparametric t tests were used to assess differences between groups. All analyses were performed using Prism 5 (GraphPad, La Jolla, CA).
Results
Exposure of Airway Epithelial Cells to DA Induces AREG Shedding
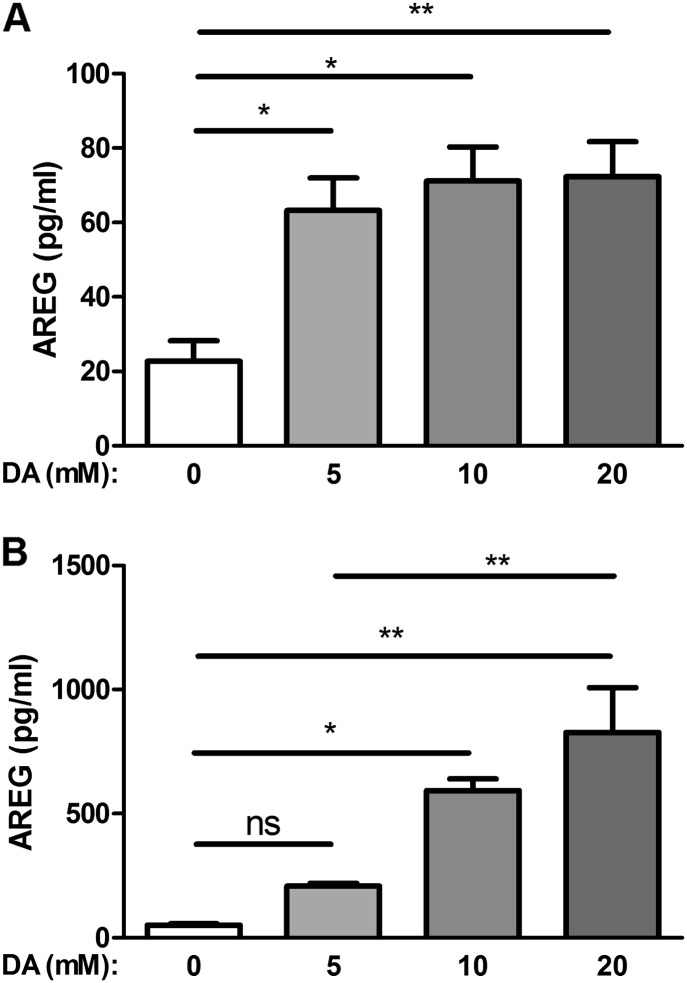
To determine the effect of DA on AREG production by NCI-H292 epithelial cells, we measured AREG protein levels by ELISA in culture supernatants at 6 and 24 hours after a 30-minute exposure to DA (5, 10, or 20 mM). AREG supernatant protein was significantly increased after DA exposure compared with control. AREG supernatant protein levels were similarly increased independent of the DA concentration tested at 6 hours after exposure (Figure 1A). AREG protein levels were significantly increased 2.8-fold (P ≤ 0.01) after 5 mM DA, and 3.1-fold (P ≤ 0.01) after 10 and 20 mM DA, compared with HBSS control. At 24 hours, AREG protein levels increased with increasing DA concentrations (Figure 1B). AREG protein levels were significantly increased compared with HBSS control, 11.7-fold (P ≤ 0.01) and 16.3-fold (P ≤ 0.01) after 10 and 20 mM DA, respectively. In addition, when AREG shedding from a fixed concentration, such as 10 mM DA, was evaluated at 6 and 24 hours, we observed a time-dependent increase with greater AREG protein levels at 24 hours as compared with 6 hours after both 10 mM (P ≤ 0.01) and 20 mM (P ≤ 0.01) (see Figure E2 in the online supplement).
Figure 1.
Diacetyl (DA) induces amphiregulin (AREG) shedding in human epithelial NCI-H292 cells. Cells were exposed to increasing concentrations of DA for 30 minutes and supernatants were assessed at 6 hours (A) or 24 hours (B). Increased AREG shedding was detected at both 6 and 24 hours. Data shown are averaged across three independent experiments. Data presented are mean (± SD), where DA-exposed groups were compared with Hanks’ balanced salt solution (HBSS) control, by one-way ANOVA with Bonferroni’s correction. **P < 0.01, *P < 0.05. ns, nonsignificant.
Increased AREG Shedding after DA Exposure by Human Pulmonary Epithelial Cells Occurs in a TACE-Dependent Manner
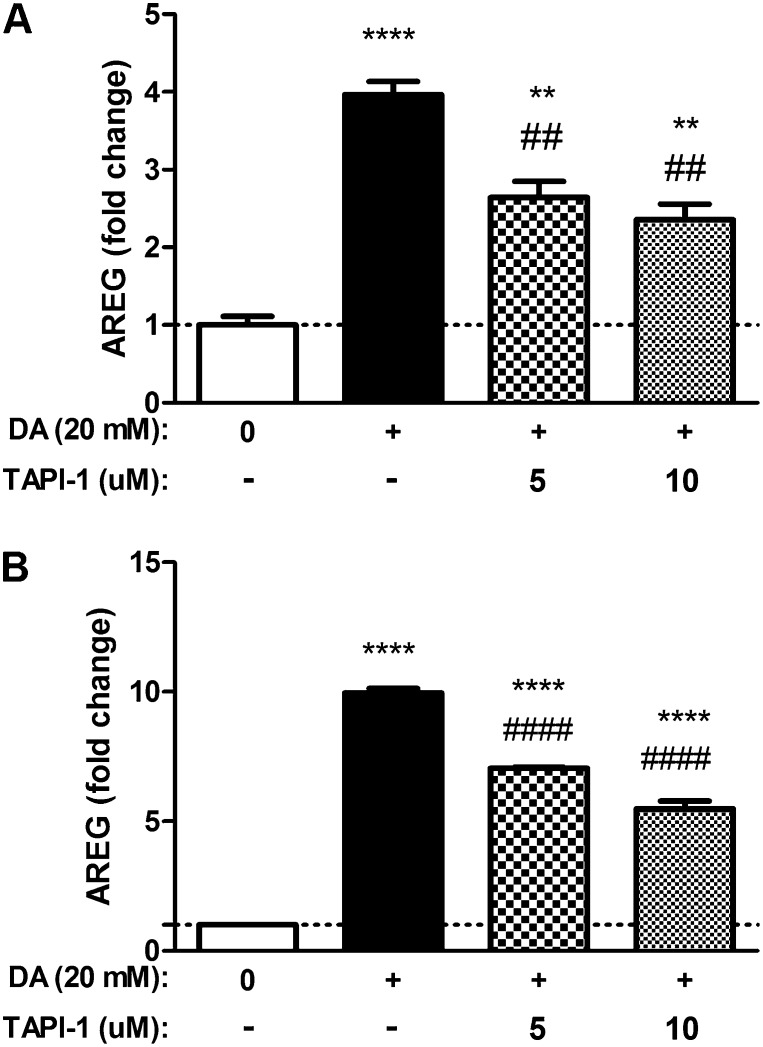
To evaluate the role of TACE in AREG shedding by NCI-H292 cells after DA exposure, we measured DA-induced AREG protein levels in the presence of the TACE inhibitor, TAPI-1. NCI-H292 cells were treated with TAPI-1 immediately after 20-mM DA exposure, and continued for the duration of the postexposure period. Compared with DA exposure alone, AREG shedding was diminished by 30% (P ≤ 0.01) at 6 hours with 10 μM TAPI (Figure 2A). Similarly, 24 hours after DA exposure AREG, shedding was reduced by 50% (P ≤ 0.0001) with 10 μM TAPI (Figure 2B).
Figure 2.
DA-induced AREG shedding by NCI-H292 epithelial cells is attenuated by TNF-α protease inhibitor (TAPI)-1. Cells were exposed to 20 mM DA for 30 minutes, followed by TAPI-1 treatment (5 or 10 μM) for 6 or 24 hours. AREG shedding was measured at 6 hours (A) and 24 hours (B). Treatment with TAPI-1 reduced AREG shedding at both 6 and 24 hours. Data shown are representative results (n = 3), reproduced over three independent experiments. Data presented are mean (± SD) from a single representative experiment, where DA-exposed groups were compared with HBSS control (*) or TAPI-1–treated DA-exposed samples were compared with DA treated alone (#), by nonparametric t test. ****,####P < 0.0001, **,##P < 0.01.
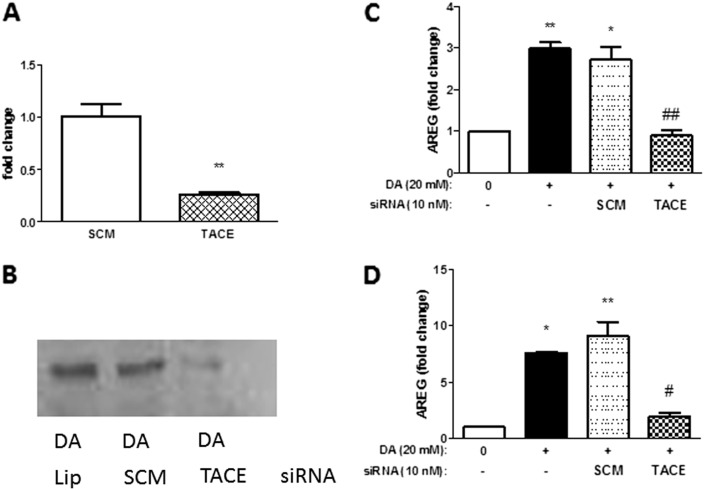
To further confirm a role for TACE mediating the effects of DA on AREG shedding, we selectively depleted TACE transcript and protein using TACE-specific siRNA, (Figures 3A and 3B). Supernatant AREG protein was measured 40 hours after siRNA transfection.
Figure 3.
DA-induced AREG shedding by airway epithelial cells is mediated by TNF-α–converting enzyme (TACE). DA-increased AREG shedding by human NCI-H292 epithelial cells is blocked by treatment with TACE small inhibitor (si)RNA. Cells were transfected with siRNA containing a nonspecific scramble sequence (SCM) or a TACE-specific sequence (TACE). Cells transfected with lipofectamine only (Lip) served as a vector control. Knockdown of TACE with TACE-specific siRNA reduced TACE transcript (A) and protein (B) levels at 40 hours. Transfected cells were treated with DA for 30 minutes, and AREG shedding was measured by ELISA in culture medium at 6 hours (C) or 24 hours (D) after exposure. In cells with attenuated levels of TACE, AREG shedding was reduced at both time points. Data presented are mean (± SD), where DA-exposed groups were compared with HBSS control (*) or TACE siRNA–treated DA-exposed samples were compared with DA treated alone (#) by nonparametric t test. *,#P < 0.05, **P < 0.01, ##P < 0.01.
AREG shedding was significantly attenuated in cells with decreased TACE expression after exposure to 20 mM DA. AREG shedding was significantly decreased by 71% (P ≤ 0.01) and by 75% (P ≤ 0.05) at 6 and 24 hours, respectively (Figures 3C and 3D). Control siRNA did not affect TACE transcript expression, protein expression, or AREG protein levels after DA exposure.
DA Exposure Increases AREG Shedding by Polarized Primary Airway Epithelial Cells
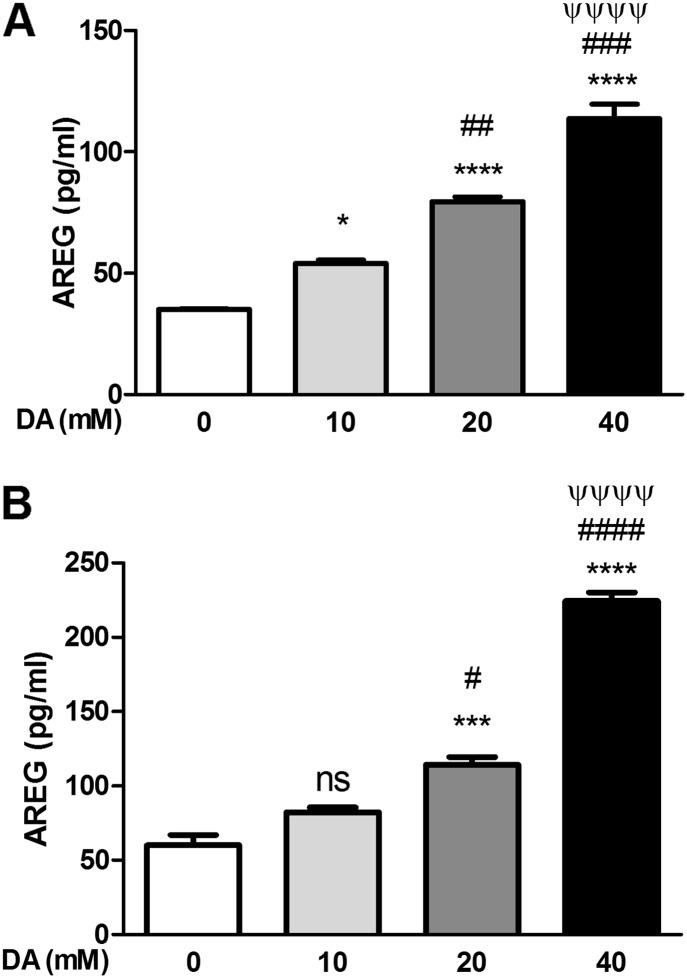
To evaluate the effects of DA on AREG shedding in a physiologically relevant ALI cell culture model, we exposed the apical side of primary differentiated NHBE cells with DA ranging from 10 to 40 mM for 30 minutes. Basolateral AREG shedding was significantly increased 6 hours after exposure (Figure 4A). Compared with HBSS control, AREG protein levels were significantly increased 1.5-fold (P ≤ 0.05), 2.3-fold (P ≤ 0.0001), and 3.2-fold (P ≤ 0.0001), after 10, 20, and 40 mM DA exposure, respectively. Similarly, 24 hours after DA exposure, basolateral AREG protein levels were significantly increased 1.3-fold (P = nonsignificant [ns]) and 3.7-fold (P ≤ 0.0001) compared with HBSS control after 10 and 40 mM DA exposure, respectively (Figure 4B). No differences in AREG shedding from the apical compartment were observed at 6 hours, whereas minimal increases were observed at 24 hours (Figures E3A and E3B, respectively).
Figure 4.
DA induces AREG shedding by primary normal human bronchial epithelial (NHBE) cells. Cells were exposed apically with increasing concentrations of DA for 30 minutes. AREG protein levels were measured in basolateral culture medium by ELISA at 6 hours (A) or 24 hours (B). Increased AREG shedding was detected at both 6 and 24 hours. Data shown are representative results (n = 3), reproduced over three independent experiments, with similar results reproduced across multiple donors (Figure E1). Data presented are mean (± SD), where DA-exposed groups were compared with HBSS control (*), the 5-mM DA group was compared with 10 and 20 mM DA (#), and 10 mM compared with 20 mM DA (ψ), using one-way ANOVA with Bonferroni’s correction ****,####,ψψψψP < 0.0001, ***,###P < 0.001, ##P < 0.01, *,#P < 0.05. ns, nonsignificant.
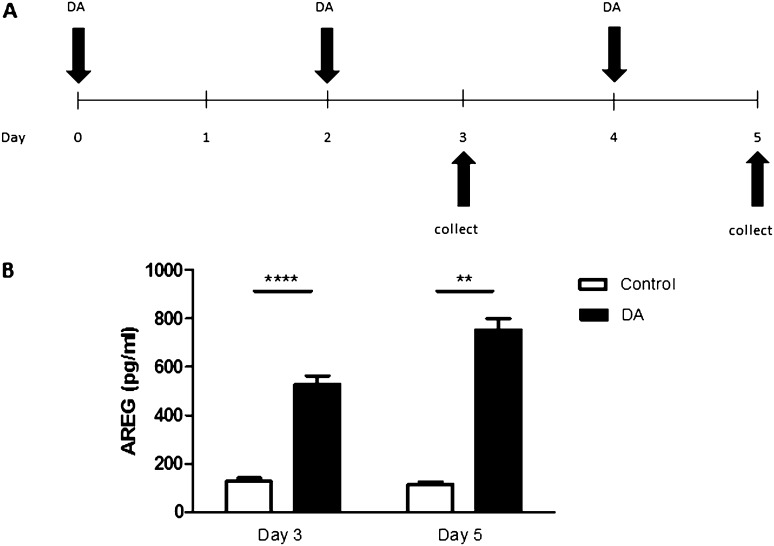
DA Vapor Increases AREG Shedding by Primary Polarized Epithelial Cells
To determine if the increase in AREG shedding after aqueous DA exposure is similar to DA vapor exposure, we exposed human EpiAirway tissues to DA vapors using vapor cups. Based on prior NHBE cell results, we only assessed AREG shedding in the basolateral compartment. Compared with control, DA vapor exposure resulted in a similar increase in basolateral AREG shedding by human EpiAirway tissue, which was significantly increased 7.1-fold (P ≤ 0.0001) at Day 3 and 7.6-fold (P ≤ 0.001) at Day 5 (Figure 5B).
Figure 5.
DA vapor exposure induces AREG shedding by primary human airway epithelial cells. (A) DA vapor exposure model: EpiAirway tissuesTM, grown at air–liquid interface (ALI), were washed and exposed to DA for 1 hour on Days 0, 2, and 4. Basolateral supernatants were collected and analyzed for AREG on Days 3 and 5. (B) DA vapor induced AREG shedding by primary human airway epithelial cells. Basolateral AREG shedding was measured by ELISA in culture medium 24 hours after the second (Day 3) or third (Day 5) exposures. Data presented are mean (± SD) for two control or three DA-treated tissue inserts derived from a single donor assayed in duplicate. ****P < 0.0001, **P < 0.01, by nonparametric t test.
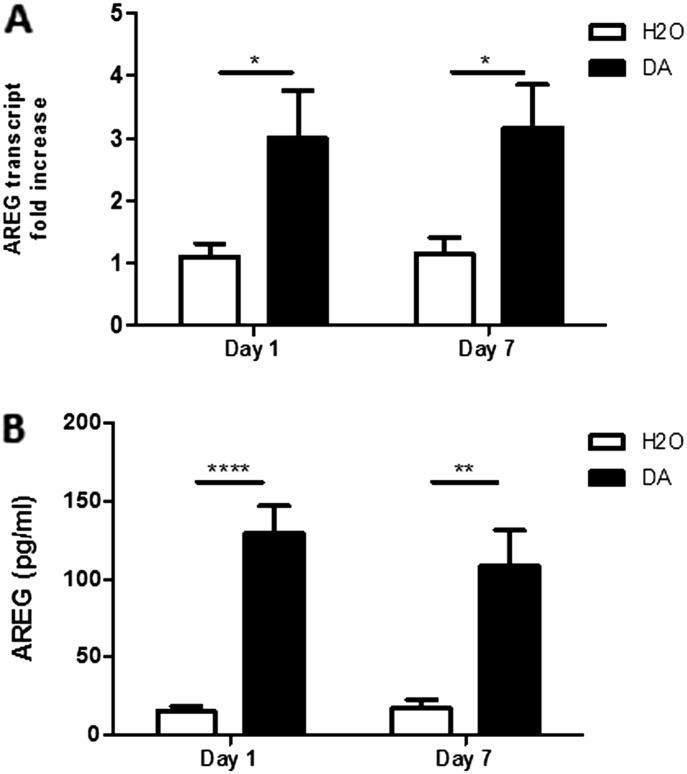
Rats Treated with DA that Develop BO Have Increased Whole-Lung AREG Transcript and BALF Protein Levels
To evaluate the in vivo relevance of our in vitro results, we assessed AREG in our established rat model, in which intratracheal instillation of DA resulted in the development of BO by Day 7. AREG transcript was significantly increased 2.9-fold (P ≤ 0.05) compared with control-treated rodents at Day 1, and remained elevated at Day 7 (P ≤ 0.05) (Figure 6A). In addition, 1 day after DA exposure, AREG BALF protein levels were 8.5-fold greater (P ≤ 0.0001) compared with control-treated rats. Similar to AREG transcript, AREG protein remained elevated at Day 7 (P ≤ 0.01; Figure 6B).
Figure 6.
AREG transcript and protein levels are increased in vivo after DA exposure. Whole-lung AREG transcripts (A) and AREG protein in bronchoalveolar lavage fluid (B) were determined at Days 1 and 7 in rats after exposure to vehicle (n = 6) or DA (n = 8). Data presented are mean (± SD). ****P < 0.0001, **P < 0.01, *P < 0.05, by nonparametric t test.
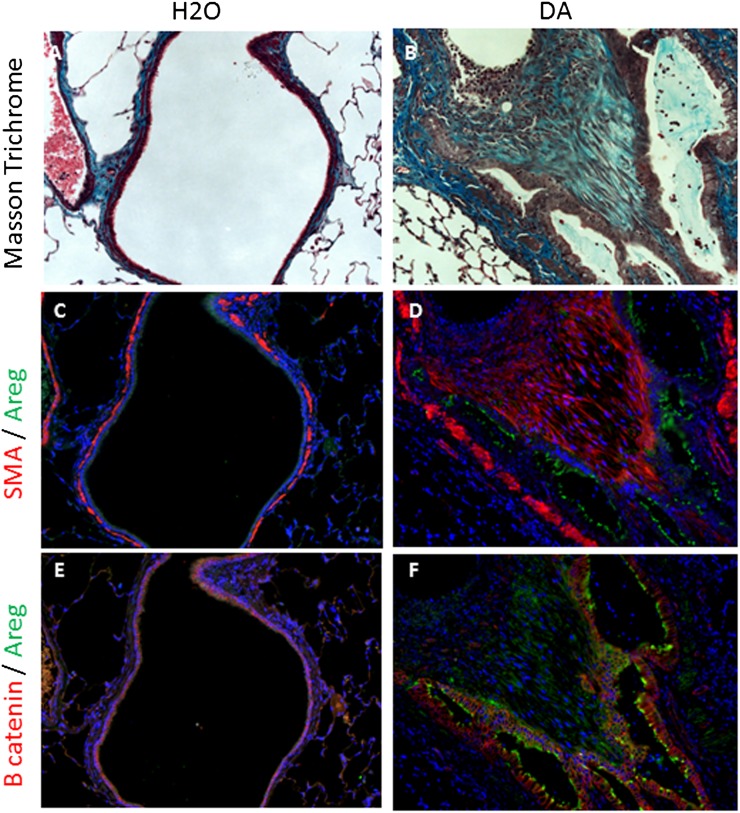
Epithelial AREG Protein Is Increased in Airways with DA-Induced Fibrosis
To further evaluate the expression of AREG in vivo, representative control and affected airways were identified by Masson trichrome in rodents treated with water or DA, respectively. These airways were further evaluated for AREG, SMA, and β-catenin expression by immunofluorescence. In contrast to control airways, fibrotic airways after DA exposure had increased peribronchial SMA as well as a predominant epithelial AREG immunodetection (Figures 7C and 7D). Epithelial β-catenin immunodetection was similar in control and fibrotic airways, but illustrated the presence of epithelial cells, which stain positive for AREG in the context of DA-induced fibrosis (Figures 7E and 7F). Similar to the large airways shown in Figure 7, smaller airways with DA-induced fibrosis also had increased AREG and SMA immunodetection compared with control airways (Figures E4C–E4F).
Figure 7.
Large airway epithelial AREG protein is overexpressed in DA-induced bronchiolitis obliterans (BO). Rodent tissue was assessed for collagen using Masson trichrome staining (A and B), AREG (green) (C–F), smooth muscle actin (SMA; red) (C and D), and β-catenin (red) (E and F), and nuclear counterstained with 4′,6-diamidino-2-phenylindole (blue) 7 days after DA exposure. Control airways (A, C, and E) illustrate minimal collagen, AREG, and SMA expression and junctional β-catenin within the airway regions. Fibrotic airways from a DA-treated rodent (B, D, and F) express increased collagen, AREG, and SMA expression within the airway epithelium. Magnification of all images is 200×.
Discussion
DA is now recognized as a pulmonary toxin causing BO (5); however, the cellular mechanisms that underlie the development of this condition are unknown. Because of the importance of AREG in various models of epithelial airway injury and lung fibrosis (10–13, 16), we hypothesized that DA would induce epithelial AREG shedding in vitro, and that DA-induced BO would be associated with AREG elevations in the lung in vivo. Consistent with this hypothesis, we make the novel observation that DA exposure results in increased AREG shedding from pulmonary epithelial cells, mediated by a TACE-dependent mechanism. We further demonstrate that DA exposure, by direct contact or vapor, results in significant AREG shedding from primary human epithelial cells grown under physiological ALI conditions. Finally, we validate the in vivo relevance of these in vitro results by demonstrating that rodents exposed to DA have increased lung tissue AREG transcript as well as increased BALF AREG protein shortly after DA instillation, and that expression is predominately localized to the airway epithelium of fibrotic airways in vitro.
There is increasing recognition that pulmonary shedding of AREG occurs after both acute and chronic injury. Recent in vitro studies have demonstrated increased AREG shedding from pulmonary epithelial cells after exposure to CSE, and particulate matter (PM), including diesel exhaust (10–12), in the acute setting similar to our in vitro results. Increased pulmonary AREG levels have also been reported in animal models of acute and chronic fibrotic lung disease, including transforming growth factor–β transgenic models (16) and bleomycin-induced lung fibrosis similar to our in vivo results (14). In addition, selective injury to the epithelial Clara cells through naphthalene or transgenic manipulation also led to an up-regulation of AREG transcript after epithelial cell injury (13). Our results reinforce that AREG shedding may be a common signal of epithelial injury across a variety of pulmonary insults. Its precise role in lung injury and repair remains uncertain and warrants further study.
Furthermore, we demonstrate that epithelial AREG shedding after DA occurs through a TACE-dependent mechanism, similar to the mechanism implicated with exposure to CSE or PM (11, 17). In the case of CSE or PM, further studies suggest that TACE activation can occur through reactive oxygen species (ROS) generation. For example, after exposure to CSE, reduced nicotinamide adenine dinucleotide phosphate oxidase ROS generation results in protein kinase C (PKC)-mediated TACE activation (17). Although studies with PM exposure did not directly demonstrate TACE activation, ROS was implicated in AREG shedding (12). Although we did not consider the potential role of ROS, our results clearly demonstrate that TACE is required for the generation of DA-induced AREG by pulmonary epithelial cells, using two independent approaches of TACE inhibition.
The functional significance of AREG shedding by airway epithelium is poorly understood. In vitro studies using CSE suggest that TACE-dependent AREG production results in EGFR activation responsible for cellular hyperproliferation, potentially contributing to cell transformation–induced lung cancer (17). PM exposures resulting in AREG shedding have been shown to increase granulocyte/macrophage colony–stimulating factor (12) and muc5AC transcript and protein (11). Other studies in the lung implicate AREG shedding in epithelial IL-8 production, a mediator of neutrophilic airway inflammation (18). Interestingly, neutrophilic inflammation is observed both in experimental DA-induced BO in rodents (5) and human studies of DA-exposed factory workers (19). Although we only observe an association in this current study, further studies are needed to determine if AREG shedding in response to DA injury contributes to the development of BO-related airway fibrosis.
Previous in vivo studies of AREG in fibrotic lung diseases have yielded conflicting results. In the case of bleomycin-induced lung fibrosis, exogenous AREG ameliorated fibrosis (14), whereas, in the case of transforming growth factor-β–induced lung fibrosis, inhibition of AREG through siRNA or small molecule inhibitors reduced fibrosis (16). Furthermore, EGFR inhibition significantly reduced bleomycin-induced fibrosis (20). These opposing results might reflect the complexity of EGFR signaling involved in different cells types, including pulmonary epithelium and fibroblasts, as well as inherent differences in the mechanisms of fibrosis in different model systems. Consistent with this point, our AREG staining in vivo did show a predominant apical expression, similar to our BALF data, but also indicated subepithelial expression, as well as within SMA-positive areas, as seen in the larger airways. Further study of the in vivo importance of AREG and EGFR signaling in the context of our BO model should help to better elucidate its role and function in fibrotic lung disease (16).
Our study has several limitations. First, most of the experiments involved direct exposures of DA to epithelial cells, rather than the vapor exposures that would represent physiological exposures similar to those encountered by workers in the occupational setting. However, to address this point, we performed experiments with a physiological exposure method, using DA as a vapor, and demonstrate results very similar to those obtained with direct contact. Second, we note that different DA doses were used in the different in vitro model systems. In these experiments, however, our intent was to demonstrate the robustness of the findings across multiple model systems rather than the specific effects of single dose, because effects can vary across the different model systems. In addition, the selection of dosing used in these experiments was consistent with previously published in vitro studies of DA, and in the case of our vapor studies, are comparable to levels of exposure reported in popcorn factory mixing vat workers (2, 21–23). Furthermore, we targeted a dose of DA below that at which cell injury or necrosis occurs, as reflected by LDH release. Third, although we demonstrate that DA induces AREG shedding in human pulmonary epithelial cells using three different cell types, including two independent primary polarized cell culture models, we pursued mechanistic studies of TACE inhibition only in the NCI-292 cell line, which provided a robust model system in which to pursue dedicated mechanistic studies, similar to that done in other injury models (10, 11).
In summary, our results are the first to demonstrate DA-induced AREG shedding in vitro in human pulmonary epithelial cells and in vivo in the context of an established rodent BO model. The TACE-dependent mechanism of AREG shedding in our studies suggests that further studies of TACE-dependent EGFR ligands, such as transforming growth factor-α and heparin-binding EGF-like growth factor are warranted in the context of DA injury. Although the biological significance of AREG after DA exposure remains unclear, previous studies implicate AREG in airway repair, lung inflammation, and fibroblast activation, suggesting that it could actively contribute to the development of BO lesions. Thus, further study is needed to determine if inhibition of AREG shedding through TACE inhibition, or more broadly, EGFR signaling, could offer protection against the development of DA-induced BO.
Footnotes
This work was supported by the Roche Organs Transplant Foundation and the Lung Transplant Foundation, by National Institutes of Health grant R01 ES016836 (J.A.V.), and by the Alpha 1 Foundation (B.M.F.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0339OC on May 9, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 2.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 3.Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharmacol. 2002;185:128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- 4.Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- 5.Palmer SM, Flake GP, Kelly FL, Zhang HL, Nugent JL, Kirby PJ, Foley JF, Gwinn WM, Morgan DL. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS ONE. 2011;6:e17644. doi: 10.1371/journal.pone.0017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 7.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor–related protein with tumor-inhibitory activity. Mol Cell Biol. 1990;10:1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DC, Sunnarborg SW, Hinkle CL, Myers TJ, Stevenson MY, Russell WE, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, et al. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann N Y Acad Sci. 2003;995:22–38. doi: 10.1111/j.1749-6632.2003.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 9.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke–induced lung cell proliferation mediated by tumor necrosis factor alpha–converting enzyme and amphiregulin. J Biol Chem. 2003;278:26202–26207. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 11.Val S, Belade E, George I, Boczkowski J, Baeza-Squiban A. Fine PM induce airway MUC5AC expression through the autocrine effect of amphiregulin. Arch Toxicol. 2012;86:1851–1859. doi: 10.1007/s00204-012-0903-6. [DOI] [PubMed] [Google Scholar]
- 12.Blanchet S, Ramgolam K, Baulig A, Marano F, Baeza-Squiban A. Fine particulate matter induces amphiregulin secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2004;30:421–427. doi: 10.1165/rcmb.2003-0281RC. [DOI] [PubMed] [Google Scholar]
- 13.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40:633–642. doi: 10.1165/rcmb.2008-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukumoto J, Harada C, Kawaguchi T, Suetsugu S, Maeyama T, Inoshima I, Hamada N, Kuwano K, Nakanishi Y. Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L131–L138. doi: 10.1152/ajplung.90576.2008. [DOI] [PubMed] [Google Scholar]
- 15.Fischer BM, Wong JK, Degan S, Kummarapurugu AB, Zheng S, Haridass P, Voynow JA. Increased expression of senescence markers in cystic fibrosis airways. Am J Physiol Lung Cell Mol Physiol. 2013;304:L394–L400. doi: 10.1152/ajplung.00091.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Lee JY, Lee CM, Cho WK, Kang MJ, Koff JL, Yoon PO, Chae J, Park HO, Elias JA, et al. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-β–induced pulmonary fibrosis. J Biol Chem. 2012;287:41991–42000. doi: 10.1074/jbc.M112.356824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemjabbar-Alaoui H, Sidhu SS, Mengistab A, Gallup M, Basbaum C. TACE/ADAM-17 phosphorylation by PKC-epsilon mediates premalignant changes in tobacco smoke–exposed lung cells. PLoS ONE. 2011;6:e17489. doi: 10.1371/journal.pone.0017489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao D, Zhan Y, Koon HW, Zeng H, Keates S, Moyer MP, Pothoulakis C. Metalloproteinase-dependent transforming growth factor-alpha release mediates neurotensin-stimulated MAP kinase activation in human colonic epithelial cells. J Biol Chem. 2004;279:43547–43554. doi: 10.1074/jbc.M401453200. [DOI] [PubMed] [Google Scholar]
- 19.Akpinar-Elci M, Stemple KJ, Enright PL, Fahy JV, Bledsoe TA, Kreiss K, Weissman DN. Induced sputum evaluation in microwave popcorn production workers. Chest. 2005;128:991–997. doi: 10.1378/chest.128.2.991. [DOI] [PubMed] [Google Scholar]
- 20.Ishii Y, Fujimoto S, Fukuda T. Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am J Respir Crit Care Med. 2006;174:550–556. doi: 10.1164/rccm.200509-1534OC. [DOI] [PubMed] [Google Scholar]
- 21.Fedan JS, Dowdy JA, Fedan KB, Hubbs AF. Popcorn worker’s lung: in vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol Appl Pharmacol. 2006;215:17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.More SS, Vartak AP, Vince R. The butter flavorant, diacetyl, exacerbates β-amyloid cytotoxicity. Chem Res Toxicol. 2012;25:2083–2091. doi: 10.1021/tx3001016. [DOI] [PubMed] [Google Scholar]
- 23.Whittaker P, Clarke JJ, San RH, Begley TH, Dunkel VC. Evaluation of the butter flavoring chemical diacetyl and a fluorochemical paper additive for mutagenicity and toxicity using the mammalian cell gene mutation assay in L5178Y mouse lymphoma cells. Food Chem Toxicol. 2008;46:2928–2933. doi: 10.1016/j.fct.2008.06.001. [DOI] [PubMed] [Google Scholar]