Abstract
Mucociliary clearance, characterized by mucus secretion and its conveyance by ciliary action, is a fundamental physiological process that plays an important role in host defense. Although it is known that ciliary activity changes with chemical and mechanical stimuli, the autoregulatory mechanisms that govern ciliary activity and mucus transport in response to normal and pathophysiological variations in mucus are not clear. We have developed a high-speed, 1-μm-resolution, cross-sectional imaging modality, termed micro-optical coherence tomography (μOCT), which provides the first integrated view of the functional microanatomy of the epithelial surface. We monitored invasion of the periciliary liquid (PCL) layer by mucus in fully differentiated human bronchial epithelial cultures and full thickness swine trachea using μOCT. We further monitored mucociliary transport (MCT) and intracellular calcium concentration simultaneously during invasion of the PCL layer by mucus using colocalized μOCT and confocal fluorescence microscopy in cell cultures. Ciliary beating and mucus transport are up-regulated via a calcium-dependent pathway when mucus causes a reduction in the PCL layer and cilia height. When the load exceeds a physiological limit of approximately 2 μm, this gravity-independent autoregulatory mechanism can no longer compensate, resulting in diminished ciliary motion and abrogation of stimulated MCT. A fundamental integrated mechanism with specific operating limits governs MCT in the lung and fails when periciliary layer compression and mucus viscosity exceeds normal physiologic limits.
Keywords: cilia, micro-optical coherence tomography, intracellular calcium, mucociliary transport
Clinical Relevance
Autoregulation of ciliary activities is a fundamental mechanism governing ciliary activity, but its role in governing mucociliary clearance (MCC) in respiratory tract is unknown. Ciliary autoregulation effectively responds to minimal perturbations of the periciliary layer induced by small changes in overlying mucus, but fails when mucus load is excessive, as can occur in human diseases that affect MCC.
Mucociliary clearance (MCC) of the lung requires coordinated interaction of three elements: motile cilia that provide the primary driving force; a well maintained periciliary liquid (PCL) layer, which is a low-density mucus gel layer that allows cilia to beat efficiently; and a more viscous mucus layer that traps inhaled particles (1). Because mucus density and the amount of mucus secretion vary with an array of stimuli, including chemical irritants, pathogens, allergens, and micro-organisms, effective MCC requires ciliary motion, and consequently the mucociliary transport (MCT) rate, to be autoregulated in response to changes in mucus load.
Previous research supports the notion of an autoregulatory mechanism governing flagella and ciliary motion in unicellular organisms (2–6) and vertebrates (7–14), respectively, which is triggered by physical contact. It has recently been discovered that airway epithelia can sense changes in mucus load by an interaction between cilia and the overlying mucus layer, and respond by regulating extracellular ATP release rates (15). However, the mechanism by which ciliary motion is regulated by physiologically relevant mucus loads under a range of normal and pathologic conditions encountered on the mucosal surface as mucus transport occurs is not well understood. Given the importance of MCT in health, and the implications of its acute and chronic failure in conditions related to ciliated epithelia (e.g., cystic fibrosis, chronic obstructive pulmonary disease, primary ciliary dyskinesia), understanding this pathway could lead to new strategies to bolster MCT activity and subvert the inability to respond to excessive mucus loading, as occurs in common and serious medical conditions.
Study of the interactions between these elements of MCC requires simultaneous and dynamic measurement of subtle, natural variations in MCT, ciliary function, including ciliary beat frequency (CBF), and PCL layer height in native, untreated epithelia. Currently available methods for investigation of MCC are inadequate for this purpose, no single technique is capable of performing all of these measurements, they typically use exogenous or nonphysiologic perturbations, many can only be applied to cell preparations, and key measurement techniques lack the potential to be used in intact tissue or in vivo (16). The detailed morphology of mucus-covered cilia and the PCL layer are particularly difficult to study dynamically; most of our knowledge of these structures is derived from electron microscopy after perfluorocarbon/osmium fixation (16–18), which is a static and destructive technique.
Optical coherence tomography (OCT) is well suited to monitoring functional anatomy of airway mucosa (19, 20). However, most OCT systems do not possess sufficient resolution to fully resolve cilia and PCL layers in normal and diseased airway epithelia. We developed a new generation of OCT, termed micro-OCT (μOCT), that provides simultaneous cross-sectional anatomical imaging and functional live motion capture of the entire MCC apparatus at a depth resolution of 1 μm (21, 22). μOCT captures several key parameters governing the function of the airway surface (airway surface liquid [ASL] depth, PCL depth, ciliary function, including beat frequency, and MCT rate) from the same series of images and without exogenous particles or labels, enabling noninvasive study of dynamic phenomena (22). In addition, the high resolution of μOCT reveals distinguishable phases of the ciliary stroke pattern, which enables us to quantify ciliary height and response to mucus load (22). In this article, we present cross-sectional μOCT images and refer to videos (see the online supplement) of MCC, and show, in detail, the interactions of the epithelial surface, PCL layer, cilia, and mucus of primary human bronchial epithelial (HBE) culture monolayers and intact, full-thickness respiratory mucosa. Analysis of these functional microanatomic data has now established that the epithelial surface dynamically responds to mucus load through a calcium-dependent pathway by altering ciliary motion, and is highly sensitive to the viscosity of the overlying mucus. This feedback-control system is operative at a specific range of mucus loading conditions, and helps explain how mucus transport in the lung is maintained during physiologically relevant insults and how it might fail in pathologic conditions.
Materials and Methods
Primary Airway Epithelial Cells and Swine Tissue Specimens
Information on the methods for procurement and growth of primary airway epithelial cells, and procurement of swine tissue specimens, is provided in the online supplement.
μOCT Measurements of ASL and PCL Depth, CBF, and MCT
Information on the μOCT technology and image acquisition and processing is provided in the online supplement. The methods used to measure ASL, PCL, MCT, and CBF from the μOCT data have been described previously (22). We confirmed that accuracy of quantitative measures were not affected by the presence of overlying mucus by demonstrating that the size of the Transwell filter support measured by μOCT was not significantly affected by the presence of mucus with increased refractive index (Figure E2 in the online supplement). Because it is known that neither endogenous nor synthetic mucus penetrate the periciliary layer when applied to the luminal surface (15, 18, 23), and we also observed that mucus does not penetrate between motile cilia to any significant extent, in these studies we equated PCL height change to ciliary height change.
Monitoring Invasion of PCL by Mucus
Information on the methods for monitoring invasion of PCL in HBE cultures by exogenous and endogenous mucus and swine tissues is provided in the online supplement.
Synthetic Mucus and Experiments Using Mucus Standards
Information on the methods for synthetic mucus preparation and experiments using mucus standards is provided in the online supplement.
Colocalized μOCT and Confocal Fluorescence Measurements
Information on the method for the construction of colocalized μOCT and confocal fluorescence system is provided in the online supplement. Well differentiated primary HBE cultures were stained with 4 μM Calcium Crimson (C-3018; Molecular Probes, Inc., Eugene, OR) in Ca2+-free Kreb-Ringer Bicarbonate Buffer (K4002; Sigma-Aldrich, Inc., St. Louis, MO) on the apical surface of the cells after being washed with DTT (10 mM) three times. The dispersing agent Pluronic F-127 (P3000; Invitrogen, Inc., Grand Island, NY) was used to facilitate cell loading. After a loading time of 30 minutes, cells were washed with PBS for 30 minutes. Intracellular calcium concentration and functional microanatomy of HBE cultures under varying mucus load was monitored simultaneously using the colocalized μOCT confocal fluorescence microscopy system (Figure E1). Spectra of fluorescence emission light were detected using an exposure time of 1 millisecond over the μOCT imaging time of 50–100 seconds. Synchronization between μOCT image acquisition and the fluorescence signal acquisition was done offline using the recorded computer time. Time series spectra were averaged over 1 second, and for each second the total fluorescence intensity was obtained by summing up intensities over the emission band of 572–642 nm. Because we found that fluorescence signal decreased significantly over the time due to photobleaching, we normalized time-lapse fluorescence data obtained during mucus invasion using a photobleaching curve obtained by fitting time-lapse fluorescence data at the basal condition (PBS only).
Statistical Analysis
Data are described using descriptive statistics as the mean (± SEM) unless described otherwise. Student’s t test or ANOVA was performed with GraphPad Prism 5.0 (La Jolla, CA) as appropriate, and an α of 0.05 was considered statistically significant. In experiments where the number of replicates were small, nonparametric statistics (i.e., Mann-Whitney) were used.
Results
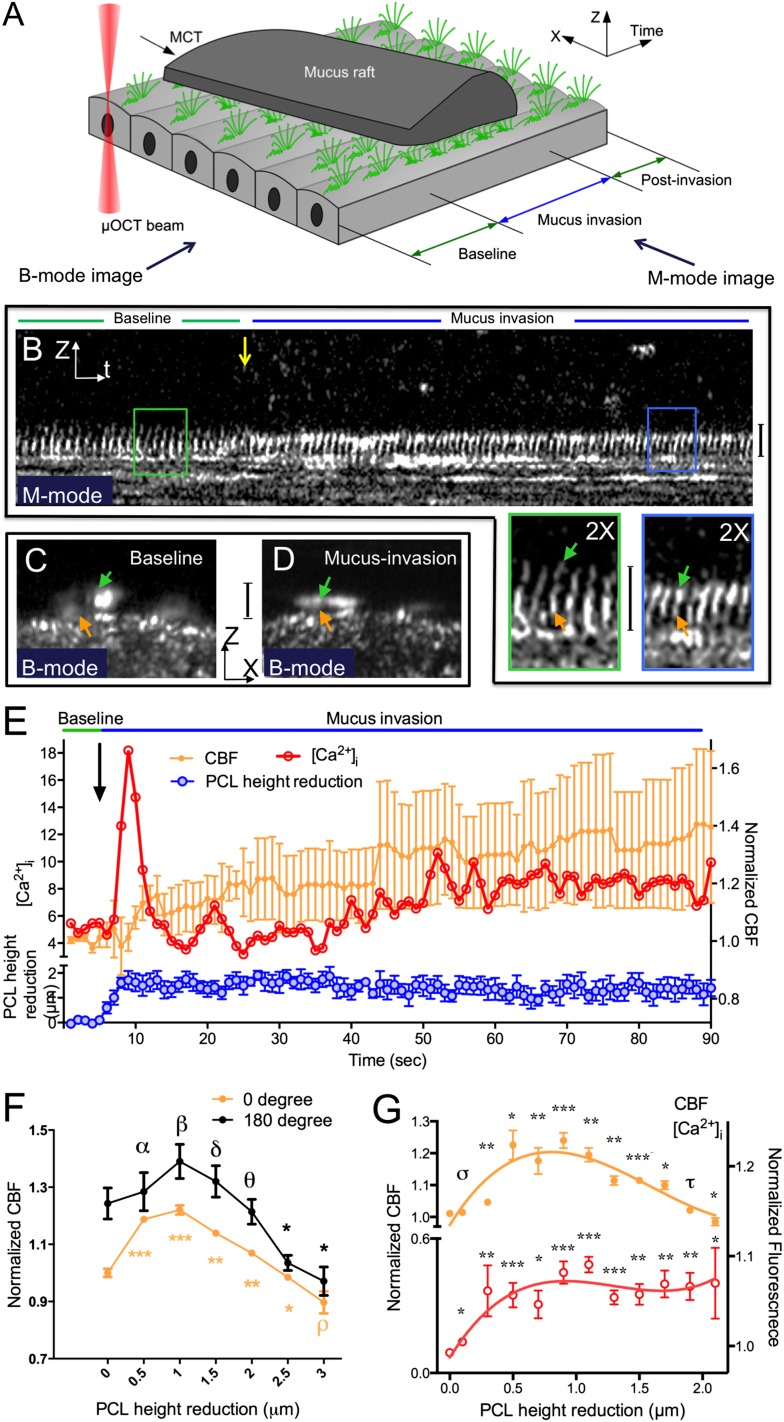
Our studies were intended to evaluate the hypothesis that the airway epithelium dynamically adjusts to mucus loading by altering ciliary beating, and that this can subsequently impact MCT. When droplets of sputum obtained from normal subjects after hypertonic saline induction were applied to normal quiescent HBE cultures distant from the imaging site (schematically depicted in Figure 1A), we found that the normal 7-μm PCL depth and cilia height were reduced immediately (henceforth termed PCL reduction [17, 24]) as the flowing mucus entered the imaging field—an event that we term “mucus invasion” (Figures 1B–1D; see also Movie E1). During these events, the PCL became compressed, but mucus did not interdigitate within the PCL; moreover, cilia tips exhibited minimal (< 100 nm) penetration into the mucus raft. At the location of initial mucus invasion, in response to the increased mucus load and PCL reduction, CBF accelerated immediately (Figures 1B and 1E). CBF was up-regulated proportionally to a reduction in PCL over a range of approximately 0–1 μm (Figure 1F), demonstrating the existence of an autoregulatory mechanism whereby small changes in mucus load reduced PCL/cilia height, which, in turn, induced augmented ciliary beating. The maximum CBF, which occurred at a PCL reduction of approximately 1 μm, was 126 (± 4)% of baseline. When the PCL reduction became greater than 1 μm, the autoregulatory response of CBF decreased monotonically (Figure 1F); when the PCL reduction exceeded 2 μm, the autoregulatory mechanism was no longer able to compensate for the mucus load, and CBF decelerated (Figure 1F). The same relationship was identified in both upright and stably inverted cells (Figure 1F), illustrating that activation of the pathway is not dependent on gravitational force.
Figure 1.
Periciliary liquid (PCL) height reduction by exogenous mucus load increases intracellular calcium concentration ([Ca2+]i) and regulates ciliary beat frequency (CBF) in human bronchial epithelial (HBE) cultures. (A) Three-dimensional (two spatial coordinates: X, Z, and time) schematic of airway cultures challenged with a mucus load. (B) A representative M-mode (Z and time coordinates) image of HBE cultures shows PCL/cilia height reduction and up-regulated CBF in response to exogenous mucus load during mucus invasion (blue line), which is indicated by decreased spacing between ciliary beating cycles over time (insets). Decreased cilia height and increased reflectance intensity of the cilia tips is also observed during mucus invasion. (C and D) B-mode (X and Z coordinates or cross-sectional) images of normal HBE cultures confirm the observations of PCL/cilia height reduction in the corresponding M-mode image shown in (B). Green arrows, effective stroke; orange arrows, recovery stroke; yellow arrow, start point of mucus addition. All vertical calibration bars, 7 μm. (E) Representative correlation between PCL/cilia height reduction, CBF, and [Ca2+]i with respect to time of mucus invasion, as observed in the experiment shown in Movie E1. Baseline CBF = 7.65 Hz. n = 5/time point; data presented are means (± SEM). (F) Summary of CBF as a function of PCL height reduction in upright (0°) and inverted (180°) HBE cultures. Baseline CBF = 10.53 Hz. n > 10/condition; data presented are means (± SEM). (G) Correlation between the normalized CBF and normalized [Ca2+]i with respect to PCL height reduction. The orange and red curves were fit to the CBF and [Ca2+]i data, respectively, to highlight the relationship between PCL and ciliary activity (third-order polynomial). Baseline CBF = 7.65 Hz. n > 10/condition; mean ± SEM. αP = 0.79, βP = 0.26, δP = 0.54, θP = 0.78, ρP = 0.15, σP = 0.54, τP = 0.40, *P < 0.05, **P < 0.005, ***P < 0.0005 versus baseline.
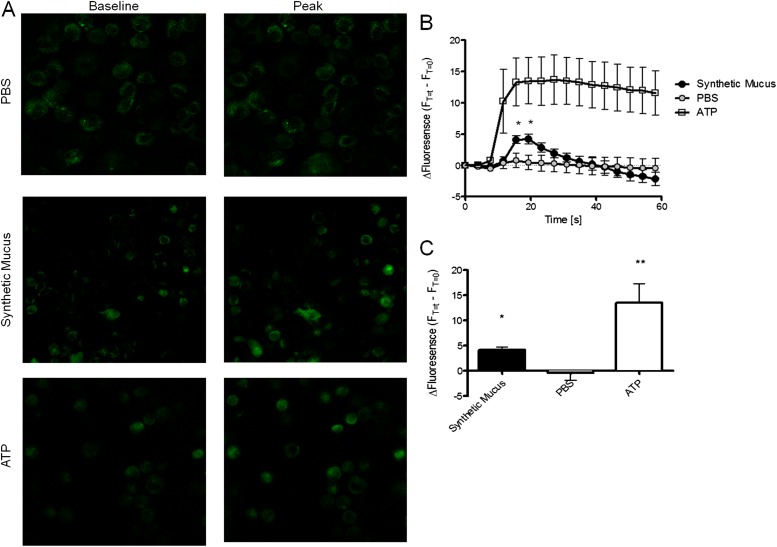
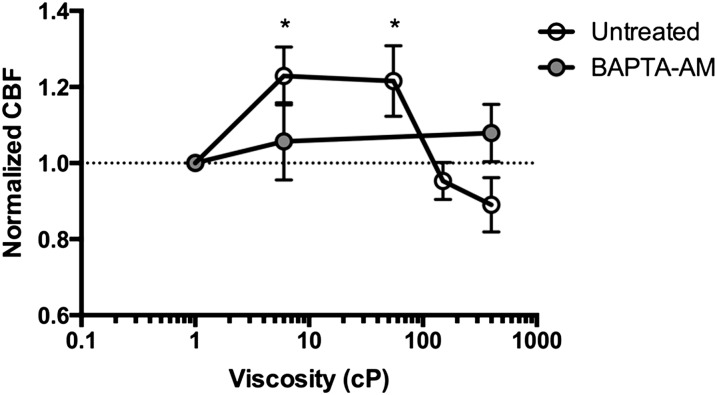
Intracellular calcium is thought to be the primary ionic modulator underlying the regulation of CBF, as demonstrated in studies of ciliary beating response to various mechanical (2, 7, 9, 11–13, 25) and chemical (26) stimuli. To evaluate the hypothesis that the autoregulatory response was mediated by calcium, we simultaneously conducted colocalized μOCT and confocal microscopy using a custom-designed imaging apparatus (Figure E1). HBE cells loaded with Ca2+-sensitive fluorescent dye demonstrated that the autoregulation of CBF in response to mucus-mediated PCL/cilia changes was tightly associated with changes in intracellular Ca2+ (Figures 1E and 1G). An intracellular Ca2+ signal was detected immediately after the onset of mucus invasion, and the CBF closely followed changes in intracellular Ca2+ (Figure 1E). Intracellular Ca2+ was also elevated in HBE cells after addition of synthetic mucus prepared in an aseptic fashion (viscosity 350 centipoise [cP]), confirming that the findings were not due to soluble mediators found in human sputum (Figure 2), and suggesting that elevated viscosity of mucus rafts may contribute to the finding. Like CBF, the intracellular Ca2+ signal increased in proportion to a reduction in PCL height over a range of approximately 0–1 μm (Figure 1G). When PCL reductions were greater than approximately 1 μm, the intracellular Ca2+ signal remained near peak levels, whereas CBF decreased despite the stimulus (Figure 1G), indicating that the autoregulatory mechanism functions at a specific operating range and cannot compensate for the excess mucus load, despite heightened calcium signaling. This finding was also demonstrated experimentally when fluids of known viscosity (i.e., rheology standards) were added to cell monolayers. CBF accelerated with addition of moderate viscosity standards (6 and 50 cP, values frequently encountered in sputum samples from healthy individuals), but fell below baseline when high viscosity standards were added (150 and 400 cP; Figure 3).
Figure 2.
Exogenous mucus stimulates intracellular calcium release in primary HBE cells. (A) Representative images of HBE monolayers loaded with Fluo-4AM to monitor [Ca2+]i and treated apically with 50 μl of PBS, synthetic mucus, and ATP (100 μM). Application of test conditions was made at the far side of the monolayer, and imaging was conducted continuously by confocal microscopy until material reached imaging field. Change in fluorescence over baseline (FT=t − FT=0) is plotted. (B) Mean fluorescent intensity over time for cells treated with synthetic mucus, PBS, and ATP. For each experiment, 3–60 cells were monitored. n = 4/condition. (C) Change in baseline fluorescence intensity at time of peak fluorescence (T = 19.44 s). *P < 0.05, **P < 0.005. Data presented are means (± SEM).
Figure 3.
Mucus viscosity affects CBF. HBE monolayers were treated with four different viscosity standards at 6 centipoises (cPs), 55 cPs, 150 cPs, and 400 cPs, 10 μl on the apical surface. Cells were imaged by micro-optical coherence tomography (μOCT) at baseline and 15 minutes after addition of the standard. CBF measured in untreated cells (open circles), and cells pretreated with 1,2-Bis(2-aminophe1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)noxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) (100 μM, filled circles). *P < 0.05 compared with baseline within condition. Data presented are means (± SEM).
To definitively establish that calcium signaling was responsible for the increase in CBF associated with mucus loading, and to determine whether this was related to mucus viscosity, we monitored the CBF response of HBE cultures in the presence and absence of the intracellular calcium chelator, 1,2-Bis(2-aminophe1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)noxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), when synthetic rheology standards were applied. Inhibition of calcium flux by pretreating airway monolayers with BAPTA-AM completely abrogated the response to CBF (Figure 3). These data confirm the notion that calcium mediates the cellular response to enhanced ciliary beating when events that induce PCL compression (such as that which occurs when the cilia encounter a mucus raft with increased viscosity) are encountered.
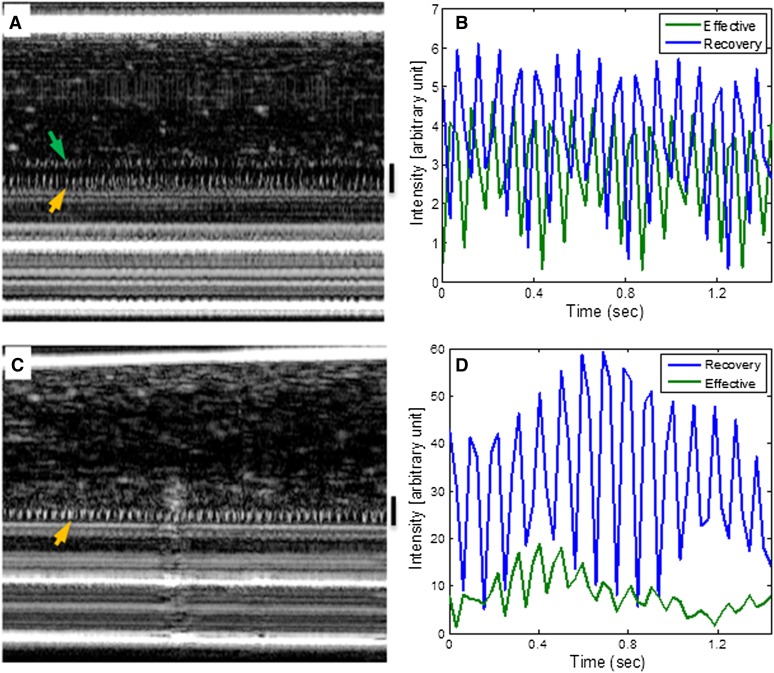
Using μOCT-enabled visualization of ciliary motion in cross-sectional view (22), we next investigated the effects of mucus loading on the ciliary motion pattern under extreme conditions, as could be expected when the epithelium encounters disease states associated with excessive mucus production and consequently mucus invasion of the PCL layer. Despite persistence of maximum calcium signaling when cilia are loaded beyond the 2-μm threshold, we observed that the effective stroke is severely diminished (as seen in the M-mode μOCT images), and the ciliary beat waveforms were severely altered (Figure 4). This observation shows that, in addition to decreased ciliary beat rate, compression (i.e., mucus invasion) of the PCL space by mucus also disrupts ciliary beat pattern, which contributes to the failure of MCT to accelerate in response to mucus loading under these circumstances.
Figure 4.
Effect of mucus load on ciliary motion pattern. (A) Representative M-mode image of HBE culture undergoing minimal ciliary height compression by mucus showing both effective stroke (green arrow) and recovery stroke (orange arrow). (B) Corresponding cilia beat waveform showing the effective stroke was intact. (C) A representative M-mode image of the same HBE culture showing loss of effective stroke when cilia height was compressed to by more than 2 μm. (D) Corresponding cilia beat waveform confirms that the waveforms of the effective stroke was lost.
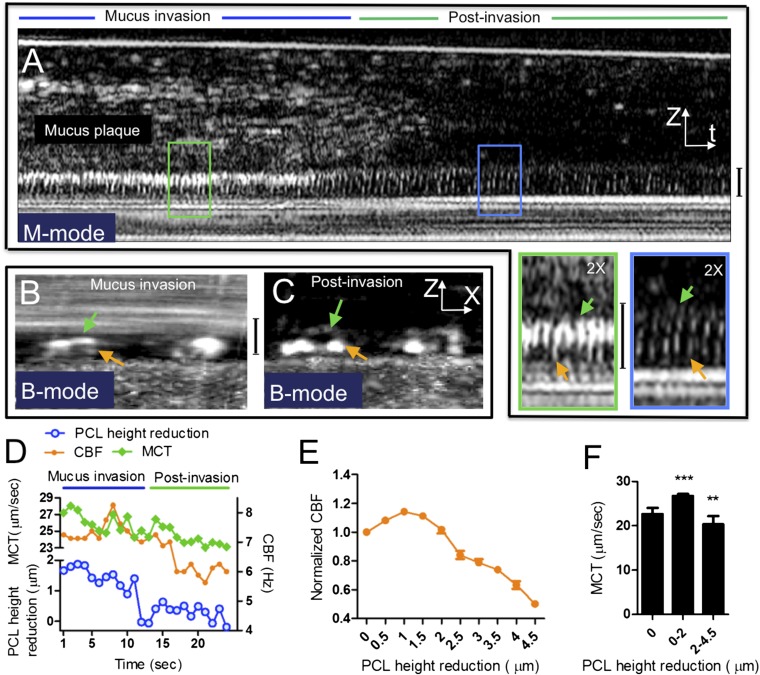
To assure that these findings were biologically relevant, and not artifacts of external mucus or sputum additions, evidence of dynamic PCL reduction was also evaluated in normal HBE cultures transporting endogenous mucus plaques, complementing studies with exogenous sputum addition. We observed that PCL/cilia height was also reduced by endogenous mucus plaques, and by as much as 4.5 μm in some areas (Figures 5A and 5B, and 0–12 seconds in Movie E2); these events were followed by restoration to the normal condition (∼ 7 μm PCL) in a postinvasion phase that occurred after the mucus was transported out of the imaging field (Figures 5A and 5C, and 12–15 s in Movie E2). As with exogenous mucus, the endogenous mucus stimulated a very similar autoregulatory response to exogenous mucus (Figures 5D and 5E); acceleration of MCT rate also followed the changes of CBF (Figures 5D and 5F), reflecting an effective response to mucus loading when the PCL reduction was between 0 and 2 μm; similarly, augmentation of MCT failed when loads exceeded 2 μm (Figures 5E and 5F).
Figure 5.
PCL height reduction by endogenous mucus plaques regulates CBF in HBE cultures. (A) A representative M-mode image of HBE cultures shows PCL/cilia height reduction and up-regulated CBF in response to an endogenous mucus plaque. Compared with the postinvasion condition (green line), CBF was up-regulated during invasion of PCL by a mucus plaque (blue line), indicated by decreased spacing between ciliary beating cycles (insets). Decreased ciliary height and increased reflectance intensity of the ciliary tips is also observed during mucus invasion. (B and C) B-mode images of normal HBE cultures confirm the observations of PCL/cilia height reduction in the corresponding M-mode image shown in Figure 2A. Green arrows, effective stroke; orange arrows, recovery stroke. All vertical calibration bars, 7 μm. (D) Quantitative analysis of the image in Figure 2A shows that CBF and mucociliary transport (MCT) up-regulation is associated with PCL/cilia height reduction with respect to time. (E) Summary of CBF as a function of PCL/cilia height reduction by endogenous mucus plaques. Baseline CBF = 5.98 Hz. (F) Summary of MCT as a function of PCL/cilia height reduction by endogenous mucus plaques. Data presented are means (± SEM). **P < 0.005, ***P < 0.0005 versus baseline.
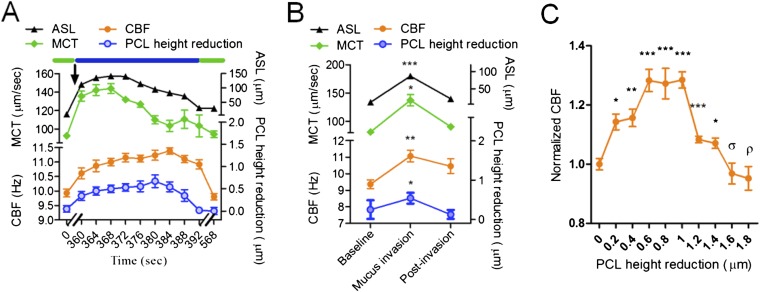
To test whether this autoregulatory mechanism also exists in intact airway tissues, we examined fresh swine tracheal tissues with μOCT under physiologic conditions. Within freshly acquired, full-thickness tracheal segments, we applied small mucus droplets derived from subjects with stable cystic fibrosis to a remote site of the epithelial surface and used μOCT to simultaneously measure the aforementioned parameters throughout the entire process of mucus entry and exit from the imaging field (Movie E3). Changes in PCL reduction, CBF, and MCT that occurred during mucus invasion were nearly proportional to ASL layer height that included the PCL and overlying mucus (mucus load); consequently, CBF and MCT were also up-regulated proportionally (Figure 6A). The autoregulatory mechanism observed in HBE cultures was found in intact respiratory mucosa over a similar operating range (Figure 6B) and failed upon excessive loading (Figure 6C); the average CBF changed proportionally with PCL height reduction in an approximate range of 0–1 μm to a maximum value of 130 (± 2.7)% at baseline (Figure 6C). As observed in HBE, the autoregulatory mechanism failed to compensate for greater mucus loads associated with PCL height reductions; CBF decreased monotonically with a PCL layer/cilia height greater than approximately 1 μm, and dropped below the baseline CBF after the PCL height was reduced by more than approximately 2 μm (Figure 6C). Similarly, stimulated MCT was abrogated when maximal loading occurred (Figure 6B).
Figure 6.
Regulation of mucociliary clearance (MCC) by mucus load in swine trachea ex vivo. (A) A representative autoregulatory process of MCT in response to a mucus load in normal swine trachea. Observations under baseline and postrecovery conditions (green bars) were made a few minutes before and after the mucus invasion (blue bar) to allow equilibriums to be established. Arrow, start point of mucus addition. (B) Summary of airway surface liquid (ASL), PCL height reduction, CBF, and MCT of five invasion experiments; data presented are means (± SEM). Note: both ASL and PCL were measured under the assumption that the refractive index is 1.33. (C) Summary of CBF as a function of PCL height reduction by exogenous mucus shows that CBF increases monotonically as PCL height reduction increases from 0–0.6 μm, maintains a maximum value of approximately 130% over the PCL height reduction range of 0.6–1.0 μm, decreases monotonically by further mucus invasion, and drops to the baseline when the PCL height reduction is over 1.6 μm. Black lines and orange lines represent ASL and CBF cures. Baseline CBF = 9.36 Hz. n = 10/condition; data presented are means (± SEM). ρP = 0.6. σP = 0.4, *P < 0.05, **P < 0.005, ***P < 0.0005 versus baseline.
Discussion
The existence of an autoregulatory mechanism that dynamically responds to mucus loads has been previously postulated, but was not possible to observe without a means for simultaneous and dynamic quantitation of cross-sectional microscopic features of the MCT apparatus in response to exposure to mucus rafts. In these studies, we used μOCT to discern that the airway epithelium dynamically responds to compression of the PCL layer imposed by high-viscosity mucus rafts in a calcium-dependent manner, and that this pathway governs stimulation of CBF and MCT in response to physiologically relevant mucus loads. This mechanism is sensitive to minimal perturbations induced by small changes in overlying mucus, fails when the mucus load is excessive, and is independent of gravitational force.
Previous studies in other model systems have suggested the potential for an autoregulatory mechanism. In unicellular organisms, the ability to adjust ciliary and flagellar activity in response to mechanical stimulation enables collision avoidance and escape from a foreign mechanical threat (2, 27–30). Evidence has accumulated to support a physical mechanism in which motile cilia and flagella are deformed on collision, triggering an avoidance reaction (2, 3) and robust flagellar beating (4–6). Because motile cilia and flagella structure and function are very well conserved across evolution, it seems likely that the regulatory mechanisms that exist in the ciliated epithelium of vertebrates would be similar. In the frog palate, it was found that the placement of endogenous mucus onto a mucus-depleted epithelium produces ciliary activity and restores normal particle transport (31). Studies in mammalian ciliated cells have demonstrated that dimpling the cells with a microprobe results in a transient CBF increase (7–9). Recently, work in mammalian airway epithelial cells has also shown that CBF is stimulated in response to changes in flow velocity and overlying fluid viscosity, complementing the findings presented here (10, 15). These reports provide support for the notion that mechanical stress caused by changes in mucus load and viscosity may stimulate ciliary activity and mucus transport through interactions between mucus and the cilia (7, 9–14, 31, 32).
Research has been conducted to identify the origin of the mechanosensor in ciliated cells that could underlie such a mechanism. Molecular channels that increase intracellular Ca2+ in response to mechanical stimulation have been found in both noncilia cell membrane (33) and cilia/flagella membrane (3, 13). However, localization of the mechanosensor site in the somatic membrane is favored, because the sensor would not be perturbed by the normal activity of the cilium/flagellum in this location, and would be ideally placed to detect mechanical stresses occurring at the ciliary base as the cilium engage overlying mucus (9). A recent study located TRP11 ion channels, a member of transient receptor potential channels, at proximal ends of flagella in Chlamydomonas where active bending is restricted, and showed that suppression of TRP11 expression results in loss of the avoiding reaction, indicating that Chlamydomonas flagella are mechanoreceptive despite constant motility (3). The results of our studies suggest that motile cilia were deformed by endogenous mucus loads, and detection of the deformation triggered Ca2+-dependent autoregulatory responses to accelerate CBF and propel the insulting mucus from the epithelial surface. Stimulation of ATP release by high-viscosity fluids may also impact mucus viscosity and consequently ciliary beating by stimulating purinergic-sensitive chloride channels, thus serving as a key second messenger (15). Further studies to isolate the specific cell types responsible for signal transduction are possible using integrated μOCT and fluorescence microscopy in future studies.
In addition to the degree of mucus load encountered by the epithelium, increased mucosal fluid viscosity also likely participates in triggering intracellular Ca2+ signaling required to induce stimulated CBF (10–13, 15). However, increased viscosity also can decrease CBF exponentially, because of increased resistance to ciliary beating (14, 34), as also demonstrated here experimentally. Our data indicate that this effect may dominate once PCL heights are reduced by more than 1–2 μm, as encountered in disease states, such as cystic fibrosis (17) and chronic bronchitis (35, 36). Thus, CBF regulation is the result of the interplay between autoregulatory response and fluid resistance. If PCL reduction is relatively small, Ca2+-dependent activation is dominant and CBF and MCT increase as PCL height decreases. If PCL is diminished beyond specific limits, the physical effect of viscosity overshadows the autoregulatory stimulation of ciliary activity, and ciliary movement through its beat cycle becomes restricted and mucus propulsion slows. It also seems plausible that hyperconcentrated mucus within the upper ASL compartment may exact an osmotic force that contributes to the PCL compression witnessed in studies involving sputum addition, which may also contribute to the phenomenon irrespective of changes in mucus viscosity (1). Given the importance of MCC in normal health, and the implications of its acute and chronic failure in respiratory diseases that include pneumonia, chronic obstructive pulmonary disease, and cystic fibrosis, as well as nonpulmonary conditions related to other ciliated epithelia (e.g., infertility and hydrocephalus), manipulation of this autoregulatory pathway could lead to new strategies to bolster MCT and subvert its failure to address these common and serious medical conditions.
Acknowledgments
Acknowledgments
The authors acknowledge Arianne Fulce, Kathy Sexton, Thurman Richardson, and the Tissue Collection and Banking Facility at University of Alabama at Birmingham (UAB) for services related to airway tissue procurement, Gina Sabbatini and Heather Hathorne for regulatory support for work with human subjects and the patients who donated their organs for these experiments, as well as assistance from the UAB Imaging Core Facility.
Footnotes
This work was supported by National Institute of Health (NIH) grants R01 HL1116213 (S.M.R. and G.J.T.), R01 HL105487 (S.M.R.), and P30 DK072482 (E.J.S.), Cystic Fibrosis Foundation grants R464-CF and SORSCH05XX0 (E.J.S.), ROWE10XX0 and CLANCY09Y2 (S.M.R.), and TEARNE07XX0 (G.J.T.), and Massachusetts General Hospital ECOR Postdoctoral Fellowship Award 2011A052538, as well as infrastructural support provided by the University of Alabama at Birmingham Center for Clinical and Translational Science (NIH contract UUL1025777).
Author Contributions: G.J.T. and L.L. developed the instrumentation; L.L., E.J.S., S.M.R., and G.J.T. conceived of the experiments; L.L., S.S., S.B.-P., G.H., K.K.C., S.E.B., C.M.F., J.A.G., W.E.G., and E.J.W. conducted the experiments; L.L., S.S., G.H., K.K.C., S.E.B., C.M.F., E.J.S., S.M.R., and G.J.T. analyzed the data; L.L., S.M.R., and G.J.T. wrote the manuscript; W.E.G., E.J.S., S.M.R., and G.J.T. contributed reagents and resources; S.M.R. and G.J.T. supervised the project; all authors read and edited the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0499MA on June 17, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogura A, Takahashi K. Artificial deciliation causes loss of calcium-dependent responses in paramecium. Nature. 1976;264:171–172. doi: 10.1038/264170a0. [DOI] [PubMed] [Google Scholar]
- 3.Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. Mechanoreception in motile flagella of Chlamydomonas. Nat Cell Biol. 2011;13:630–632. doi: 10.1038/ncb2214. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa R, Shingyoji C. Induction of beating by imposed bending or mechanical pulse in demembranated, motionless sea urchin sperm flagella at very low ATP concentrations. Cell Struct Funct. 2007;32:17–27. doi: 10.1247/csf.06035. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi S, Shingyoji C. Bending-induced switching of dynein activity in elastase-treated axonemes of sea urchin sperm—roles of Ca2+ and ADP. Cell Motil Cytoskeleton. 2009;66:292–301. doi: 10.1002/cm.20360. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi S, Shingyoji C. Mechanism of flagellar oscillation—bending-induced switching of dynein activity in elastase-treated axonemes of sea urchin sperm. J Cell Sci. 2008;121:2833–2843. doi: 10.1242/jcs.031195. [DOI] [PubMed] [Google Scholar]
- 7.Lansley AB, Sanderson MJ. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+ Biophys J. 1999;77:629–638. doi: 10.1016/S0006-3495(99)76919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990;1:585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanderson MJ, Dirksen ER. Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: implications for the regulation of mucociliary transport. Proc Natl Acad Sci USA. 1986;83:7302–7306. doi: 10.1073/pnas.83.19.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Button B, Boucher RC, Univ NCVLG. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163:189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winters SL, Davis CW, Boucher RC. Mechanosensitivity of mouse tracheal ciliary beat frequency: roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am J Physiol Lung Cell Mol Physiol. 2007;292:L614–L624. doi: 10.1152/ajplung.00288.2005. [DOI] [PubMed] [Google Scholar]
- 12.Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA. Trpv4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. Trpv4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci USA. 2008;105:12611–12616. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NT, Villalon M, Royce FH, Hard R, Verdugo P. Autoregulation of beat frequency in respiratory ciliated cells—demonstration by viscous loading. Am Rev Respir Dis. 1991;144:1091–1094. doi: 10.1164/ajrccm/144.5.1091. [DOI] [PubMed] [Google Scholar]
- 15.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal. 2013;6:ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antunes MB, Cohen NA. Mucociliary clearance—a critical upper airway host defense mechanism and methods of assessment. Curr Opin Allergy Clin Immunol. 2007;7:5–10. doi: 10.1097/ACI.0b013e3280114eef. [DOI] [PubMed] [Google Scholar]
- 17.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson MJ, Sleigh MA. Ciliary activity of cultured rabbit tracheal epithelium: beat pattern and metachrony. J Cell Sci. 1981;47:331–347. doi: 10.1242/jcs.47.1.331. [DOI] [PubMed] [Google Scholar]
- 19.Jonas S, Bhattacharya D, Khokha MK, Choma MA. Microfluidic characterization of cilia-driven fluid flow using optical coherence tomography–based particle tracking velocimetry. Biomed Opt Express. 2011;2:2022–2034. doi: 10.1364/BOE.2.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oldenburg AL, Chhetri RK, Hill DB, Button B. Monitoring airway mucus flow and ciliary activity with optical coherence tomography. Biomed Opt Express. 2012;3:1978–1992. doi: 10.1364/BOE.3.001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, Tearney GJ. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med. 2011;17:1010–1014. doi: 10.1038/nm.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Chu KK, Houser GH, Diephuis BJ, Li Y, Wilsterman EJ, Shastry S, Dierksen G, Birket SE, Mazur M, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One. 2013;8:e54473. doi: 10.1371/journal.pone.0054473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleigh MA. Adaptations of ciliary systems for the propulsion of water and mucus. Comp Biochem Physiol A Physiol. 1989;94:359–364. doi: 10.1016/0300-9629(89)90559-8. [DOI] [PubMed] [Google Scholar]
- 24.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naitoh Y. Reversal response elicited in nonbeating cilia of paramecium by membrane depolarization. Science. 1966;154:660. doi: 10.1126/science.154.3749.660. [DOI] [PubMed] [Google Scholar]
- 26.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings HS. Studies on reactions to stimuli in unicellular organisms. II.—The mechanism of the motor reactions of paramecium. Am J Physiol. 1899;2:311–341. [Google Scholar]
- 28.Jennings HS. Studies on reactions to stimuli in unicellular organisms. V.—On the movements and motor reflexes of the flegellata and ciliata. Am J Physiol. 1900;3:229–260. [Google Scholar]
- 29.Naitoh Y, Eckert R. Ionic mechanisms controlling behavioral responses of paramecium to mechanical stimulation. Science. 1969;164:963. doi: 10.1126/science.164.3882.963. [DOI] [PubMed] [Google Scholar]
- 30.Naitoh Y, Eckert R. Ciliary orientation—controlled by cell membrane or by intracellular fibrils. Science. 1969;166:1633. doi: 10.1126/science.166.3913.1633. [DOI] [PubMed] [Google Scholar]
- 31.Spungin B, Silberberg A. Stimulation of mucus secretion, ciliary activity, and transport in frog palate epithelium. Am J Physiol Cell Physiol. 1984;247:C299–C308. doi: 10.1152/ajpcell.1984.247.5.C299. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. Ip3 sensitizes trpv4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Cell Biol. 2008;181:143–155. doi: 10.1083/jcb.200712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura A, Machemer H. Distribution of mechanoreceptor channels in the paramecium surface-membrane. J Comp Physiol. 1980;135:233–242. [Google Scholar]
- 34.Gheber L, Korngreen A, Priel Z. Effect of viscosity on metachrony in mucus propelling cilia. Cell Motil Cytoskeleton. 1998;39:9–20. doi: 10.1002/(SICI)1097-0169(1998)39:1<9::AID-CM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]