Abstract
Bronchopulmonary dysplasia (BPD), a common chronic respiratory disease that occurs after premature birth, is believed to be secondary to oxidative damage from hyperoxia and inflammation, which leads to impaired alveolar formation and chronic lung dysfunction. We hypothesized that extracellular superoxide dismutase (SOD)3, an antioxidant uniquely targeted to the extracellular matrix (ECM) and alveolar fluid, might have a different response (down-regulation) to hyperoxic injury and recovery in room air (RA), thereby contributing to the persistent airspace injury and inflammation. We used a murine BPD model using postnatal hyperoxia (O2) (4 or 5 d) followed by short-term recovery (14 d) in RA, which mimics the durable effects after injury during alveolar development. This was associated with significantly increased mRNA expression for antioxidant genes mediated by nuclear factor erythroid 2–related factor (Nrf2) in the O2 (n = 4) versus RA group (n = 5). SOD3, an Nrf2-independent antioxidant, was significantly reduced in the O2-exposed mice compared with RA. Immunohistochemistry revealed decreased and disrupted SOD3 deposition in the alveolar ECM of O2-exposed mice. Furthermore, this distinct hyperoxic antioxidant and injury profile was reproducible in murine lung epithelial 12 cells exposed to O2. Overexpression of SOD3 rescued the injury measures in the O2-exposed cells. We establish that reduced SOD3 expression correlates with alveolar injury measures in the recovered neonatal hyperoxic lung, and SOD3 overexpression attenuates hyperoxic injury in an alveolar epithelial cell line. Such findings suggest a candidate mechanism for the pathogenesis of BPD that may lead to targeted interventions.
Keywords: extracellular superoxide dismutase; bronchopulmonary dysplasia; hyperoxia, murine lung epithelial 12; Nrf-2
Clinical Relevance
We establish that reduced superoxide dismutase (SOD)3 expression correlates with alveolar injury measures in the recovered neonatal hyperoxic lung and that SOD3 overexpression attenuates hyperoxic injury in an alveolar epithelial cell line. Such findings suggest a candidate mechanism for the pathogenesis of bronchopulmonary dysplasia that may lead to targeted interventions.
Bronchopulmonary dysplasia (BPD), or lung disease of prematurity, causes significant morbidity and mortality in the perinatal period (1). In addition, long-term sequelae, such as reactive airway disease (2), asthma (2), and chronic obstructive lung disease (3), can develop from this early life injury. The pathogenesis of BPD is multifactorial but is punctuated by inflammation and oxidative damage to biomolecules, ultimately compromising alveolar development and repair (4, 5). Such damage is thought to be in part due to the relative deficiency of antioxidant defenses in the premature infant during the transition from the relative hypoxic in utero environment to the normoxic postnatal environment (6). Thus, therapies that inhibit inflammation and/or augment the perinatal antioxidant milieu are being explored in animal models and in premature infants at high risk for BPD. Despite the fact that interventions such as systemic steroids and surfactant therapy have improved perinatal mortality, the incidence of BPD has not been substantially reduced (7).
One class of antioxidants proposed as a candidate treatment for a variety of inflammatory lung diseases is the superoxide dismutase (SOD) family (8). These enzymes catalyze the conversion of superoxide anions into hydrogen peroxide and oxygen. Three SODs have been identified in rodents and humans (SOD1–3), but extracellular SOD (SOD3, ECSOD) is the only one with extracellular distribution and with predominant lung expression. In fact, SOD3 accounts for the majority of SOD activity in airways and lung vessels (9, 10). For this reason, SOD3 might provide targeted protection for the extracellular milieu in the vertebrate airspace. Transgenic mice deficient in SOD3 display increased lung superoxide, marked inflammatory cell infiltration, increased arterial–alveolar gradient, respiratory acidosis, histological changes similar to those observed in adult respiratory distress syndrome, and high mortality (11). Oury and colleagues showed reduced SOD3 activity and protein expression but preserved RNA expression in the lungs of adult mice exposed to hyperoxia (12), suggesting that defective SOD3 expression may confer susceptibility to lung injury. Unfortunately, the pattern of SOD regulation with various neonatal lung insults remains largely unresolved, especially in the setting of murine neonatal hyperoxia exposure.
From a therapeutic standpoint, pharmacologic and genetic augmentation of SOD3 in the lung has been shown to ameliorate a wide range of lung injuries in animal models, including emphysema (13) and bleomycin-induced pulmonary fibrosis (14). In the neonatal lung, Auten and colleagues (15) and Ahmed and colleagues (4) showed that neonatal hyperoxic lung injury is attenuated with transgenic constitutive overexpression of SOD3 in the lung. Because overexpression preceded the injury in both studies, it was unclear whether the SOD3 had a protective or a therapeutic effect. Clinical trials using recombinant human Cu-Zn SOD intratracheally, an analog to SOD1, have shown no reduction in mortality or in the incidence of BPD (16). No clinical studies using SOD3 augmentation in the neonatal setting for premature infants at risk for BPD have been pursued. Accordingly, a careful assessment of differential antioxidant expression in the neonatal hyperoxic lung, a model for BPD, will provide a preclinical context for targeted enzyme replacement in the BPD setting.
Although alterations in the antioxidant milieu are thought to contribute to BPD development and progression, no careful analysis of the changes in antioxidant expression, deposition, and activity in a murine model is available. Neonatal mice deficient in nuclear factor erythroid 2–related factor (Nrf2), a master regulator of many antioxidants, are more susceptible to hyperoxic lung injury (17). Because many antioxidants, including SOD1, are regulated by the Nrf2 transcription factor, we considered whether all SODs were regulated coordinately in this setting. We specifically sought to characterize the expression of SODs and known Nrf2-regulated antioxidant genes in the lungs of neonatal mice exposed to postnatal hyperoxia (O2) followed by short-term recovery in room air (RA). This model recapitulates selective aspects of the chronic sequelae seen in premature infants with BPD (i.e., airspace enlargement, cell death, and oxidative stress) and thereby may have greater clinical relevance than models examined immediately after perinatal O2 exposure. Using such a model, we investigate the expression and deposition of SOD3, the only matrix-localized antioxidant, in the context of lung injury.
We found that neonatal mice exposed to O2 followed by recovery display discordant antioxidant induction compared with RA mice. These lungs show enlarged airspaces accompanied by enhanced cytokine levels, inflammation, and measures of oxidative stress and cell death. Whereas SOD1, SOD2, and other known Nrf2-regulated antioxidants are up-regulated, SOD3 is down-regulated. We further show fragmented deposition of SOD3 accompanied by abnormal partitioning of the enzyme in the airspace of injured lung. Whole cell studies confirm SOD3 down-regulation with O2 exposure in an alveolar cell line. These findings validate the use of cross-platform models (in vivo and whole cell) to investigate pathways that contribute to neonatal lung disease. Some of these data have been presented previously in abstract form (18).
Materials and Methods
Animal Studies
Pups (24 h old) from timed-pregnant C57Bl/6 female mice (Jackson Laboratory, Bar Harbor, ME) were placed in an O2 (85–90% FiO2) or RA chamber for 5 days followed by RA recovery for 14 days. Experiments were performed according to institutional guidelines for animal care and use at Johns Hopkins University School of Medicine. Nursing dams alternated daily between RA and O2-exposed litters. Balanced litter sizes were maintained by culling pups. Animals were anesthetized and killed with isoflurane inhalation. Body weights, gender, bronchoalveolar lavage fluid (BALF), and lungs for histology and frozen tissue were collected. Further details regarding the BALF collection methodology are provided in the online supplement.
Morphometry
Morphometric studies were performed as previously described (19, 20) on mice exposed to O2 for 4 days. Further details are provided in the online supplement
Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously described (20). Further details are provided in the online supplement.
Cytokine ELISAs
Cytokine measurements in lung tissue lysates (4 d exposure) were analyzed at the Cytokine Core Laboratory at the University of Maryland. Further details are provided in the online supplement.
Total Glutathione Levels
Total glutathione (GSH) and oxidized GSH (GSSG) levels were measured using the GSH-GloTM Glutathione Assay (Promega, Madison, WI). Further details are provided in the online supplement.
Apoptosis
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was performed using the TdT-FragEL DNA Fragmentation Detection Kit (Calbiochem, San Diego, CA) per the manufacturer’s protocol. Quantitation was performed as described above.
Western Blot Analysis
Western blot was performed as previously described (20). Further details are provided in the online supplement.
Quantitative Real-Time PCR Analysis
Real-time PCR was performed as previously described (19). Further details are provided in the online supplement.
SOD Total Activity
SOD total activity was determined by the Superoxide Dismutase Assay Kit II (Calbiochem). Further details are provided in the online supplement.
Cell Culture and Hyperoxia Exposure
Murine lung epithelial (MLE)-12 cell line (ATCC, Rockville, MD) is a transformed clonal line representative of alveolar epithelial type II cells. MLE-12 cells were cultured in DMEM/F12 media containing 10% FBS and penicillin/streptomycin (100 U/ml) following the published protocol (21). Cells at 80% confluence were exposed to hyperoxia (95% O2/5% CO2) using a modular incubator chamber (Billups-Rothenberg, del Mar, CA) or RA-5%CO2 for 12 or 24 hours and harvested for cell counting, proliferation assay, RNA extraction, or protein extraction. A hemocytometer was used to count total cells.
MLE-12 SOD3 Overexpressor Transient Transfection
Cells were transiently transfected using a pIRES-hrGFP-1a vector containing mouse SOD3 cDNA. Further details are provided in the online supplement.
MTT-Based Proliferation Assay
The CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) was used to determine the number of viable cells in proliferation. Further details are provided in the online supplement.
5-Bromo-2′-deoxyuridine ELISA
The Amersham Cell Proliferation Biotrak ELISA System (GE Healthcare, Amersham, UK) was used to measure cell proliferation to confirm any significant findings from the MTT assay. Details are provided in the online supplement.
Statistics
Student’s t test was used to determine differences between groups with respect to the readouts of MLI, SOD activity, immunoblotting, proliferation assays, apoptosis assays, oxidative stress assays, nitrotyrosine staining, and TUNEL staining using SigmaPlot 11 (Systat Software, San Jose, CA). Values for measurements are expressed as means ± SEM, and P values for significance are designated at < 0.05.
Results
Acute O2 followed by RA Recovery in Neonatal Mice Leads to Increased Airspace Size and Inflammation
The airspace (AS) defects resulting from neonatal O2 in mice can be variable, dependent upon the timing of exposure, length of exposure, percent hyperoxia, and inbred mouse strain used (22–24). Few groups have incorporated a recovery phase after short-term O2 exposure (24–26), a modification that we submit best mimics the current clinical situation in which neonates are subjected to a severe short-lived perinatal stress but receive little long-term supplemental oxygen (given concerns about oxygen toxicity). This constellation causes transient injury and durable changes in lung architecture and function, leading to adverse chronic outcomes (4, 24, 26). To establish whether our BPD model induced adequate lung injury, we measured AS size and markers of inflammation in BALF and in lung tissue. Four days of O2 exposure followed by RA recovery resulted in significant AS enlargement, as demonstrated by an increase in the MLI in the O2 group (Figure 1A). Pups exposed to O2 also showed a significant increase in total cell counts in BALF compared with the RA group (Table 1). Although there was a trend of increased macrophages and neutrophils in the O2 group, this was not significantly different from the RA controls (data not shown). There was also no significant difference in BALF protein concentration between the two groups. In addition to increased cells in the BALF of O2-exposed mice, there was an elevation of cytokine levels measured in lung tissue lysates (Table 1). Although TGF-β levels were borderline significantly increased in O2-exposed mice compared with RA controls (RA: 764 ± 80 pg/ml; O2: 1,169 ± 163 pg/ml [P = 0.05]), IL-6 and TNF-α levels were significantly increased in the O2-exposed mice. We wanted to determine if there was a dysregulation in anti-inflammatory cytokines and found that IL-10 levels were significantly higher in the O2 group compared with RA controls.
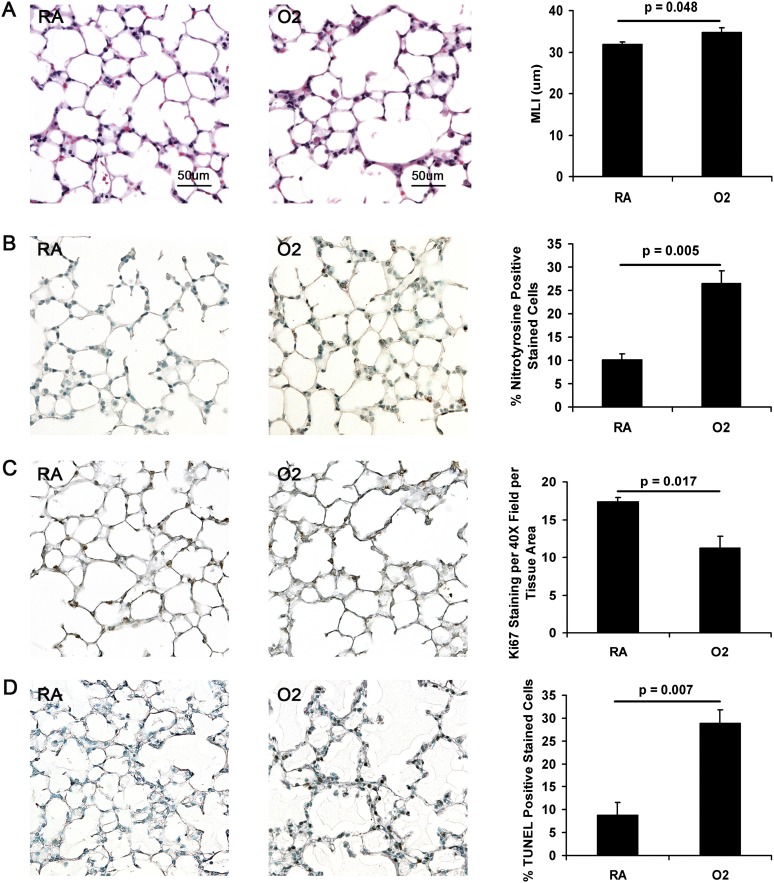
Figure 1.
Measurements of lung injury in room air (RA)- versus hyperoxia (O2)-exposed neonatal mice. (A) Morphometry. Representative images of RA- and O2-exposed (4 d) neonatal mice after RA recovery (original magnification: ×20). O2-exposed mice (n = 4) have significant airspace enlargement compared with RA mice (n = 5) as measured by the mean linear intercept (MLI). (B–D) Representative images of RA- and O2-exposed (5 d) neonatal mice after RA (original magnification: ×20; n = 3 for each group). (B) O2-exposed mice demonstrate increased nitrotyrosine staining compared with RA mice, indicating increased oxidative stress. (C) Ki67 staining. O2-exposed mice have decreased Ki67 staining compared with RA mice, a measure of proliferation. (D) O2-exposed mice have significantly increased TUNEL staining compared with RA mice.
Table 1.
Measurements of Inflammation in Room Air–exposed versus Hyperoxia-exposed Neonatal Mice*
| Readout | RA (n) | O2 (n) | P Value |
|---|---|---|---|
| BALF | |||
| Total cell counts | 1.98 × 106 ± 8.4 × 104 (6) | 2.94 × 106 ± 2.1 × 104 (4) | 0.001 |
| Protein concentration, μl/μl | 0.08 ± 0.01 (6) | 0.11 ± 0.02 (6) | NS |
| Cytokines (lung tissue lysates), pg/ml | |||
| IL-1β | 29.6 ± 4.4 (6) | 44.9 ± 6.6 (6) | 0.08 |
| IL-6 | 9.2 ± 1.1 (6) | 17.5 ± 1.7 (6) | 0.002 |
| TNF-α | 2.4 ± 0.3 (6) | 4.0 ± 0.6 (6) | 0.045 |
| IL-10 | 3.8 ± 1.0 (6) | 8.3 ± 1.3 (6) | 0.019 |
| TGF-β | 764 ± 80 (6) | 1169 ± 163 (6) | 0.05 |
Definition of abbreviations: BALF, bronchoalveolar lavage fluid; O2, hyperoxia; NS, not significant; RA, room air.
Four days of RA versus O2 exposure followed by 14 d RA recovery.
Hyperoxic Injury followed by RA Recovery in Neonatal Mice Leads to Increased Markers of Oxidative Stress, Proliferation, and Apoptosis
Oxidative stress was measured by IHC for nitrotyrosine and 8-hydroxyguanine and GSH/GSSG levels in tissue lysates. IHC for nitrotyrosine and 8-hydroxyguanine revealed significant increased staining in the O2 versus the RA group (Figure 1B and Figure E2 in the online supplement, respectively). On the other hand, GSH/GSSG levels in lung tissue lysates showed no significant difference between the two exposure groups (Figure E3). Whereas GSH/GSSG levels were unaffected by hyperoxia, proliferation as measured by Ki67 staining was significantly reduced on IHC (Figure 1C). As a measure of cell death, TUNEL staining was significantly increased in the O2 group compared with RA controls (Figure 1D). However, there were no detectable levels of cleaved caspase-3 protein expression in either group (data not shown). Protein expression of prosurvival mediators, such as pSTAT-3, pAKT, and pERK, were not significantly different between the RA and O2 groups (data not shown). Taken together, these studies demonstrate persistently enhanced oxidative stress despite RA recovery that is also accompanied by increased cell death and reduced proliferation.
Acute O2 Exposure followed by RA Recovery in Neonatal Mice Is Associated with Decreased and Fragmented SOD3 Deposition within the ECM
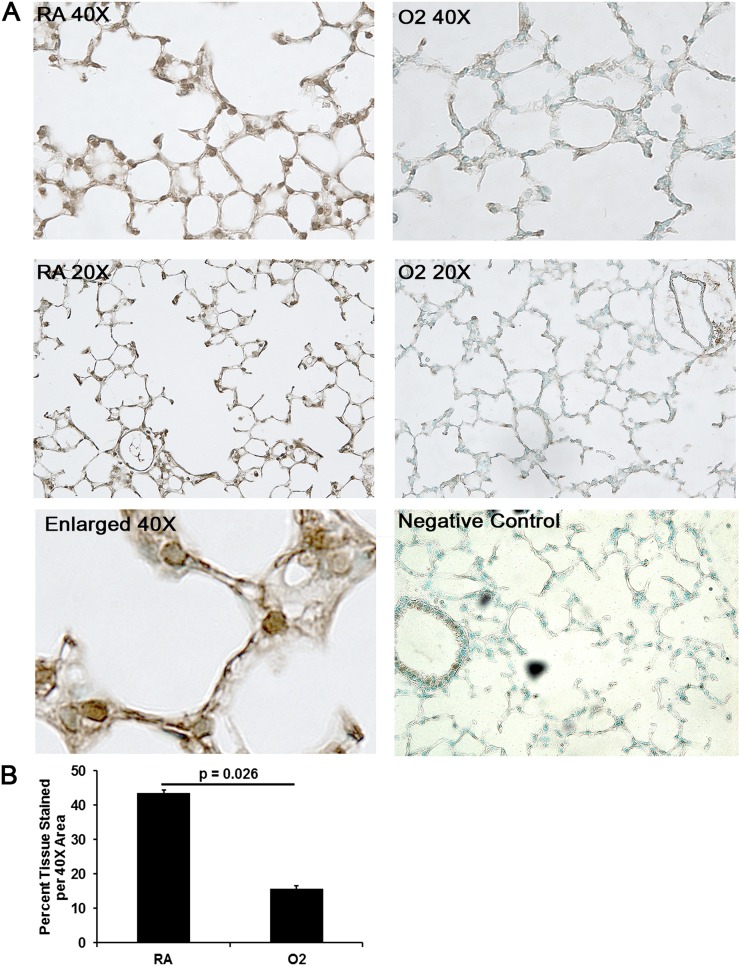
Adult O2 without a recovery phase results in reduced SOD3 levels and activity in the lung (12). We were interested in whether the lung-abundant and ECM-associated antioxidant SOD3 displays altered deposition in our neonatal O2 model. In adult O2, SOD3 deposition is significantly diminished along the alveolar epithelial lining (12). IHC staining revealed qualitatively decreased and fragmented deposition of SOD3 within the ECM, the site of maximal SOD3 activity, and along the alveolar epithelium (Figure 2) in mice exposed to O2 for 4 days followed by RA recovery compared with RA controls (n = 3 per group). Although there appears to be a difference in deposition of SOD3, total SOD3 protein expression in lung tissue lysates was not significantly different between the two exposure groups (Figure E1). In addition, total SOD activity (a reflection of the activity of all SODs) in lung tissue lysates did not differ between O2- versus RA-exposed (5 d) mice (RA: n = 5, 0.11 ± 0.01 U/ml; O2: n = 4, 0.12 ± 0.01 U/ml [P = NS]). Total SOD activity in whole lung tissue lysates is confounded by compartmental differences in expression of SOD isoforms. Nonetheless, these data suggest that the most prominent and consequential defect in SOD3 expression attending neonatal hyperoxia occurs in the AS compartment.
Figure 2.
Immunohistochemistry (IHC) of extracellular superoxide dismutase (SOD)3 in RA- versus O2-exposed mice. (A) Brown 3,3'-diaminobenzidine staining denotes positive staining of SOD3 on 20× and 40× magnification. Representative images of RA-exposed mice demonstrate continuous staining of SOD3 along the extracellular matrix, whereas O2-exposed (5 d) mice demonstrate fragmented and decreased deposition of SOD3 along the extracellular matrix. (B) Quantitative IHC confirms reduced SOD3 deposition in O2-exposed lungs (n = 3 per group) (P = 0.026).
The Durable Effects of Hyperoxic Lung Injury Are Associated with a Lack of Induction of SOD3 mRNA Expression
Nrf2 is a major antioxidant transcription factor that has been found to mediate the expression of several phase II antioxidant genes, such as heme oxygenase-1 (Ho-1), glutamate-cysteine ligase catalytic subunit (Gclc), and NAD(P)H dehydrogenase [quinine]-1 (Nqo1) (27–29). Nrf2 has also been reported to mediate expression of SOD1 and SOD2 (30), and we asked whether the induction of Nrf2 antioxidants was maintained during the late AS enlargement and inflammation observed in mice exposed to neonatal O2. Such mice demonstrated increased mRNA expression of all three phase II antioxidant genes (Table 2). Similar results were found for SOD1 and SOD2 mRNA expression, but SOD3 was significantly reduced in the O2 group compared with RA controls (Table 2). These data suggest that the SOD superfamily regulation in the neonatal O2 setting is discordant and that SOD3 down-regulation solely corresponds with the architectural, inflammatory, and biochemical injury measures observed in neonatal mice exposed to O2 followed by RA recovery.
Table 2.
mRNA Gene Expression by Real-Time PCR in Room Air–exposed versus Hyperoxia-exposed Neonatal Mice*
| Gene | RA (n = 5) | O2 (n = 4) | P Value |
|---|---|---|---|
| Nrf2-mediated antioxidant genes (FC) | |||
| Ho-1 | 1.06 ± 0.17 | ↑ 5.48 ± 1.12 | 0.003 |
| Gclc | 1.08 ± 0.24 | ↑ 3.22 ± 0.38 | 0.002 |
| Nqo-1 | 1.017 ± 0.10 | ↑ 9.09 ± 0.90 | <0.001 |
| SOD antioxidant genes (FC) | |||
| SOD1 | 1.039 ± 0.15 | ↑ 3.96 ± 0.98 | <0.001 |
| SOD2 | 1.01 ± 0.06 | ↑ 1.86 ± 0.03 | <0.001 |
| SOD3 | 1.01 ± 0.07 | ↓ 0.36 ± 0.06 | <0.001 |
Definition of abbreviations: FC, fold change; Gclc, glutamate-cysteine ligase catalytic subunit; Ho-1, heme oxygenase-1; Nqo-1, NAD(P)H dehydrogenase [quinine]-1; Nrf2, nuclear factor erythroid 2; O2, hyperoxia; RA, room air; SOD, superoxide dismutase.
Five days RA versus O2 exposure followed by 14-d RA recovery.
Hyperoxia Exposure in MLE-12 Cells Is Associated with Decreased Cell Survival and Proliferation and Increased Markers of Oxidative Stress
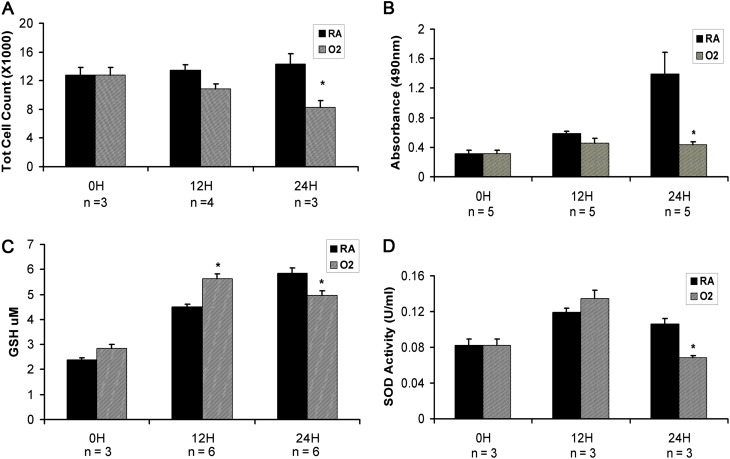
Because SOD3 is expressed by alveolar epithelial cells (31), we considered whether hyperoxia resulted in epithelial cell oxidative stress possibly secondary to disturbances in SOD expression. We used MLE-12 cells, a murine transformed alveolar epithelial cell line, to examine cell-type–specific hyperoxic responses. We found that total cell counts decreased with greater O2 exposure times and were significantly less compared with RA controls at 24 hours of O2 exposure in MLE-12 cells (Figure 3A). As a measure of proliferation, we used an MTT-based proliferation assay and found attenuated proliferation with increasing O2 exposure times, which became significantly reduced compared with RA controls at 24 hours of O2 exposure (Figure 3B). This finding was also confirmed with the 5-bromo-2′-deoxyuridine proliferation assay at 24 hours of O2 exposure (data not shown). Measurements of oxidative stress, which included GSH levels and total SOD activity, were similarly reduced at 24 hours of O2 exposure. For example, whereas GSH levels were initially increased at 12 hours of O2 exposure as a likely compensatory response to oxidative injury, total GSH levels eventually decreased significantly at 24 hours of O2 exposure, indicating the likely consumption of the antioxidant in the setting of increasing O2 exposure (Figure 3C). Total SOD activity was also decreased at this same time point (Figure 3D). Thus, oxidative stress and cell survival findings in the MLE-12 hyperoxia system recapitulate the findings in mice exposed to neonatal O2 followed by RA recovery.
Figure 3.
Measurements of injury in murine lung epithelial 12 cells. (A) Total cell counts were significantly lower at 24 hours of O2 exposure. (B) Using the MTT-based proliferation assay, proliferation was significantly reduced at 24 hours of of O2 exposure. (C) Total Glutathione (GSH) was significantly increased at 12 hours of O2 exposure but significantly decreased at 24 hours of O2 exposure. (D) Total SOD activity was significantly reduced at 24 hours of O2 exposure. *P < 0.05.
Hyperoxia Exposure in MLE-12 Cells Is Associated with Increased Protein Expression for Markers of Cell Death and Dysregulated Expression for Markers of Cell Cycle Growth and Mediators of Survival
To explore the mechanism of impaired cell survival in O2-exposed MLE-12 cells, we investigated mediators of apoptosis and cell cycle growth/regulation by Western blot and densitometric analysis (Table 3). Results demonstrated that proliferating cell nuclear antigen (PCNA) was significantly increased at 24 hours of O2 exposure, whereas the tumor suppressor gene p21 was significantly increased at 12 hours of O2 exposure. Increased markers for cell death included cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase (PARP) at 24 hours of O2 exposure. Bax expression was not significantly increased between groups at either exposure time. Protein expression for mediators of survival varied. At 12 hours of O2 exposure, pSTAT3 and pAKT were increased, whereas at 24 hours of O2 exposure, only pAKT was increased with O2. This panel indicates a robust induction of cell death and cell survival signaling during the early phase of O2 exposure in MLE-12 cells, which culminates in reduced cell survival.
Table 3.
Protein Expression of Markers for Cell Cycle Regulation, Apoptosis, and Mediators of Survival
| Protein | 12 Hours |
24 Hours |
||||
|---|---|---|---|---|---|---|
| RA (n) | O2 (n) | P Value | RA (n) | O2 (n) | P Value | |
| Cell cycle | ||||||
| PCNA | 0.42 ± 0.02* (4) | 0.45 ± 0.01 (3) | NS | 0.42 ± 0.01 (4) | 0.52 ± 0.02 (3) | <0.001 |
| p21 | 1.71 ± 0.06 (3) | 2.07 ± 0.03 (3) | 0.005 | 1.94 ± 0.01 (3) | 1.41 ± 0.29 (3) | NS |
| Apoptosis | ||||||
| Cleaved PARP | 0.66 ± 0.01 (3) | 0.75 ± 0.05 (4) | NS | 0.73 ± 0.05 (3) | 0.97 ± 0.04 (4) | 0.012 |
| Cleaved caspase-3 | 0.73 ± 0.02 (3) | 0.85 ± 0.05 (3) | NS | 0.68 ± 0.04 (3) | 0.83 ± 0.03 (3) | 0.044 |
| Bax | 0.54 ± 0.05 (3) | 0.69 ± 0.03 (3) | NS | 0.54 ± 0.03 (3) | 0.67 ± 0.05 (3) | NS |
| Mediators of cell survival | ||||||
| pAKT | 1.01 ± 0.02 (4) | 1.15 ± 0.05 (4) | 0.039 | 1.75 ± 0.11 (3) | 2.55 ± 0.17 (3) | 0.017 |
| pERK | 0.83 ± 0.09 (5) | 0.93 ± 0.06 (3) | NS | 0.46 ± 0.04 (3) | 0.79 ± 0.13 (3) | NS |
| pSTAT3 | 0.70 ± 0.03 (6) | 0.80 ± 0.03 (5) | 0.049 | 1.01 ± 0.04 (3) | 1.02 ± 0.02 (3) | NS |
Definition of abbreviations: NS, not significant; O2, hyperoxia; PARP, poly(ADP-ribose) polymerase; PCNA, proliferating cell nuclear antigen; RA, room air.
Units are in net pixel density after normalization to actin control or total protein for pAKT, pERK, and pSTAT3.
In Vitro Hyperoxic Injury Is Associated with a Lack of Induction of SOD3 mRNA Expression Despite Increased Expression of Nrf2-Mediated Antioxidant Genes
Given our findings of discordant induction of SOD antioxidants in the lungs of neonatal mice exposed to O2, we pursued a survey of these genes by real-time PCR in MLE-12 cells exposed to O2 compared with RA controls. When the relative fold-change was compared between groups within each time point, the Nrf2-mediated antioxidant genes Ho-1 and Nqo1 had increased mRNA expression after 24 hours of O2 exposure (Table 4). Similar results were seen for SOD1 and SOD2 mRNA expression (Table 4). However, SOD3 had significantly decreased mRNA expression at both time points of O2 exposure. Such results are similar to our in vivo data (Table 2). Taken together, our whole cell studies of epithelial cell hyperoxia selectively replicate our findings in the murine neonatal O2 model, suggesting that the defects in oxidative stress and cell survival that punctuate the in vivo model can be partially modeled using a cell system.
Table 4.
mRNA Gene Expression by Real-Time PCR in Murine Lung Epithelial 12 Cells at 12 and 24 Hours of Room Air versus Hyperoxia Exposure
| Gene | 12 Hours |
24 Hours |
||||
|---|---|---|---|---|---|---|
| RA (n = 3) | O2 (n = 3) | P Value | RA (n = 3) | O2 (n = 3) | P Value | |
| Nrf2-mediated antioxidant genes (FC) | ||||||
| Ho-1 | 1.39 ± 0.09 | 1.61 ± 0.01 | NS | 1.00 ± 0.02 | 2.15 ± 0.12 | <0.001 |
| Gclc | 1.53 ± 0.10 | 1.41 ± 0.05 | NS | 1.02 ± 0.02 | 1.27 ± 0.10 | NS |
| Nqo-1 | 1.48 ± 0.05 | 1.71 ± 0.08 | NS | 0.77 ± 0.04 | 1.69 ± 0.08 | <0.001 |
| SOD antioxidant genes (FC) | ||||||
| SOD1 | 1.63 ± 0.02 | 2.36 ± 0.11 | 0.002 | 1.43 ± 0.02 | 2.06 ± 0.13 | 0.009 |
| SOD2 | 1.76 ± 0.03 | 1.54 ± 0.20 | NS | 1.24 ± 0.03 | 1.83 ± 0.21 | 0.009 |
| SOD3 | 1.00 ± 0.04 | 0.57 ± 0.07 | 0.006 | 1.00 ± 0.09 | 0.71 ± 0.08 | 0.01 |
Definition of abbreviations: FC, fold change; Gclc, glutamate-cysteine ligase catalytic subunit; Ho-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2; NS, not significant; Nqo-1, NAD(P)H dehydrogenase [quinine]-1; O2, hyperoxia; RA, room air; SOD, superoxide dismutase.
In Vitro SOD3 Overexpression Attenuates Cell Death and Increases Proliferation Despite Hyperoxia Exposure
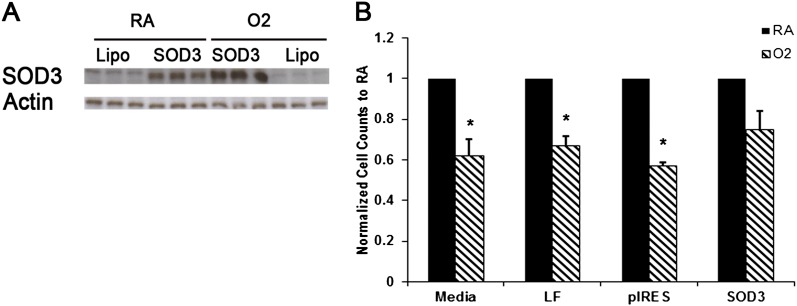
To determine whether increased levels of SOD3 in vitro could ameliorate selected injury parameters we found increased in the setting of hyperoxia, we transiently transfected MLE-12 cells with an SOD3-overexpressing plasmid (Figure 4A). Total cell counts and Western blot for PARP cleavage was assessed. We found that although total cell counts were decreased in the setting of O2 versus RA exposure, cell survival was preserved in MLE-12 cells transfected with SOD3 compared with MLE-12 cells treated with media, lipofectamine, or vector alone (Figure 4B). Higher levels of SOD3 expression and the vector control were associated with increased cell toxicity, suggesting that a therapeutic window for augmentation exists in a whole cell system (data not shown). In summary, increased levels of SOD3 promote MLE-12 cell survival under hyperoxic conditions by increasing proliferation, as evidenced by higher cell counts, suggesting that SOD3 augmentation may provide significant protective and therapeutic effects upon oxidative injury of lung epithelial cells.
Figure 4.
Cell death and proliferation in MLE-12 cells overexpressing SOD3. (A) There was significantly increased SOD3 protein expression in MLE-12 cells transiently transfected with SOD3 DNA compared with lipofectamine only–treated cells. (B) Total cell survival was significantly lower in O2-exposed cells in media, lipofectamine, or vector alone but was unchanged in cells overexpressing SOD3 (n = 3 per group). *P < 0.05.
Discussion
In this study, we found that neonatal mice exposed to O2 followed by RA recovery demonstrated significant AS disease, increased markers of inflammation and apoptosis, and decreased proliferation. In addition, we showed that, despite an appropriate compensatory increase in the mRNA expression of Nrf2-mediated antioxidants, the durable effects of hyperoxic lung damage was associated with a lack of induction in SOD3 mRNA expression and a qualitative decreased deposition of SOD3 along the alveolar ECM. We show here that an O2-induced alteration in expression of a largely lung-specific antioxidant correlates with the durable manifestations of neonatal O2 lung injury. A similar antioxidant profile of decreased SOD3 mRNA expression was evident in our whole cell model of O2 exposure. These findings provide proof-of-concept evidence that (1) a major site of antioxidant protection during the neonatal period is in the alveolar epithelial cell compartment and (2) alveolar epithelial cell lines can be used as convenient model systems to examine critical events attending O2 lung injury.
Although most BPD animal studies have looked at the acute effects of O2 exposure (15, 23, 24, 32), our model of recovery after injury provides valuable insight into the late phase after acute O2 damage. It not only mimics the clinical setting for neonates at risk for developing BPD, but it also gives a timeframe to determine the ideal therapeutic window. Unfortunately, no consensus exists on the best protocol for such exposure/recovery maneuvers. For example, Bozyk exposed C57Bl/6 neonatal mice to 14 days of 75% O2 versus RA (23), and Li exposed neonatal TGF-β1 overexpressors versus wild-type (WT) mice to 100% O2 for 7 days of O2 exposure only or 4 days of O2 exposure with RA recovery for 10 days (24). Another group that included a recovery phase was McGrath-Morrow and colleagues, who exposed p21 knockout and WT neonatal mice to O2 for 4 days followed by RA recovery for 2 or 6 weeks (25). Clearly, the timing of exposure, length of exposure, percent hyperoxia, and inbred mouse strain used are highly variable between studies. The protocol used here is advantaged by the clear confluence of late injury measures, architectural defects, and discordant antioxidant regulation. As such, our approach is an attractive platform for intervention studies.
Do other studies, with and without recovery phases, show similar results? Although most studies with a recovery phase demonstrate persistent changes in lung architecture and function (4, 24–26), data are limited on surveys of contemporaneous inflammatory, apoptotic, proliferative, and oxidative stress profile in a murine model of BPD and especially in describing the attendant antioxidant profile (17, 33). In a study by Auten and colleagues, hyperoxia markedly impaired bronchiolar and alveolar epithelial proliferation at postnatal day 3 and 5, as measured by 5-bromo-2′-deoxyuridine uptake or Ki67 labeling (15). These studies were performed without a recovery phase. This is similar to our results in that Ki67 was reduced in the O2 group compared with RA. However, although we noted a significant decrease in mRNA SOD3 expression in the O2 group, Auten and colleagues found that mouse SOD3 mRNA expression was unaffected by O2 and did not comment on the expression of other antioxidants (15). Furthermore, we found that there was fragmented and decreased deposition of SOD3 along the alveolar ECM, an observation in neonatal mice that to our knowledge has not been reported by other investigators. We submit that the combination of reduced expression and reduced deposition that we report here culminate in a critical impairment in antioxidant protection in the AS, conferring susceptibility to O2 injury.
Why does SOD3 behave differently from other antioxidant genes? A plausible scheme is that SOD3 is regulated on two levels: gene expression and tissue deposition. In this way, alterations in SOD3 gene expression that compromise tissue homeostasis can be partially modulated by preserved or even enhanced tissue deposition of the secreted protein onto the matrix. From a gene expression standpoint, the mechanism by which O2 might dampen SOD3 expression is obscure. Whereas many antioxidant genes harbor antioxidant response elements suggestive of Nrf2 as the upstream regulator, the specifics of SOD3 gene regulation are poorly understood but are thought to be in part regulated by Sp1 and Sp3 transcription factors (34). An active area of inquiry in our laboratory is to identify the upstream mediators of SOD3 expression that converge on tissue injury cascades. From a matrix deposition standpoint, SOD3 contains a heparin-binding domain that is sensitive to proteolysis, which allows it to exist in relatively high concentrations in specific regions of the ECM and on cell surfaces. In adult mice exposed to acute O2 for 72 hours, there is proteolytic clearance of SOD3 from the lung during O2, which is believed to contribute to the oxidant–antioxidant imbalance in lung tissue and BALF (12). Another possibility is that the underlying ECM is altered in the setting of O2 due to an imbalance between exaggerated elastin (35) and collagen (22, 32) deposition and activation of metalloproteases/collagenases (32, 36). Human SOD3 is known to bind to heparan sulfate (37) but has also been shown to bind to ECM components, such as type I collagen (38) and fibulin-5 (39), in mice. In the present study, we found decreased and fragmented deposition of SOD3 along the ECM in O2-exposed neonatal mice, which is similar to findings in TSK mice, a murine model of genetic emphysema with known altered fibrillin-1 incorporated into the lattice of the ECM (20). Thus, our neonatal O2 model may well exhibit alterations in the alveolar matrix scaffold that compromise the deposition of critical protective factors such as SOD3.
We present data showing a corresponding pattern of detailed injury measures in neonatal mouse lung and MLE-12 cells exposed to O2. Our findings of increased cell death and increased cleaved caspase-3 protein expression in MLE-12 cells exposed to O2 are similar to previous studies (40, 41). However, no survey of Nrf2-mediated antioxidant expression in MLE-12 cells has been reported. In terms of the effects of O2 in A549 cells, a human lung epithelial tumor cell line, Gehen and colleagues found an increase in p21 protein expression, a tumor suppressor gene, with a decrease in PCNA protein expression after 72 hours of O2 exposure (42). We reported similar results with increased p21 protein expression at 24 and 48 hours of O2 exposure but no significant difference in PCNA protein at similar time points (43). Pagano and colleagues noted increased LDH levels, a measure of cell death, with 72 hours of O2 but no significant difference in cleaved PARP protein expression (44). However, none of these studies described the expression of Nrf2-mediated antioxidant and SOD genes in response to O2 or attempted to conflate findings in an in vivo model with the in vitro studies as we report here.
Lung epithelial cells can be used as testing platforms for SOD augmentation with potential extension to more complex animal models. In A549 cells overexpressing SOD1 or SOD2, AP-1 activation and IL-8 levels were significantly reduced in the setting of O2 compared with controls (45). Koo and colleagues found that MLE-12 cells overexpressing SOD2 and glutathione peroxidase had increased cell viability at 48 and 72 hours of O2 exposure (46). Ilizarov and colleagues found similar results in MLE-12 cells overexpressing SOD2 alone or SOD2 and catalase after 5 and 10 days of O2 exposure (47). Translating these findings of using either SOD1 or SOD2 to the successful clinical treatment of BPD in neonates has proven to be more challenging. Davis and colleagues studied the 1-year follow-up of premature infants who received rh-SOD1 intratracheally every 48 hours during intubation or for up to 1 month postnatally and found that there was no difference in the incidence of BPD between the treatment and control groups (16). However, our studies here and previously published (20) suggest that the SODs may still have distinct antioxidant roles in the lung and that SOD3 might be uniquely involved in airspace protection during hyperoxic injury.
Whether or not an increase specifically in SOD3 levels can prevent or repair damage caused by O2 has yet to be determined. However, pursuing such a route may be pivotal given that our study demonstrates that the lack of SOD3 induction is associated with lung injury despite increased expression of SOD1, SOD2, and Nrf2-mediated antioxidant genes. Furthermore, our findings of SOD3 overexpression in vitro provide some insight that increased levels of SOD3 can decrease cell death and increase proliferation. In adult mice, Folz and colleagues found that the overexpression of SOD3 in the airways of mice attenuated the hyperoxic lung injury response, decreased morphologic evidence of lung damage, reduced numbers of recruited inflammatory cells, and reduced lung wet/dry ratio (48). In neonatal mice constitutively overexpressing SOD3 in ATII cells, 21 days of postnatal O2 resulted in preserved alveolar volume and density when compared with WT mice (4). Auten and colleagues reported that lung-specific constitutive overexpression of SOD3 in neonatal mice partially rescued the reduced alveolar and airway epithelial proliferation observed in WT mice exposed to 3 days of O2 (15). Whether augmentation of SOD3 is effective after neonatal lung injury is established is unknown. Accordingly, an inducible murine model of SOD3 expression would be useful in determining whether increased SOD3 levels initiated postnatally can repair O2 lung injury.
From our study, we propose that such a detailed in vivo analysis of the antioxidant profile at a postinjury time point at which durable effects are observed creates an important foundation for possible therapeutic targeting. We also submit that the development of a parallel whole cell model with corresponding injury measures provides a strong mechanistic platform, one in which we were able to demonstrate attenuation of apoptosis and increased proliferation with SOD3 overexpression. Distinct from our findings in the murine model, however, we suspect that the protective effects of SOD3 on hyperoxic MLE-12 cells are likely to occur with several antioxidants. We also recognize the drawbacks of using an established tumor cell line in studying the in vitro effects of hyperoxia, including that such cells may be more resistant to cell death and oxidative stress. Because such models are highly manipulable, often used, and widely reported, they can still provide valuable information in elucidating certain pathways involved in O2 injury. Although primary AEII cells would be a more accurate model to study O2 in vitro, the yield is often low during the isolation process, thus making analyzing various readouts difficult. However, there have been a few studies that have isolated murine primary AE II cells (40, 49, 50) with varying O2 exposure times between 24 to 48 hours. Although most described the overall cell survival and proliferation in such settings, none commented on the antioxidant profile associated with their findings.
Our study presents novel findings of decreased lung SOD3 mRNA expression and airspace deposition in the setting of neonatal O2 exposure followed by RA recovery, a murine BPD model with evidence of AS injury, inflammation, apoptosis, and decreased proliferation. This model not only validates the value of having a recovery phase after injury but, more importantly, demonstrates that the lack of induction of SOD3 is associated with the durable effects of neonatal O2 lung injury despite an appropriate compensatory increase in Nrf2-mediated antioxidant genes, SOD1, and SOD2. Furthermore, these results were reproducible in an in vitro model of epithelial cell hyperoxia, with improvement in injury markers with SOD3 overexpression. Future studies are needed to determine if increasing SOD3 levels initiated postnatally can rescue the lung from injury and prevent damage found in BPD.
Footnotes
The work was supported by National Institutes of Health grant R01HL085372 (E.R.N.) and the Stetler Foundation Grant (H.K.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2013-0043OC on March 27, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev. 2009;85(Suppl):S1–S3. doi: 10.1016/j.earlhumdev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Northway WH, Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, Eichler I, Lamm RL, Brown BW., Jr Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323:1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 3.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med. 2003;167:400–405. doi: 10.1164/rccm.200202-108OC. [DOI] [PubMed] [Google Scholar]
- 5.Randell SH, Mercer RR, Young SL. Neonatal hyperoxia alters the pulmonary alveolar and capillary structure of 40-day-old rats. Am J Pathol. 1990;136:1259–1266. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23:161–166. doi: 10.1097/MOP.0b013e3283423e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, Blackmon L, Jobe A. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117:S52–S56. doi: 10.1542/peds.2005-0620I. [DOI] [PubMed] [Google Scholar]
- 8.Davis JM, Rosenfeld WN, Richter SE, Parad MR, Gewolb IH, Spitzer AR, Carlo WA, Couser RJ, Price A, Flaster E, et al. Safety and pharmacokinetics of multiple doses of recombinant human CuZn superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics. 1997;100:24–30. doi: 10.1542/peds.100.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Oury TD, Chang LY, Marklund SL, Day BJ, Crapo JD. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab Invest. 1994;70:889–898. [PubMed] [Google Scholar]
- 10.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 11.Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, et al. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L777–L784. doi: 10.1152/ajplung.00011.2002. [DOI] [PubMed] [Google Scholar]
- 13.Yao H, Arunachalam G, Hwang JW, Chung S, Sundar IK, Kinnula VL, Crapo JD, Rahman I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc Natl Acad Sci USA. 2010;107:15571–15576. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- 15.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L32–L40. doi: 10.1152/ajplung.00133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld WNorth American Recombinant Human CuZnSOD Study Group. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase Pediatrics 2003111469–476. [DOI] [PubMed] [Google Scholar]
- 17.Cho HY, van Houten B, Wang X, Miller-Degraff L, Fostel J, Gladwell W, Perrow L, Panduri V, Kobzik L, Yamamoto M, et al. Targeted deletion of Nrf2 impairs lung development and oxidant injury in neonatal mice. Antioxid Redox Signal. 2012;17:1066–1082. doi: 10.1089/ars.2011.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poonyagariyagorn HK, Metzger S, Dikeman D, Lopez-Mercado A, Malinina A, McGrath-Morrow S, Neptune ER. Extracellular superoxide dismutase (sod3) deficiency contributes to durable effects of neonatal hyperoxic lung injury [abstract] Am J Respir Crit Care Med. 2012;185:A1278. [Google Scholar]
- 19.Podowski M, Calvi C, Metzger S, Misono K, Poonyagariyagorn H, Lopez-Mercado A, Ku T, Lauer T, McGrath-Morrow S, Berger A, et al. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. J Clin Invest. 2012;122:229–240. doi: 10.1172/JCI46215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podowski M, Calvi CL, Cheadle C, Tuder RM, Biswals S, Neptune ER. Complex integration of matrix, oxidative stress, and apoptosis in genetic emphysema. Am J Pathol. 2009;175:84–96. doi: 10.2353/ajpath.2009.080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neptune ER, Podowski M, Calvi C, Cho JH, Garcia JG, Tuder R, Linnoila RI, Tsai MJ, Dietz HC. Targeted disruption of NeuroD, a proneural basic helix-loop-helix factor, impairs distal lung formation and neuroendocrine morphology in the neonatal lung. J Biol Chem. 2008;283:21160–21169. doi: 10.1074/jbc.M708692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buczynski BW, Yee M, Paige Lawrence B, O'Reilly MA. Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1078–L1087. doi: 10.1152/ajplung.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, Pryhuber GS, Moore BB, Hershenson MB. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS ONE. 2012;7:e31336. doi: 10.1371/journal.pone.0031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Choo-Wing R, Sun H, Sureshbabu A, Sakurai R, Rehan VK, Bhandari V. A potential role of the JNK pathway in hyperoxia-induced cell death, myofibroblast transdifferentiation and TGF-beta1-mediated injury in the developing murine lung. BMC Cell Biol. 2011;12:54. doi: 10.1186/1471-2121-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath-Morrow SA, Cho C, Soutiere S, Mitzner W, Tuder R. The effect of neonatal hyperoxia on the lung of p21Waf1/Cip1/Sdi1-deficient mice. Am J Respir Cell Mol Biol. 2004;30:635–640. doi: 10.1165/rcmb.2003-0049OC. [DOI] [PubMed] [Google Scholar]
- 26.Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- 27.Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Choi YK, Lee KS, Cho DH, Baek YY, Lee DK, Ha KS, Choe J, Won MH, Jeoung D, et al. Functional dissection of Nrf2-dependent phase II genes in vascular inflammation and endotoxic injury using Keap1 siRNA. Free Radic Biol Med. 2012;53:629–640. doi: 10.1016/j.freeradbiomed.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:Quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, Biswal S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLOS Comput Biol. 2008;4:e1000166. doi: 10.1371/journal.pcbi.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, Crapo JD. Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am J Respir Cell Mol Biol. 1997;17:393–403. doi: 10.1165/ajrcmb.17.4.2826. [DOI] [PubMed] [Google Scholar]
- 32.Chetty A, Cao GJ, Severgnini M, Simon A, Warburton R, Nielsen HC. Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L584–L592. doi: 10.1152/ajplung.00441.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, O'Reilly M, Biswal S. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L565–L573. doi: 10.1152/ajplung.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelko IN, Folz RJ. Sp1 and Sp3 transcription factors mediate trichostatin A-induced and basal expression of extracellular superoxide dismutase. Free Radic Biol Med. 2004;37:1256–1271. doi: 10.1016/j.freeradbiomed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics. 2000;106:1452–1459. doi: 10.1542/peds.106.6.1452. [DOI] [PubMed] [Google Scholar]
- 36.Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE. Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics. 2003;111:766–776. doi: 10.1542/peds.111.4.766. [DOI] [PubMed] [Google Scholar]
- 37.Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD. Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun. 2000;275:542–548. doi: 10.1006/bbrc.2000.3327. [DOI] [PubMed] [Google Scholar]
- 38.Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, Jacobsen C, Bowler RP, Fattman CL, Crapo JD, et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen AD, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95:1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 40.De Paepe ME, Mao Q, Chao Y, Powell JL, Rubin LP, Sharma S. Hyperoxia-induced apoptosis and Fas/FasL expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L647–L659. doi: 10.1152/ajplung.00445.2004. [DOI] [PubMed] [Google Scholar]
- 41.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced bax activation and cell death in alveolar epithelial cells. J Biol Chem. 2004;279:6753–6760. doi: 10.1074/jbc.M310145200. [DOI] [PubMed] [Google Scholar]
- 42.Gehen SC, Vitiello PF, Bambara RA, Keng PC, O'Reilly MA. Downregulation of PCNA potentiates p21-mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L716–L724. doi: 10.1152/ajplung.00135.2006. [DOI] [PubMed] [Google Scholar]
- 43.McGrath-Morrow SA, Stahl J. Growth arrest in A549 cells during hyperoxic stress is associated with decreased cyclin B1 and increased p21(Waf1/Cip1/Sdi1) levels. Biochim Biophys Acta. 2001;1538:90–97. doi: 10.1016/s0167-4889(00)00142-7. [DOI] [PubMed] [Google Scholar]
- 44.Pagano A, Pitteloud C, Reverdin C, Metrailler-Ruchonnet I, Donati Y, Barazzone Argiroffo C. Poly(ADP-ribose)polymerase activation mediates lung epithelial cell death in vitro but is not essential in hyperoxia-induced lung injury. Am J Respir Cell Mol Biol. 2005;33:555–564. doi: 10.1165/rcmb.2004-0361OC. [DOI] [PubMed] [Google Scholar]
- 45.Joseph A, Li Y, Koo HC, Davis JM, Pollack S, Kazzaz JA. Superoxide dismutase attenuates hyperoxia-induced interleukin-8 induction via AP-1. Free Radic Biol Med. 2008;45:1143–1149. doi: 10.1016/j.freeradbiomed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Koo HC, Davis JM, Li Y, Hatzis D, Opsimos H, Pollack S, Strayer MS, Ballard PL, Kazzaz JA. Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2005;288:L718–L726. doi: 10.1152/ajplung.00456.2003. [DOI] [PubMed] [Google Scholar]
- 47.Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, Pollack S, Horowitz S, Davis JM. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol. 2001;24:436–441. doi: 10.1165/ajrcmb.24.4.4240. [DOI] [PubMed] [Google Scholar]
- 48.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lozon TI, Eastman AJ, Matute-Bello G, Chen P, Hallstrand TS, Altemeier WA. PKR-dependent CHOP induction limits hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300:L422–L429. doi: 10.1152/ajplung.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturrock A, Seedahmed E, Mir-Kasimov M, Boltax J, McManus ML, Paine R., III GM-CSF provides autocrine protection for murine alveolar epithelial cells from oxidant-induced mitochondrial injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L343–L351. doi: 10.1152/ajplung.00276.2011. [DOI] [PubMed] [Google Scholar]