Abstract
NF-κB and IL-6, a NF-κB downstream mediator, play a central role in the inflammatory response of tissues. We aimed to determine the role of the classical NF-κB pathway in severe pulmonary arterial hypertension (PAH) induced by SU5416 and chronic hypoxia (SuHx) in rats. Tissue samples from patients with idiopathic PAH (iPAH) and control subjects were investigated. SuHx rats were treated from Days 1 to 3, 1 to 21, and 29 to 42 with the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) and/or from Days 1 to 21 with anti–IL-6 antibody. Nuclear staining for NF-κB, an indicator of the activation of the classical NF-κB pathway, was detected in pulmonary arterial lesions of patients with iPAH and SuHx rats. NF-κB inhibition with PDTC prevented and reduced pulmonary arterial obliteration without reducing muscularization. However, the elevated lung levels of IL-6 were not reduced in PDTC-treated SuHx animals. PDTC treatment prevented or reduced apoptosis of pulmonary artery wall cells and pulmonary arterial obliteration. IL-6 inhibition had only a partial effect on apoptosis and obliteration. Pulmonary arterial media wall thickness was not affected by any of these treatments. Preventive and therapeutic PDTC treatment promoted immune regulation by increasing the number of perivascular CD4+ T cells, in particular regulatory T cells (early treatment), and by reducing the number of perivascular CD8+ T lymphocytes and CD45RA+ B lymphocytes. Therapeutic PDTC treatment further preserved right ventricular function in SuHx animals. Inhibition of NF-κB may represent a therapeutic option for pulmonary arterial obliteration via reduced vessel wall cell apoptosis and improved regulation of the immune system.
Keywords: pulmonary hypertension, lymphocytes, apoptosis, inflammation, proliferation
Clinical Relevance
This work demonstrates an important role for a central proinflammatory pathway (nuclear factor-κB) in the development and progression of pulmonary vascular lesions in severe pulmonary arterial hypertension. Inhibition of this pathway is shown to have protective effects on pulmonary vascular remodeling and to prevent the development of right heart failure in the context of severe pulmonary hypertension with luminal obliteration.
Severe pulmonary arterial hypertension (PAH) is a disease of the pulmonary arteries that is characterized by progressive obliteration of the small pulmonary arteries, increased pulmonary vascular resistance, and right ventricular failure (1). Because no curative treatment is available for the progressive pulmonary arterial vasculopathy, novel treatment strategies are needed (2). Levels of proinflammatory cytokines and chemokines are increased in lung tissue and blood of patients with PAH, and the accumulation of leukocytes has been found in the perivascular region of remodeling pulmonary arteries (2–6). Although inflammation is considered as an important contributor to vascular remodeling in PAH, the relevance of inflammation for pulmonary arterial obliteration in severe PAH is unclear.
NF-κB is a central regulator of inflammation. Activation of the classical NF-κB pathway induces expression of various genes encoding proinflammatory cytokines and chemokines, such as IL-6, and NF-κB can therefore promote vascular inflammation by chemotaxis of leukocytes (7, 8). The classical NF-κB pathway requires the release of the p65/p50 subunit from the inhibitor of κB complex and the translocation of p65/p50 heterodimers to the nucleus (7). The role of NF-κB in PAH is incompletely understood: pulmonary artery smooth muscle cells and macrophages derived from patients with idiopathic PAH (iPAH) show increased NF-κB activation, and NF-κB inhibition ameliorates monocrotaline (MCT)-induced PAH (9–13), but it is unclear whether NF-κB contributes to the obliterative overgrowth of cells in pulmonary arterial obliteration, as found in severe PAH.
We hypothesized that classical NF-κB signaling is essential for the lumen obliteration of pulmonary arteries and that NF-κB activation promotes pulmonary vascular remodeling via the modulation of cell growth and inflammation and the impairment of immune regulation. Here, we demonstrate that the classical NF-κB pathway is activated in the plexiform lesions of patients with iPAH and in pulmonary arterial lesions of rats with severe obliterative PAH induced by the vascular endothelial growth factor receptor inhibitor SU5416 and chronic hypoxia (SuHx). We further show that inhibition of NF-κB with pyrrolidine dithiocarbamate (PDTC) prevented apoptosis of pulmonary artery wall cells, pulmonary arterial obliteration, and in part PAH but failed to ameliorate pulmonary arterial muscularization. In established SuHx PAH, PDTC treatment reduced pulmonary arterial obliteration and preserved the function of the right ventricle (RV). PDTC treatment promoted immune regulation by increasing the perivascular accumulation of CD4+ T cells, in particular regulatory T (T reg) cells, and by reducing the perivascular accumulation of CD8+ T and CD45RA+ B lymphocytes. Inhibition of the NF-κB downstream target IL-6 alone prevented pulmonary arterial obliteration to a lesser degree than PDTC or PDTC+IL-6 inhibition. Pulmonary arterial muscularization was not affected by any of these treatments.
Materials and Methods
Human Tissue
Human deidentified lung tissue samples were obtained from the Department of Pathology, University of Colorado Denver. The collection of human tissue samples was approved by the local institutional research ethics board at the University of Colorado Denver. The use of deidentified tissue samples was approved as non-human subjects research by the Office of Research Subjects Protection at Virginia Commonwealth University.
Animal Experiments
All animal experiments were approved by the institutional animal care and use committee and were performed according to the Guidelines for the Care and Use of Laboratory Animals (revision 1996). SuHx-induced PAH was established, and animals were killed by exsanguination after hemodynamic measurements (14). The right lung was snap frozen for molecular biology studies and/or sampled for flow cytometry. The left lung was inflated with 0.5% low-melting agarose (20 cm H2O), formalin-fixed (48 h), and paraffin embedded for histology. The RV was cut in half by a transversal section. One part was snap frozen for molecular biology studies, and the other part was formalin fixed and paraffin embedded for histology. Naive animals were kept at room air.
SuHx animals received 100 mg/kg/d PDTC (Sigma) or vehicle (saline) five times a week intraperitoneally from Day 1 to Day 3, from Day 1 to Day 21, and from Day 29 to Day 42 after SU5416. Controls included naive rats, naive rats treated with vehicle or PDTC from Day 1 to Day 21, and rats housed under conditions of chronic hypoxia and treated with vehicle or PDTC from Day 1 to Day 21. In an additional set of experiments, SuHx animals received PDTC and 5 μg/animal of a control goat IgG three times a week; 5 μg/animal anti–IL-6 antibody (ab) (goat anti-rat) (AF506; R&D Systems, Minneapolis, MN) three times a week; and saline, PDTC and 5 μg/animal anti–IL-6 ab, or control goat IgG and saline. At Day 21, the animals were killed for tissue harvest. For vasodilator experiments, 10 mg/kg of the Rho kinase inhibitor Fasudil (Sigma-Aldrich, St. Louis, MO) was infused over 10 minutes under continuous registration of invasive hemodynamics into SuHx animals treated with vehicle or PDTC from Day 1 to Day 21 as previously described (15). For each animal, the right ventricular systolic pressure (RVSP) was recorded at steady state before Fasudil infusion and at steady state during Fasudil infusion, and the difference in RVSP between steady state before and during Fasudil injection was calculated.
Histology and Morphometry
Details regarding histology, immunohistochemistry (IHC), immunofluorescence (IF), morphometry, IHC quantification, and confocal microscopy are provided in the online supplement.
Protein Lysate Preparation, Western Blot, and ELISA
Details regarding protein isolation, Western blot, and ELISA are provided in the online supplement.
Flow Cytometry
Details regarding flow cytometry are provided in the online supplement.
Statistical Analysis
Data are presented as mean ± SEM. Groups were compared with two-tailed unpaired Student’s t test or one-way ANOVA followed by Newman-Keuls’ multiple comparison test. Statistics and graphs were done with Prism 5.0 (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
Results
NF-κB Activity in Obliterative PAH
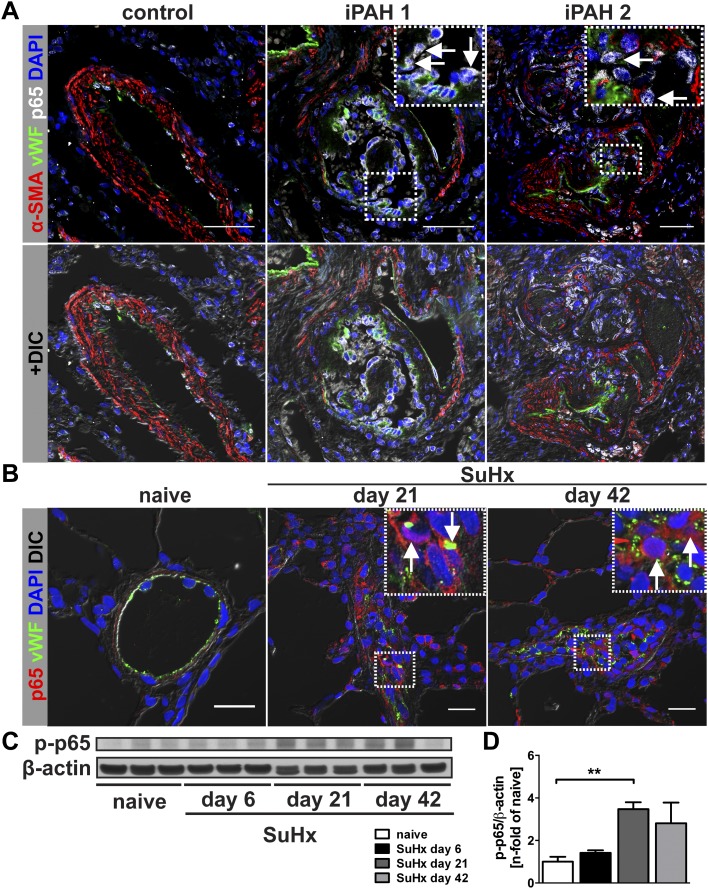
To determine NF-κB activity in pulmonary arterial lesions of patients with iPAH and SuHx animals, we investigated by triple IF and IHC whether nuclear staining (7) for the NF-κB p65 is more frequent in obliterative lung vascular lesions. We found strong nuclear p65 staining in the pulmonary arterial lesions of patients with iPAH that was commonly localized to von Willebrand Factor–positive (vWF+) and, to a lesser degree, to α-smooth muscle actin–positive (α-SMA+) cells. Occasional nuclear staining of vWF+ endothelial cells (ECs) was found in pulmonary arteries of control tissue (Figure 1A and see Figures E1–E3 in the online supplement). Multiple pulmonary artery lesion cells, in particular vWF+ cells, exhibited nuclear p65 IHC staining in SuHx lungs at Days 21 and 42, whereas nuclear p65 staining was rarely detected in pulmonary artery ECs of control rats (Figures 1B and E4–E6). We detected increased expression of phospho-p65 (p-p65), which indicates NF-κB activity (7), by Western blot in the lung tissue homogenate of SuHx animals at Days 21 and 42 (Figures 1C and 1D).
Figure 1.
NF-κB lung vascular lesions of patients with human idiopathic pulmonary arterial hypertension (iPAH) and animals with SU5416 and chronic hypoxia (SuHx)-induced severe pulmonary arterial hypertension (PAH). (A) Confocal microscopy images of triple immunofluorescence (IF) staining for von Willebrand factor (vWF), α-smooth muscle actin (α-SMA), and p65 shows strong nuclear NF-κB p65 expression in vWF+ cells of plexiform lesions in lung tissue of two patients with iPAH (arrows) but not in a pulmonary artery of a control subject without pulmonary vascular disease. The upper panels shows overlays of four fluorescence channels in pseudocolors (including DAPI for nuclear counterstaining), and the lower panel shows the images additionally merged with a differential interference contrast (DIC) image to demonstrate the tissue architecture. Inserts in iPAH1 and iPAH2 images show the region indicated by a dotted box in more detail. Scale bar, 50 μm. (B) In representative optical sections of double IF staining for vWF and p65 obtained by confocal microscopy, SuHx animals at Days 21 and 42 had strong nuclear NF-κB p65 expression in vWF+ pulmonary artery lesion cells. No increase in p65 expression was found in pulmonary arteries of naive animals. Scale bar, 20 μm. The images show overlay of red, green, and blue fluorescence channels in pseudocolors with DIC images to demonstrate tissue architecture. (C) Representative Western blot of phospho-p65 (p-p65) in naive control, SuHx Day 6, Day 21, and Day 42 animals. β-actin was used as loading control. (D) Semiquantitative densitometric analysis of p-p65 Western blots. Densitometric values were normalized versus β-actin and expressed as n-fold of the results of naive control animals. Each bar: mean ± SEM (n = 3 animals per group). **P < 0.01.
To determine whether inflammation was present in the SuHx lungs, we demonstrated by Western blot that the levels of cytokines/chemokines CC chemokine ligand 2 (CCL2) and IL-6, which have been implicated in pulmonary arterial remodeling (16–21), were elevated in SuHx lung tissue protein lysate (Figure E7).
The NF-κB Inhibitor PDTC Prevented Pulmonary Arterial Obliteration without Affecting Muscularization
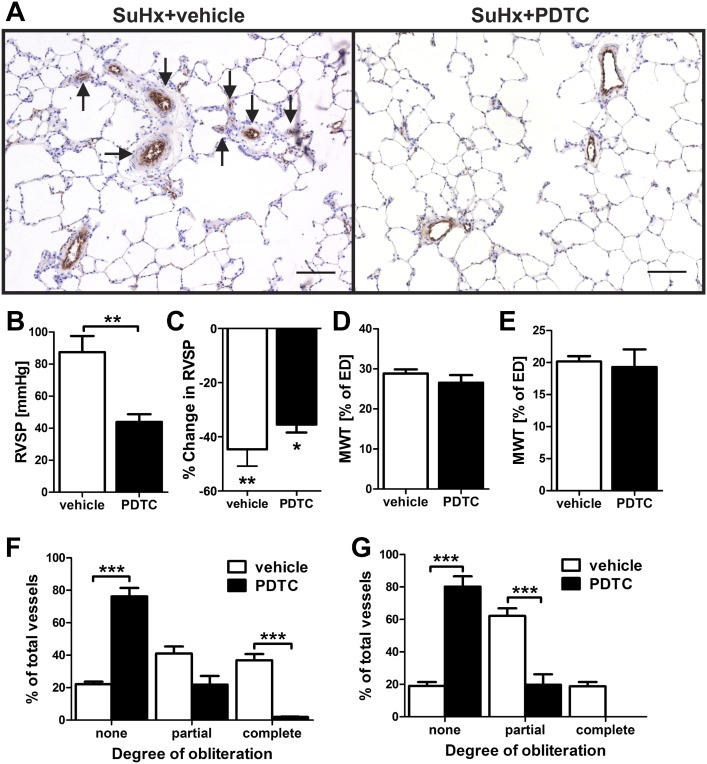
Based on our findings of increased NF-κB activity in the pulmonary artery lesions of the SuHx model, we investigated whether inhibition of the classical NF-κB pathway with PDTC prevents pulmonary arterial remodeling and PAH. Pulmonary arterial obliteration was almost completely prevented and RVSP was decreased in SuHx+PDTC-treated animals, and the residual PAH was highly reactive to infusion of the vasodilator Fasudil, a Rho-kinase inhibitor (15) (Figure 2). In vehicle-treated SuHx animals, there was a similar degree of %RVSP reduction after Fasudil infusion (Figure 2). We did not find a reduction of pulmonary artery media wall thickness (MWT), right ventricular hypertrophy index, or echocardiographically estimated cardiac output in SuHx+PDTC animals as compared with vehicle-treated SuHx animals (Figures 2 and E8). In naive rats or rats housed under conditions of chronic hypoxia, PDTC treatment did not reduce RVSP, right ventricular hypertrophy index, cardiac output, and MWT (Figures E9 and E10). No obliteration of pulmonary arteries was detected in naive or chronic hypoxic rats treated with PDTC (Figure E10). In addition, there was no reduction in cardiac output of SuHx rats at Day 21 with or without PDTC when compared with naive animals (Figures E8 and E9).
Figure 2.
The NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) prevents obliteration of pulmonary arteries and severe PAH. The animals and animal tissues were investigated at Day 21 after SU5416 administration. (A) Representative images of sections with vWF immunohistochemical staining show that, whereas SuHx+vehicle animals had multiple obliterated vessels (arrows), no obliteration was detected in SuHx+PDTC-treated animals. Scale bar, 100 μm. (B) Right ventricular systolic pressure (RVSP) in SuHx+vehicle- and SuHx+PDTC-treated animals. (C) Percentage change in RVSP after Fasudil infusion in SuHx+vehicle- and SuHx+PDTC-treated animals (n = 4). *P < 0.05 and **P < 0.01, both versus pre-Fasudil. (D and E) Media wall thickness (MWT) was not reduced in the small (D) and medium-sized (E) pulmonary arteries of SuHx+PDTC-treated animals versus SuHx+vehicle-treated animals. (F and G) Pulmonary arterial obliteration was reduced in the small (F) and medium-sized (G) pulmonary arteries of SuHx+PDTC-treated animals versus SuHx+vehicle-treated animals. (B, D–G) n = 6 animals per group. **P < 0.01 and ***P < 0.0001.
PDTC Reduced Pulmonary Artery Wall Cell Apoptosis and NF-κB Activity
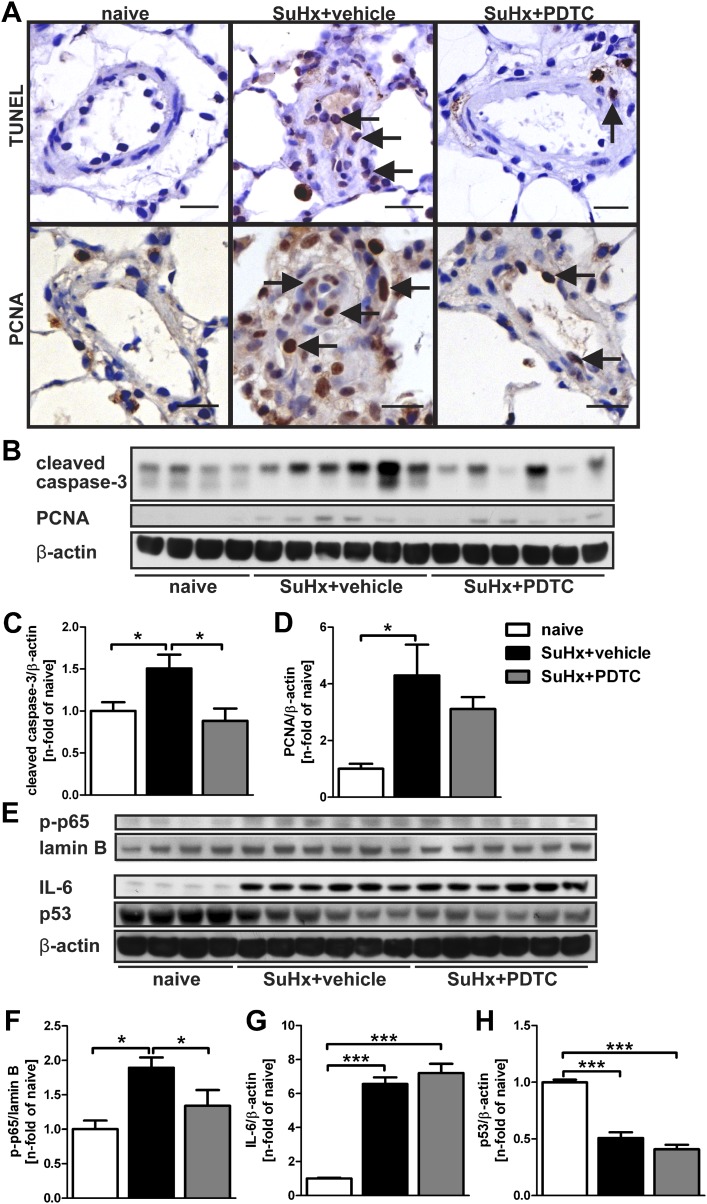
We then examined the effect of preventive PDTC treatment on pulmonary artery wall cell apoptosis and proliferation. We detected, by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and IHC for proliferating cell nuclear antigen (PCNA), that PDTC reduced apoptosis and proliferation in the pulmonary artery wall and perivascular region of SuHx animals at Day 21 (Figure 3A; Table 1). To identify whether early apoptosis was inhibited by PDTC, we investigated SuHx animals treated with vehicle or PDTC from Day 1 to Day 3. At Day 3, PDTC treatment reduced the number of TUNEL+ and cleaved caspase-3+ cells in the pulmonary artery wall and perivascular region (Figure E11). In accordance with the histological findings of less apoptosis and proliferation in pulmonary artery walls, we found reduced levels of cleaved caspase-3 and, as a trend, PCNA in the lung tissue protein lysate of SuHx+PDTC animals at Day 21 (Figures 3B–3D).
Figure 3.
PDTC treatment ameliorates apoptosis and proliferation, as well as NF-κB activity, but not IL-6 levels in the SuHx model. All animal tissues were investigated at Day 21 after SU5416 administration. (A) PDTC treatment reduced the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ (arrows, top row) and proliferating cell nuclear antigen (PCNA)+ (arrows, bottom row) cells in the pulmonary arteries of SuHx animals. Scale bar, 20 μm. (B) Representative Western blots of cleaved caspase-3 and PCNA from lung whole cell lysate indicate a trend toward reduction of PCNA levels after PDTC treatment and a significant reduction in caspase-3 cleavage after PDTC treatment in SuHx animals. (C and D) Semiquantitative densitometry analysis of cleaved caspase-3 (C) and PCNA (D) Western blots. (E) Western blots of p-p65 in nuclear lung lysate (lamin B as loading control) and IL-6 and p53 in whole cell lung lysate (β-actin as loading control). (F–H) Semiquantitative densitometric analysis of Western blots (E) demonstrate that nuclear levels of p-p65 are reduced by PDTC treatment in SuHx lungs (F). Whereas IL-6 levels are still significantly elevated in SuHx+PDTC animals (G), p53 levels remain decreased after PDTC treatment in the lungs of SuHx rats (H). Densitometric values were normalized to lamin B (F) or β-actin (C, D, G, and H) and expressed as n-fold of the results of naive animals. Each bar: mean ± SEM (n = 4 animals per group for naive controls and n = 6 animals per group for SuHx+vehicle and SuHx+PDTC). *P < 0.05 and ***P < 0.0001.
Table 1.
Comparison of Inflammatory, Apoptotic, and Proliferative Cells After Preventive and Therapeutic Pyrrolidine Dithiocarbamate Treatment in Lung Vessels in the SU5416 and Chronic Hypoxia Model (n = 3 Animals Per Group)
| Cells | naïve | SuHx + vehicle, Day 21 | SuHx + PDTC, Day 21 | SuHx + vehicle, Day 42 | SuHx + PDTC, Day 42 |
|---|---|---|---|---|---|
| CD4, n positive cells/vessel | 3.67 ± 0.03 | 1.80 ± 0.12* | 4.33 ± 0.62† | 2.03 ± 0.29* | 3.97 ± 0.22‡ |
| CD8, n positive cells/vessel | 2.93 ± 0.13 | 3.33 ± 0.23 | 1.43 ± 0.12§,|| | 3.60 ± 0.15¶ | 2.03 ± 0.12*,** |
| CD45RA, n positive cells/vessel | 0.70 ± 0.15 | 1.70 ± 0.17* | 0.77 ± 0.12† | 3.40 ± 0.21§ | 2.63 ± 0.26§,†† |
| TUNEL, n positive cells/vessel | 0.40 ± 0.21 | 6.50 ± 0.98* | 1.43 ± 0.33† | 25.40 ± 1.52§ | 16.33 ± 1.15§,** |
| PCNA, n positive cells/vessel | 1.10 ± 0.31 | 18.27 ± 3.33¶ | 5.57 ± 0.24‡‡ | 27.53 ± 5.87§ | 9.67 ± 1.11‡ |
Definition of abbreviations: PCNA, proliferating cell nuclear antigen; PDTC, pyrrolidine dithiocarbamate; SuHx, SU5416 and chronic hypoxia; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
P < 0.01 versus naive.
P < 0.01 versus SuHx+vehicle, Day 21.
P < 0.01 versus SuHx+vehicle, Day 42.
P < 0.0001 versus naive.
P < 0.0001 versus SuHx+vehicle, Day 21.
P < 0.05 versus naive.
P < 0.0001 versus SuHx+vehicle, Day 42.
P < 0.05 versus SuHx+vehicle, Day 42.
P < 0.05 versus SuHx+vehicle, Day 21.
Whereas p-p65 levels, which indicate successful inhibition of classical NF-κB activity, were reduced by PDTC treatment (Figures 3E and 3F), IL-6 levels were still elevated in the lung tissue after PDTC treatment (Figures 3E and 3G). We further detected a reduction of p53 protein levels in the lung of SuHx animals at Day 21, and p53 expression was not augmented by preventive PDTC treatment (Figures 3E and 3H).
PDTC Improved Immune Regulation in the Pulmonary Artery
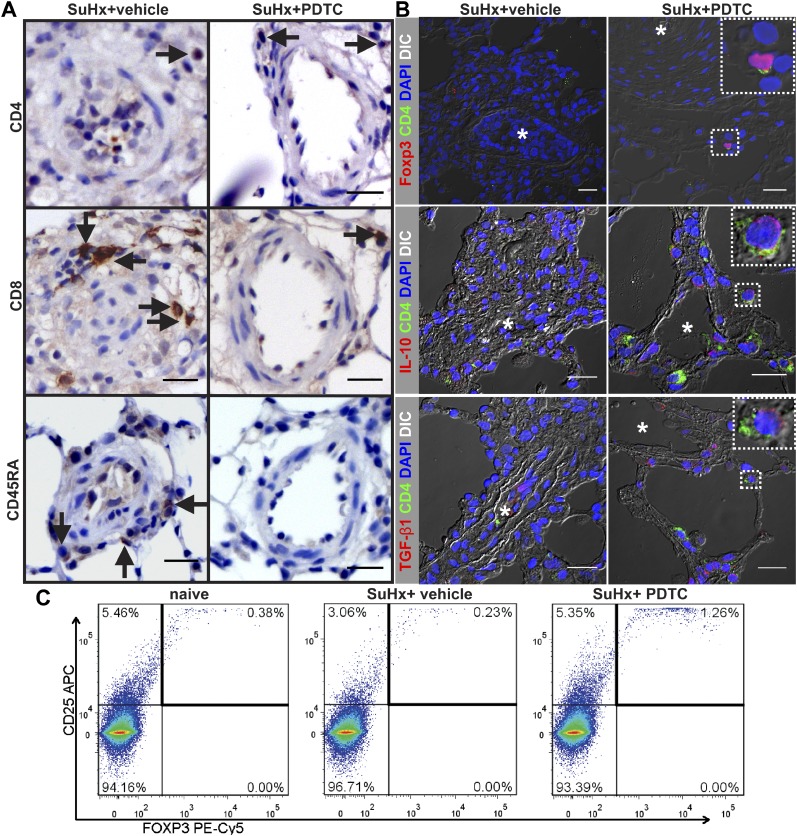
To examine the effects of preventive PDTC treatment on perivascular lymphocyte accumulation, we performed IHC for CD4, CD8, and CD45RA. PDTC treatment resulted in restoration of the number of perivascular CD4+ cells in the nonobliterated pulmonary arteries (Figure 4A; Table 1). We found that SuHx+PDTC-treated animals had smaller perivascular infiltrates and less CD8+ and CD45RA+ cells around the pulmonary arteries than SuHx+vehicle-treated rats (Figure 4A; Table 1). Because the CD4+ cell population contains Treg cells, we examined by flow cytometry and IF staining whether PDTC treatment would increase the pulmonary and perivascular presence of Treg cells in pulmonary arteries. To identify the localization of Treg cells, we examined by IF staining the presence of CD4+ Forkhead box P3+ (Foxp3+), CD4+ IL-10+ cells, and CD4+ transforming growth factor (TGF)-β1+ cells around pulmonary arteries. Treg cells express the anti-inflammatory cytokines TGF-β1 and IL-10 as important cofactors to maintain self-tolerance (22, 23). Whereas CD4+ Foxp3+, CD4+ IL-10+, and CD4+ TGF-β1+ cells were largely absent from the pulmonary arterial lesions in SuHx+vehicle animals, CD4+ Foxp3+, CD4+ TGF-β1+, and CD4+ IL-10+ Treg cells were more frequently found in the perivascular region and close proximity of pulmonary arteries after PDTC treatment (Figure 4B). We found by flow cytometry that, whereas CD25+ Foxp3+ Treg cells were reduced among CD4+ cells in the lungs of SuHx+vehicle animals as compared with naive animals, PDTC-treated SuHx rats had a higher lung content of Treg cells than SuHx+vehicle and naive animals (Figure 4C).
Figure 4.
PDTC treatment improves immune regulation in the SuHx model of severe PAH. Animal tissues were investigated at Day 21 after SU5416 administration. (A) Representative CD4, CD8, and CD45RA immunohistochemistry (IHC) shows increased number of perivascular CD4+ T cells in SuHx animals treated with PDTC (SuHx+PDTC) as compared with SuHx+vehicle animals, whereas the number of perivascular CD8+ T lymphocytes and CD45RA+ B lymphocytes was reduced in SuHx+PDTC. Arrows indicate representative cells with positive staining. Scale bar, 20 μm. (B) CD4+ Forkhead box protein 3+ (Foxp3+), CD4+ IL-10+, and CD4+ transforming growth factor (TGF)-β1+ regulatory T cells (representative cells in inserts) are frequently found in the perivascular region of pulmonary arteries in a SuHx+PDTC animal but not in the perivascular region of obliterated pulmonary arteries in SuHx+vehicle rats. Images of optical sections were obtained with confocal microscopy. Overlay with DIC image is shown for each image merged from pseudocolored single channels to demonstrate tissue architecture. The inserts show CD4+ Foxp3+, CD4+ IL-10+, and CD4+ TGF-β1+ cells outlined by dotted boxes in more detail. Asterisks indicate the original vessel lumen. Nuclear counterstaining with DAPI. Scale bar, 20 μm. (C) Representative flow cytometry dot plots of CD25+ Foxp3+ T reg cells after gating of lung cells for CD4+ cells.
IL-6 Inhibition and PDTC Treatment Prevented Obliteration of Pulmonary Arteries but Not Increased Muscularization
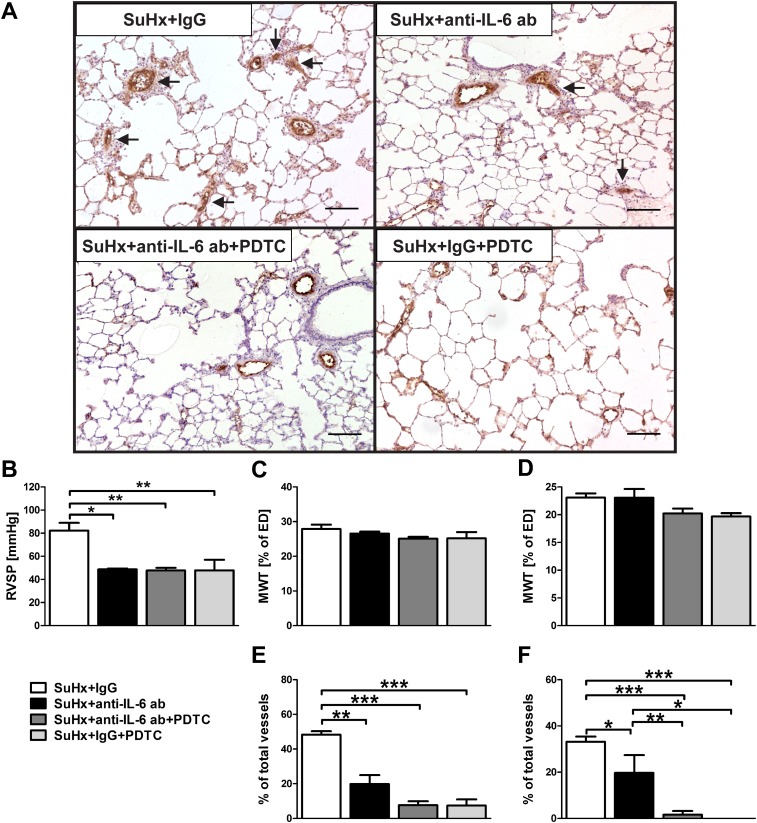
PDTC treatment prevented pulmonary arterial obliteration but not the increase in pulmonary artery MWT. IL-6 protein levels were still elevated in PDTC-treated SuHx animals, and IL-6 has been shown to be important in the increased pulmonary arterial muscularization of chronic hypoxic mice (19, 21). Therefore, we examined whether IL-6 inhibition reduces pulmonary artery MWT in combination with PDTC and prevents pulmonary arterial obliteration and muscularization when administered alone. IL-6 inhibition prevented PAH development in SuHx animals to the same degree as PDTC treatment and partially reduced pulmonary arterial obliteration but, similar to PDTC treatment, did not reduce MWT (Figures 5 and E12). The combination of IL-6 inhibition and NF-κB inhibition had similar effects on PAH development, MWT, and pulmonary arterial obliteration as PDTC treatment alone and did not reduce pulmonary artery MWT (Figures 5 and E12).
Figure 5.
IL-6 neutralization and PDTC treatment did not prevent increased pulmonary arterial muscularization. All animals and animal tissue were investigated at Day 21 after SU5416 administration. (A) Representative images of vWF IHC show that IL-6 inhibition resulted in less obliterated pulmonary arteries (arrows) in SuHx animals, whereas PDTC with or without IL-6 inhibition almost completely prevented pulmonary arterial obliteration. Scale bar, 100 μm. (B) RVSP was reduced by IL-6 inhibition and PDTC treatment with or without IL-6 inhibition. (C and D) Quantification of MWT in small (25 μm < external diameter [ED] < 50 μm) (C) and medium-sized (50 μm ≤ ED < 100 μm) (D) pulmonary arteries in SuHx animals after IL-6 inhibition and/or PDTC treatment. (E and F) Quantification of the fraction of completely obliterated small (E) and medium-sized (F) pulmonary arteries in SuHx animals after IL-6 inhibition with or without PDTC treatment. (B–F) Mean ± SEM (n = 4 animals per group). *P < 0.05, **P < 0.01, and ***P < 0.0001.
IL-6 Inhibition and PDTC Treatment Reduced Apoptosis, Proliferation, and Inflammation in the Lung
We examined whether IL-6 and NF-κB inhibition reduces apoptosis and proliferation of pulmonary artery wall cells. We detected that PDTC treatment, and to a lesser degree IL-6 inhibition, reduced the appearance of TUNEL+ and PCNA+ cells in pulmonary artery wall and perivascular region (Figure E13). We confirmed our findings by Western blots for cleaved caspase-3 and PCNA from lung tissue protein lysates and found that protein levels of cleaved caspase-3 and PCNA were reduced in the SuHx+IgG+PDTC and SuHx+anti–IL-6 ab+PDTC groups and, to a lesser extent, in the SuHx+anti–IL-6 ab group (Figure E13).
We then investigated whether inhibition of NF-κB and IL-6 reduces lung inflammation and found that PDTC treatment and/or IL-6 inhibition prevented the accumulation of granulocytes in the lung following the SuHx protocol (Figure E14). PDTC treatment, but not IL-6 inhibition, reduced the lung concentration of CCL2 significantly (Figure E15).
PDTC Treatment, but Not IL-6 Inhibition, Improved Immune Regulation in the Lung
Because PDTC treatment improved immune regulation in SuHx lungs, we investigated whether inhibition of IL-6 also induces a switch in the balance between CD4+ T helper cells, CD8+ cytotoxic T lymphocytes, and CD45RA+ B lymphocytes in the lungs of SuHx animals. We detected by flow cytometry that the fraction of CD4+ CD8− cells among the CD45+ CD3+ T lymphocytes in the lung was significantly reduced in the lungs of SuHx+IgG animals and remained reduced in SuHx+anti–IL-6 ab and SuHx+anti–IL-6 ab+PDTC animals (Figure E16). In the lungs of SuHx+IgG+PDTC rats, the CD4+ CD8− cell fraction was restored to the level of naive animals (Figure E16). However, the fraction of all CD4+, and in particular of CD4+ CD8+ double-positive T cells among the CD3+ cell fraction, was significantly increased in the lungs of SuHx+anti–IL-6 ab+PDTC-treated animals (Figure E17). In contrast, the fraction of CD8+ CD4− cytotoxic T lymphocytes in the lung was not changed in the SuHx+IgG and SuHx+anti–IL-6 ab groups but was significantly reduced in the SuHx+anti–IL-6 ab+PDTC and SuHx+IgG+PDTC groups (Figure E16). Although there was a small (not significant) increase in the fraction of CD45RA+ B lymphocytes among CD45+ CD3− cells in the lungs of animals in the SuHx+IgG and SuHx+anti–IL-6 ab groups, we measured a strong reduction in the fraction of CD45RA+ B cells in the lungs of SuHx+anti–IL-6 ab+PDTC and SuHx+IgG+PDTC animals (Figure E16).
Therapeutic Treatment with PDTC Reduced Pulmonary Arterial Obliteration but Not Muscularization and PAH
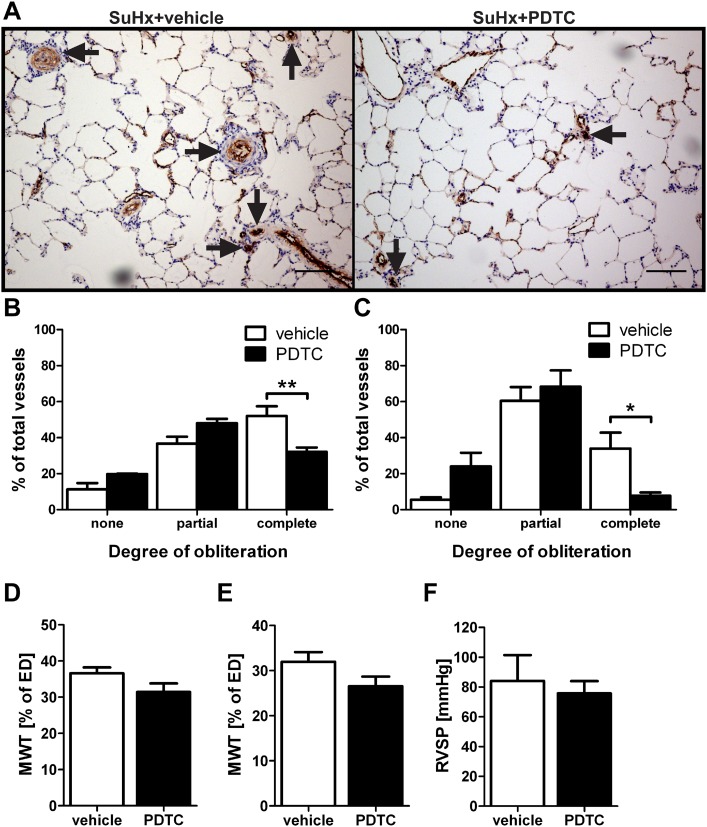
To be able to better translate our preclinical findings to the clinical situation, we evaluated the therapeutic potential of PDTC in SuHx animals with established pulmonary vascular disease and treated SuHx rats from Day 29 to Day42 after SuHx when the animals were housed under normoxic conditions. We detected less completely obliterated pulmonary arteries in PDTC-treated SuHx animals than in SuHx+vehicle animals (Figures 6A–6C). Similar to the prevention study, there was no significant effect of PDTC treatment on MWT of SuHx animals when treated from Day 29 to Day 42 (Figures 6D and 6E). In contrast to the prevention trial, there was no significant effect of PDTC treatment on RVSP of SuHx rats treated from Day 29 to Day 42 (Figure 6F).
Figure 6.
Therapeutic treatment with PDTC from Day 29 to Day 42 ameliorates pulmonary arterial obliteration, but not pulmonary arterial muscularization and pulmonary hypertension, in SuHx rats. All animals and animal tissues were investigated at Day 42 after SU5416 administration. (A) Representative IHC for vWF shows that in SuHx animals treated with vehicle, multiple pulmonary arteries were occluded by vWF+ cells (arrows). In SuHx rats treated with PDTC, less vascular obliteration was detected, mainly in small pulmonary arterioles (arrows). Scale bar, 100 μm. (B and C) Pulmonary arterial obliteration was reduced in the small (B) and medium-sized (C) pulmonary arteries of SuHx+PDTC-treated animals versus SuHx+vehicle-treated animals. (D and E) MWT was not reduced in the small (D) and medium-sized (E) pulmonary arteries of SuHx+PDTC-treated animals versus SuHx+vehicle-treated animals. (F) RVSP in SuHx+vehicle- and SuHx+PDTC-treated animals. (B–F) Each bar: mean ± SEM (n = 3 animals per group). *P < 0.05 and **P < 0.01.
Therapeutic Treatment with PDTC Reduced Apoptosis and Improved Immune Regulation in the Pulmonary Artery Wall
To identify whether late treatment with PDTC had effects on apoptosis and proliferation in the pulmonary artery wall, we investigated the number of TUNEL+ and PCNA+ cells in the pulmonary artery wall of SuHx rats treated with PDTC or vehicle from Day 29 to Day 42. We found that the numbers of TUNEL+ and PCNA+ cells in the pulmonary artery wall of SuHx+vehicle rats was higher in the therapeutic treatment group than in the SuHx+vehicle rats of the prevention treatment and that PDTC treatment from Day 29 to Day 42 reduced the number of TUNEL+ and PCNA+ cells in the pulmonary artery wall (Table 1).
We also quantified the number of CD4+, CD8+, and CD45RA+ cells in the pulmonary artery wall/perivascular infiltrate of SuHx rats after treatment with vehicle or PDTC from Day 29 to Day 42 after SU5416 administration. We detected increased numbers of CD4+ T cells in and around pulmonary arteries of SuHx+PDTC rats as compared with SuHx+vehicle rats (Table 1). In contrast, we found reduced numbers of CD8+ T cells and CD45RA+ B cells in pulmonary artery wall and in the perivascular region of SuHx+PDTC-treated animals (Table 1).
Therapeutic PDTC Treatment Improved Cardiac Output, Capillary Density, and RV Apoptotic Index in SuHx Animals
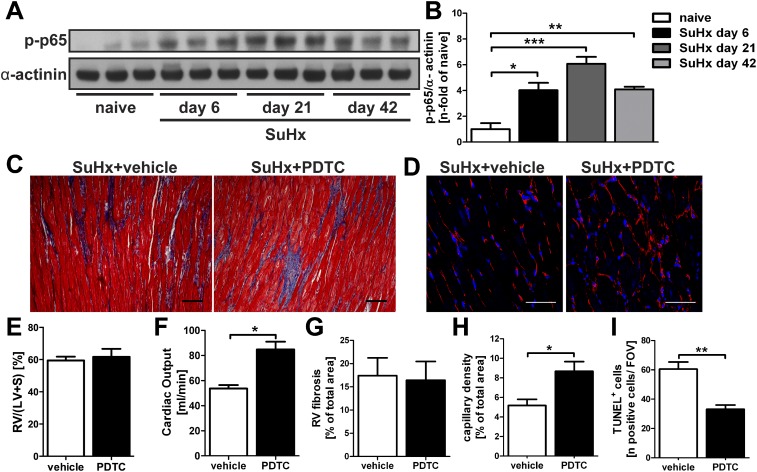
Because RV dysfunction is an important indicator of mortality in patients with established severe PAH (24), we have characterized the functional and structural effects of therapeutic PDTC treatment in SuHx animals with established severe PAH and developing RV failure (25, 26). We investigated whether increased activity of the canonical NF-κB pathway was found in the RV of SuHx rats by Western blot for p-p65, and we detected increased NF-κB activity at Days 6, 21, and 42 in SuHx rats (Figures 7A and 7B). After therapeutic treatment (Days 29–42 after SU5416 administration), there was no change in the degree of RV fibrosis and RV hypertrophy, but we detected a significantly increased cardiac output, elevated RV capillary density, and decreased RV apoptotic index (Figures 7C–7I). Hence, PDTC treatment improved RV function and ameliorated some of the structural changes that occur in the RV of SuHx animals.
Figure 7.
Effect of therapeutic treatment with PDTC on right ventricular function and structure in SuHx animals. (A) Representative Western blot showing the protein levels of p-p65 in the right ventricle (RV) of naive animals and SuHx animals at Days 6, 21, and 42. α-Actinin was used as loading control. (B) Semiquantitative densitometric analysis of p-p65 Western blot in (A). Densitometric values were normalized versus α-actinin and expressed as n-fold of the results of naive control animals. (C) Representative images of Masson’s Trichrome stained images indicate fibrosis in the RV of SuHx+vehicle and SuHx+PDTC rats in blue color. Scale bar, 20 μm. (D) Representative pseudocolored images of RV sections labeled with Texas Red–conjugated tomato lectin obtained by confocal microscopy indicating the capillarization of RVs derived from SuHx+vehicle and SuHx+PDTC rats. Scale bar, 20 μm. RV hypertrophy index (E) and cardiac output (F) in SuHx animals after therapeutic treatment with vehicle or PDTC. Quantification of RV fibrosis (G), RV capillary density (H), and the number of TUNEL+ cells per field of view (FOV) in the RV (I). Animal tissues in (C–I) were investigated at Day 42 after SU5416 administration. (B, E–I) Each bar: mean ± SEM (n = 3 animals per group). *P < 0.05, **P < 0.01, and ***P < 0.0001.
Discussion
Severe PAH is a disease characterized by progressive obliteration of the small pulmonary arteries (1). The contribution of inflammation to the pulmonary arterial remodeling in severe, obliterative PAH is not fully understood (2). The classical NF-κB pathway is a central regulator of cell growth and inflammation (7), and we hypothesized that NF-κB signaling contributes to obliterative pulmonary arterial remodeling. Here, we demonstrate activation of the classical NF-κB pathway in plexiform lesions of patients with iPAH and pulmonary arterial lesions of SuHx rats. We further show that NF-κB inhibition reduced pulmonary arterial obliteration without ameliorating pulmonary arterial muscularization in preventive and therapeutic studies and that PDTC decreased PAH in preventive studies. Pulmonary arterial obliteration was almost completely ameliorated in prevention studies by inhibition of NF-κB or inhibition of NF-κB and IL-6 and, to a lesser degree, by IL-6 inhibition alone. NF-κB inhibition promoted the regulation of the immune system by increasing the perivascular number of CD4+ T cells and Treg cells and by reducing the number of perivascular CD8+ T lymphocytes and CD45RA+ B lymphocytes. NF-κB inhibition also preserved RV function in established severe SuHx-induced PAH.
Previous reports have suggested that NF-κB activity was increased in macrophages, pulmonary artery ECs, or smooth muscle cells of patients with iPAH (9, 27) and in the pulmonary artery ECs of rats with MCT-induced PAH (11). Here, we show evidence for increased activity of the classical NF-κB pathway predominantly in the vWF+ cells in the remodeled pulmonary arteries of patients with iPAH and of SuHx rats. Whereas NF-κB inhibition almost completely prevented pulmonary arterial obliteration, the increased MWT was not influenced by PDTC, and the residual PAH in SuHx+PDTC-treated animals was highly reactive to vasodilator infusion, similar to SuHx+vehicle rats (15). In stark contrast to our results, previous studies have shown that NF-κB inhibition reduces MCT-induced muscularization in rats (10, 11, 13). It needs to be considered in this context that pulmonary arterial remodeling in the rat MCT model is characterized by extensive inflammation and pulmonary arterial muscularization but not by obliteration of pulmonary arteries (28) and may represent a model for a different pathobiological entity.
We detected elevated levels of the cytokine IL-6, a downstream target of NF-κB (8) that has been implicated in the pathobiology of iPAH and in pulmonary arterial remodeling in mice (19, 21, 29), in the lung tissue of SuHx+PDTC rats. We hypothesized that the elevation in IL-6 levels in the lung tissue contributes to the persistence of pulmonary arterial muscularization in PDTC-treated SuHx rats. Pathways in addition to NF-κB (8) have been implicated in the regulation of IL-6 expression. We have detected a significant reduction in the lung protein level of p53 in vehicle-treated and PDTC-treated SuHx animals. p53 is a central regulator of cell growth that has been shown to act as a repressor of IL-6 transcription (30). Indeed, it has been recently shown that activation of lung p53 prevented and reversed experimental pulmonary hypertension (31). IL-6 inhibition partially prevented PAH and angio-obliteration in SuHx rats but did not reduce pulmonary arterial muscularization. The effects of combined treatment with anti–IL-6 ab and PDTC were similar to PDTC alone. Our findings are in accordance with previous results indicating a contribution of IL-6 to pulmonary arterial obliteration (21). IL-6 blockade had a smaller reductive effect on apoptosis and proliferation of PA lesion cells than NF-κB inhibition. These findings correlate with a less pronounced effect of IL-6 inhibition on pulmonary arterial obliteration. IL-6 inhibition reduced PAH to a similar degree as PDTC treatment, and we conclude that the reduction of angio-obliteration after IL-6 inhibition, although not as complete as with PDTC, may be sufficient to decrease PAH to a similar level. Improved endothelial function may account for the reduction in PAH (32). Various other potential explanations may be suggested for the persisting pulmonary arterial muscularization after PDTC treatment or IL-6 inhibition, such as direct effects of hypoxia on pulmonary artery wall cells, increased expression of CXC chemokine ligand 12, persistence of decreased p53 expression, and the contribution of bone marrow–derived cells (31, 33, 34).
The central translational finding of our experiments using inhibition of NF-κB was that pulmonary arterial obliteration was completely prevented or, in the treatment study in established SuHx PAH, significantly ameliorated. In severe PAH, post-EC apoptotic overgrowth of pulmonary artery lesion cells and ongoing inflammation are accepted as important contributors to pulmonary arterial obliteration (2). Here, we show that NF-κB inhibition reduced pulmonary artery wall cell apoptosis and lesion cell overgrowth as well as inflammation in the SuHx model. Because NF-κB modulates apoptosis and proliferation depending on cell type and environment (35, 36), it is likely that the direct antiapoptotic effects of NF-κB inhibition on ECs contribute to the reduced apoptosis that we have observed in SuHx+PDTC animals (35). Less EC apoptosis would then decrease the proliferation of pulmonary artery lesion cells and pulmonary arterial obliteration (37). This concept is supported by our findings that PDTC treatment reduced apoptosis very early on (at Day 3) and later in established severe PAH (Day 42) in the SuHx model. PDTC may also reduce EC apoptosis by inhibiting the ongoing inflammatory response, as demonstrated by our findings of reduced levels of the NF-κB downstream mediator CCL2, a monocyte/macrophage chemoattractant relevant to PAH pathobiology (38), and of decreased accumulation of granulocytes in the lungs of SuHx animals after PDTC treatment. In contrast, IL-6 inhibition only marginally reduced pulmonary artery lesion cell apoptosis and proliferation. IL-6 inhibition decreased granulocyte accumulation but did not reduce the lung level of CCL2 and therefore only partially diminished the inflammatory response. The effects on pulmonary artery wall cell apoptosis and angio-obliteration may be less pronounced with IL-6 inhibition than with PDTC treatment because IL-6 inhibition only interfered with a part of NF-κB downstream signaling (8).
Because we detected accumulation of lymphocytes in the perivascular infiltrate of pulmonary arteries in the SuHx model, similar to human iPAH (5, 39), we have investigated whether inhibition of NF-κB and IL-6 alters the balance between lymphocyte fractions. We detected in SuHx lungs a reduced number of perivascular CD4+ T lymphocytes, which contain Treg cells, which are CD4+ T cells that prevent an overactive immune response (40). In contrast, we detected unchanged numbers of perivascular CD8+ T lymphocytes and increased numbers of perivascular CD45RA+ B lymphocytes in SuHx lungs. Preventive and therapeutic PDTC treatment increased the number of CD4+ T cells while reducing the number of CD8+ T cells and B lymphocytes. Preventive NF-κB inhibition induced in particular the Treg lung fraction and the appearance of perivascular Treg cells. However, the elevated number of Treg cells cannot account for the total increase of the CD4+ T cell fraction, and the CD4+ cell fraction of rats also contains CD25− Foxp3− cells that have immune regulatory function in addition to Treg cells (41). Because the classical NF-κB pathway is important for the development and activation of mature B and T lymphocytes (42), it is not surprising that NF-κB inhibition alters the immune balance via regulation of the lymphocyte fractions. Although it has been shown that CD4+ T lymphocytes, in particular Treg cells, protect against angio-obliterative PAH (40), there are conflicting data in the literature regarding the role of NF-κB in Treg cell development. Whereas NF-κB signaling has been suggested to be required for the expression of Foxp3 (43), another publication identified a natural inhibitor of NF-κB that induces Foxp3 expression during Treg cell development (44). Our data indicate that the results of PDTC treatment on Treg cell accumulation in the SuHx model may be rather similar to the natural inhibitor of NF-κB. B lymphocytes have been suggested as important contributors to pulmonary arterial obliteration through ab deposition (18, 45), and CD8+ cytotoxic T cells can mediate endothelial damage (46). The increase in perivascular CD4+ T cells, and in particular Treg cells, paired with the reduction of perivascular CD8+ T cells and CD45RA+ B lymphocytes, may prevent the ongoing EC apoptosis. The consequence would be the prevention of a vicious circle of EC apoptosis, lymphocyte accumulation, and lesion cell proliferation, as previously suggested (40).
IL-6 inhibition alone had no effect on the balance between lymphocyte populations in the lungs. One potential explanation for the less pronounced effect of IL-6 inhibition on vascular remodeling is the lacking effect on immune regulation. However, the combination of NF-κB inhibition and IL-6 inhibition did not lead to an increase in the number of CD4+ CD8− T cells, as found with PDTC treatment alone, but instead highly increased the number of CD4+CD8+ double-positive T cells in the lung. Such CD4+ CD8+ double-positive T cells can be detected outside of the thymus, and elevated numbers of these double-positive cells have been shown in autoimmune diseases (e.g., systemic sclerosis) and in graft versus host disease after bone marrow transplantation (47, 48). Recently, it has been shown that CD4+ CD8+ double-positive T cells can exert regulatory cell function by inhibiting the alloresponse in an in vitro assay in the context of graft versus host disease after bone marrow transplantation (48). Hence, the combination of NF-κB and IL-6 inhibition may also have promoted immune regulation, likely via increasing the CD4+ CD8+ double-positive T cell population.
The second major finding of high translational value in our experiments was a protective effect of PDTC on RV function and structure when used in a therapeutic treatment protocol in established severe PAH in SuHx rats. Whereas cardiac output was reduced in SuHx+vehicle animals in accordance with previously published literature (25), cardiac output was significantly improved in PDTC-treated SuHx rats. It is controversial whether NF-κB inhibition is beneficial or not in the left heart (49). It appears that although short-time activation of NF-κB may be protective for the left heart, sustained NF-κB activation may promote apoptotic cardiac cell death and the progression to left heart failure (49, 50). Indeed, we found a sustained elevation of NF-κB activation in the RV of SuHx rats associated with increased apoptotic index and RV dysfunction. Although no effect on the degree of RV fibrosis was seen after PDTC treatment, we detected improved capillary density and reduced RV apoptotic index after PDTC treatment. The higher cardiac output in PDTC-treated SuHx rats may also explain the finding that PAH was not reduced in SuHx animals after therapeutic treatment with PDTC, although the degree of pulmonary arterial obliteration was ameliorated (24).
There are potential limitations of our study. (1) The NF-κB/proteasome inhibitor PDTC may induce off-target effects not exclusively related to NF-κB inhibition, and (2) we have not proven that CD4+ T cells and Treg cells are required for the protective effects of PDTC treatment.
In summary, our data indicate that NF-κB has a central role in promoting pulmonary arterial obliteration, inflammation, and RV dysfunction and reducing immune regulation in the context of severe obliterative PAH. The NF-κB downstream mediator IL-6 has a partial effect on pulmonary arterial obliteration and inflammation but does not influence immune regulation. In conclusion, NF-κB inhibition may be a valid therapeutic target for severe obliterative PAH and associated RV dysfunction, but the detailed mechanism and the level of NF-κB inhibition have to be established because of the fundamental importance of NF-κB signaling for host defense and repair processes (7).
Footnotes
This work was supported by the CTSA of the Virginia Commonwealth University (UL1RR031990 from the National Center for Research Resources), by the AD Williams’ Funds of the Virginia Commonwealth University (L.F.), by the Victoria Johnson Center Research Funds, by National Institutes of Health/National Heart, Lung and Blood Institute grant HL114816 (L.F.) (study part on human tissue) and American Heart Association grant 13SDG16360018 (L.F.). Confocal microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from National Institutes of Health-National Institute of Neurological Disorders and Stroke Center core grant 5P30NS047463. Flow cytometry was performed at the VCU Massey Cancer Center Flow Cytometry Shared Resource, supported, in part, with funding from National Institutes of Health-National Cancer Institute Cancer Center Support Grant P30 CA016059.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0355OC on March 31, 2014
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erzurum S, Rounds SI, Stevens T, Aldred M, Aliotta J, Archer SL, Asosingh K, Balaban R, Bauer N, Bhattacharya J, et al. Strategic plan for lung vascular research: an NHLBI-ORDR workshop report. Am J Respir Crit Care Med. 2010;182:1554–1562. doi: 10.1164/rccm.201006-0869WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(Suppl):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Price LC, Wort SJ, Perros F, Dorfmüller P, Huertas A, Montani D, Cohen-Kaminsky S, Humbert M. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 5.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman A, Fazal F. Blocking nf-kappab: an inflammatory issue. Proc Am Thorac Soc. 2011;8:497–503. doi: 10.1513/pats.201101-009MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasier AR. The nuclear factor-κb–interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang J-Y, et al. A functional single-nucleotide polymorphism in the trpc6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Kaminski PM, Edwards JG, Yeh A, Wolin MS, Frishman WH, Gewitz MH, Mathew R. Pyrrolidine dithiocarbamate restores endothelial cell membrane integrity and attenuates monocrotaline-induced pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1250–L1259. doi: 10.1152/ajplung.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, Yamamoto H, Imanaka-Yoshida K, Shimpo H, Mizoguchi A, et al. A nuclear factor-kappab inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest. 2007;132:1265–1274. doi: 10.1378/chest.06-2243. [DOI] [PubMed] [Google Scholar]
- 12.Kimura S, Egashira K, Chen L, Nakano K, Iwata E, Miyagawa M, Tsujimoto H, Hara K, Morishita R, Sueishi K, et al. Nanoparticle-mediated delivery of nuclear factor kappab decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension. 2009;53:877–883. doi: 10.1161/HYPERTENSIONAHA.108.121418. [DOI] [PubMed] [Google Scholar]
- 13.Hosokawa S, Haraguchi G, Sasaki A, Arai H, Muto S, Itai A, Doi S, Mizutani S, Isobe M. Pathophysiological roles of nf-kb in pulmonary arterial hypertension: effects of synthetic selective nf-kb inhibitor imd-0354. Cardiovasc Res. 2013;99:35–43. doi: 10.1093/cvr/cvt105. [DOI] [PubMed] [Google Scholar]
- 14.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the vegf receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 15.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto S, Yamaoka T, Nishida K, Takeshita A, Egashira K, Sueishi K. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2002;283:H2021–H2028. doi: 10.1152/ajpheart.00919.2001. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived cc chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–1047. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno S, Farkas L, Al Husseini A, Farkas D, Gomez-Arroyo J, Kraskauskas D, Nicolls MR, Cool CD, Bogaard HJ, Voelkel NF. Severe pulmonary arterial hypertension induced by su5416 and ovalbumin immunization. Am J Respir Cell Mol Biol. 2012;47:679–687. doi: 10.1165/rcmb.2012-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selimovic N, Bergh C-H, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J. 2009;34:662–668. doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- 21.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. Tgf-beta 1 plays an important role in the mechanism of cd4+cd25+ regulatory t cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 23.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory t cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, et al. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 Suppl):D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 26.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, et al. Pgc1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price LC, Caramori G, Perros F, Meng C, Gambaryan N, Dorfmuller P, Montani D, Casolari P, Zhu J, Dimopoulos K, et al. Nuclear factor κ-b is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS ONE. 2013;8:e75415. doi: 10.1371/journal.pone.0075415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 30.Santhanam U, Ray A, Sehgal PB. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA. 1991;88:7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouraret N, Marcos E, Abid S, Gary-Bobo G, Saker M, Houssaini A, Dubois-Rande JL, Boyer L, Boczkowski J, Derumeaux G, et al. Activation of lung p53 by nutlin-3a prevents and reverses experimental pulmonary hypertension. Circulation. 2013;127:1664–1676. doi: 10.1161/CIRCULATIONAHA.113.002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin ii type 1 receptor. Circ Res. 2004;94:534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 33.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (himf/fizz1/relmα) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L582–L593. doi: 10.1152/ajplung.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farkas D, Kraskauskas D, Drake JI, Alhussaini AA, Kraskauskiene V, Bogaard HJ, Cool CD, Voelkel NF, Farkas L. Cxcr4 inhibition ameliorates severe obliterative pulmonary hypertension and accumulation of c-kit+ cells in rats. PLoS ONE. 2014;9:e89810. doi: 10.1371/journal.pone.0089810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright CJ, Agboke F, Muthu M, Michaelis KA, Mundy MA, La P, Yang G, Dennery PA. Nuclear factor-κb (nf-κb) inhibitory protein iκbβ determines apoptotic cell death following exposure to oxidative stress. J Biol Chem. 2012;287:6230–6239. doi: 10.1074/jbc.M111.318246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS. Nf-κb controls cell growth and differentiation through transcriptional regulation of cyclin d1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 38.Itoh T, Nagaya N, Ishibashi-Ueda H, Kyotani S, Oya H, Sakamaki F, Kimura H, Nakanishi N. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology. 2006;11:158–163. doi: 10.1111/j.1440-1843.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 39.Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 40.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, et al. Regulatory t cells limit vascular endothelial injury and prevent pulmonary hypertension / novelty and significance. Circ Res. 2011;109:867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillebrands JL, Whalen B, Visser JT, Koning J, Bishop KD, Leif J, Rozing J, Mordes JP, Greiner DL, Rossini AA. A regulatory cd4+ t cell subset in the bb rat model of autoimmune diabetes expresses neither cd25 nor foxp3. J Immunol. 2006;177:7820–7832. doi: 10.4049/jimmunol.177.11.7820. [DOI] [PubMed] [Google Scholar]
- 42.Gerondakis S, Siebenlist U. Roles of the nf-κb pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long M, Park S-G, Strickland I, Hayden MS, Ghosh S. Nuclear factor-κb modulates regulatory t cell development by directly regulating expression of foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Schuster M, Glauben R, Plaza-Sirvent C, Schreiber L, Annemann M, Floess S, Kühl Anja A, Clayton Linda K, Sparwasser T, Schulze-Osthoff K, et al. Iκbns protein mediates regulatory t cell development via induction of the foxp3 transcription factor. Immunity. 2012;37:998–1008. doi: 10.1016/j.immuni.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of t cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007;75:1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, Gratwohl A. Endothelial injury mediated by cytotoxic t lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 47.Parel Y, Chizzolini C. Cd4+ cd8+ double positive (dp) t cells in health and disease. Autoimmun Rev. 2004;3:215–220. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Eljaafari A, Yuruker O, Ferrand C, Farre A, Addey C, Tartelin ML, Thomas X, Tiberghien P, Simpson E, Rigal D, et al. Isolation of human cd4/cd8 double-positive, graft-versus-host disease-protective, minor histocompatibility antigen-specific regulatory t cells and of a novel hla-dr7-restricted hy-specific cd4 clone. J Immunol. 2013;190:184–194. doi: 10.4049/jimmunol.1201163. [DOI] [PubMed] [Google Scholar]
- 49.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of nf-kappab in the heart: to be or not to nf-kappab. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 50.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte nf-kappab p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res. 2011;89:129–138. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]