Abstract
Background:
Extracellular stimulation of cells with growth factors such as epidermal growth factor (EGF) induces cell proliferation and cell transformation. Although fibroblast growth factor (FGF) is a well-known family member of growth factors and acts as a ligand of FGF receptor (FGFR), a receptor tyrosine kinase, in cytoplasmic membrane, the tumor promoter potential of FGF has not been clearly understood.
Methods:
The role of FGF as a tumor promoter was determined measuring its effects of cell proliferation and transformation by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium and anchorage-independent cell transformation assays, respectively. The antibody specificity of phospho-RSK2 Tyr529 was determined by Western blotting using a purified FGFR kinase domain in vitro and the membrane fraction of JB6 Cl41 cells ex vivo. The signaling pathways mediated by FGF or EGF were determined by the comparisons of phosphorylation inhibitory efficacy using signaling inhibitors including kaempferol.
Results:
FGF acted as a tumor promoter. FGF induced cell proliferation by stimulation of G1/S cell cycle transition, and anchorage-independent cell transformation in JB6 Cl41 cells. FGF-induced FGFR phosphorylation was suppressed by kaempferol treatment in a dose dependent manner. Interestingly, FGF stimulation utilized a non-canonical signaling pathway to activate RSK2 and activating transcription factor (ATF)-1, which was not transduced by EGF stimulation. Importantly, kaempferol inhibited tyrosine phosphorylation of FGFR by FGF stimulation and nuclear accumulation of phospho-ATF-1 at Ser63. Moreover, although kaempferol, 4’-N-benzoyl staurosporine (PKC412), 2-(2’-amino-3’-methoxyphenyl)oxanaphthalen-4-one (PD98059) and 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)buta-diene (U0126) inhibited EGF-induced anchorage-independent cell transformation in JB6 Cl41 cells, FGF-induced cell transformation in soft agar was only inhibited by PKC412 and kaempferol, but not by PD98059 and U0126.
Conclusions:
FGF acts as a tumor promoter and dual inhibition of kaempferol on the kinase activities of FGFR3 and RSK2 suppresses the FGF-induced neoplastic cell transformation through a non-canonical signaling pathway which is not utilized by EGF stimulation.
Keywords: Fibroblast growth factor, Fibroblast growth factor receptor, Kaempferol, Cell transformation, Signaling pathway
INTRODUCTION
The mammalian fibroblast growth factor (FGF) family consists of 18 ligands that bind to four highly conserved transmembrane tyrosine kinase receptors (FGF receptor [FGFR] 1–4).1 The specific binding of FGF to the respective receptor plays essential roles by induction of dimerization and auto-phosphorylation at the kinase domain(s) in cytosolic face, resulting in the activation of the intracellular signaling pathway,2 cell proliferation, migration and survival.3–5 The epidermal growth factor receptor (EGFR) family also belongs to a receptor tyrosine kinase family and is comprised of four members (EGFR, ErbB2, ErbB3 and ErbB4).6 The EGFR signaling pathway is triggered by interaction between EGFR and epidermal growth factor (EGF) known as a tumor promoter, and the intracellular signaling produced by EGF and EGFR interaction is mainly mediated through the Ras-Raf-MEK-MAPK and PI3K-PTEN-AKT signaling pathways.7 Notably, the EGFR signaling pathway plays key roles in cell proliferation, cell transformation, angiogenesis and metastasis.8,9 Although FGF induces cell proliferation, the role of FGF in cell transformation has not been clearly understood.
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), a major flavonoid component of plants and dietary foods including broccoli, cabbage, tomato and kale, contains the antioxidant, anti-inflammatory, antimicrobial, anti-cancer, cardioprotective, neuroprotective, antidiabetic, antioste-oporotic, estrogenic/antiestrogenic, anxiolytic, analgesic and an-tiallergic activities.10–15 Previous studies have demonstrated the chemopreventive/chemotherapeutic effects of kaempferol in various human cancers including those of the ovary, prostate, bladder, colon and skin.10,11,16–18 Our previous results demonstrated that kaempferol directly targets the NH2-terminal kinase domain of RSK2, resulting in the inhibition of EGF-induced cell proliferation, neoplastic cell transformation and RSK2-mediated cancer cell proliferation.19,20 Recent studies demonstrated that kaempferol inhibited MSK1 kinase activity by targeting an active pocket,21 suggesting that kaempferol may have other target molecules. In this study, we demonstrated that FGF strongly induced anchorage-independent cell transformation by stimulation of G1/S cell cycle transition. Moreover, we found that kaempferol inhibited the phosphorylation of FGFR, resulting in inhibition of FGF-induced cell proliferation and transformation by suppression of a non-canonical signaling pathway which is different from the signaling pathway induced by EGF stimulation in cell proliferation and transformation.
MATERIALS AND METHODS
1. Reagents and antibodies
Tris, NaCl, sodium dodecyl sulfate (SDS) and buffer preparations were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture medium and other supplements were purchased from Life Science Technologies (Rockville, MD, USA). Antibodies for the Western blot analysis were from Cell Signaling Technology (Beverly, MA, USA), Santa Cruz Biotechnology (Santa Cruz, CA, USA) or Upstate Biotechnology (Lake Placid, NY, USA). Human FGF and EGF were purchased from BD Sciences (San Jose, CA, USA). Kaempferol, 4‘-N-benzoyl staurosporine (PKC412), 2-(2’-amino-3’-methoxyphenyl)oxanaphthalen-4-one (PD98059) and 1,4-dia-mino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126) were obtained from Sigma-Aldrich. The antibodies against total activating transcription factor (ATF)-1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and pho-spho-ATF1 antibody was obtained from Abcam (Cambridge, MA, USA).
2. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay
JB6 Cl41 cells (1 × 103) were seeded into 96-well plates in 100 μl of 5% fetal bovine serum-minimal essential medium, cultured for 2 hours at 37°C in a 5% CO2 incubator, and then at 0 hour, the absorbance was measured at optical density of 492 nm and 690 nm using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium-based Cell Titer 96® Aqueous One Solution according to manufacturer’s suggested protocols (Promega, Madison, WI, USA). Briefly, the cells in each well were added 20 μl of MTS solution and incubated for 1 hour at 37°C in a 5% CO2 incubator. The reaction was stopped by adding 25 ul of 10% SDS solution to each well and the absorbance was measured immediately at 492 and 690 nm. The inhibitory effects of kaempferol on the cell proliferation were evaluated by comparing the absorbance of a vehicle-treated control group over 72 hours at 24-hour intervals.
3. Anchorage-independent cell transformation assay
EGF-or FGF-induced cell transformation was examined in the JB6 Cl41 cells. Briefly, cells (8 × 103/ml) were exposed to EGF or FGF (0.1–10 ng/ml) in 1 ml of 0.3% basal medium Eagle agar containing 10% fetal bovine serum. The cultures were maintained in a 37°C, 5% CO2 incubator for 2 weeks and the colonies were scored using an ECLIPSE Ti inverted microscope and the NIS-Elements AR (V. 4.0) computer software program (NIKON Instruments Korea, Seoul, Korea) as described previously.22
4. Cell cycle analysis
JB6 Cl41 cells (4 × 105) were seeded into 60-mm cell culture dishes and cultured overnight at 37°C in a 5%, CO2 incubator. To examine the cell cycle under normal cell culture conditions, JB6 Cl41 cells were treated with the indicated concentrations of compounds in complete cell culture medium for 12 hours. To explore the effects of chemical compounds on the cell cycle transition induced by FGF, JB6 Cl41 cells were pretreated with indicated concentration of a compound for 30 minutes and co-stimulated with FGF (1 ng/ml) and a indicated compound for 12 hours. The cells were trypsinized, fixed and then stained with propidium iodide (20 μg/ml) for 15 minutes at 4°C. The cell cycle distribution was measured by fluorescence activated cell sorting flow cytometry (BD FACSCaliburTM flow cytometer, Franklin Lakes, NJ, USA).
5. Western blotting
Samples containing equal amount of protein were resolved by 8–10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked in a blocking buffer containing 5% skim milk and probed with specific antibodies against phospho-ERK, total-ERK, phospho-RSK, total-RSK, phospho-RSK2 Tyr529, phospho-ATF-1, and total-ATF-1 (Cell Signaling Technology, Beverly, MA, USA). Western blots were visualized with an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ, USA) using a Chemidoc XRS imager system (Bio-Rad Laboratories, Hercules, CA, USA).
6. Immunocytofluorescence
JB6 Cl41 cells (2 × 104) were seeded into four-chamber culture slides and cultured for 12 hours. The cells were starved overnight, pretreated with the indicated concentrations of kaempferol for 30 minutes and then co-treated with FGF (10 ng/ml) and indicated concentrations of kaempferol for 30 minutes. The cells were fixed with 4% formalin, permeabilized, and hybridized with the indicated specific antibodies overnight at 4°C in a humidified chamber. The proteins were visualized by hybridization with secondary antibody conjugated with Alexa-568 under an ECLIPSE Ti inverted fluorescence microscope (NIKON Instruments Korea, Seoul, Korea).
7. In vitro kinase assay
His-RSK2-327-740 truncated fusion proteins were purified from uridine 5’-diphospho-N-acetylmuramoyl-L-alanyl-D-gluta-mate:L-lysine ligase using the Ni 2+-nitrilotriacetic acid agarose beads (Qiagen Korea Ltd., Seoul, Korea). Active kinase domain of FGFR (20 ng), and 100 μM of cold adenosine triphosphate were combined with the indicated concentrations of kaempferol in 20 μl of the reaction mixture. The kinase reaction was carried out at 30°C for 30 minutes and stopped by adding 6X SDS-sample buffer and boiling. The proteins were resolved in SDS-polyacrylamide gel electrophoresis and visualized by Western blotting using the specific antibodies as indicated.
RESULTS
1. Fibroblast growth factor acted as a tumor promoter
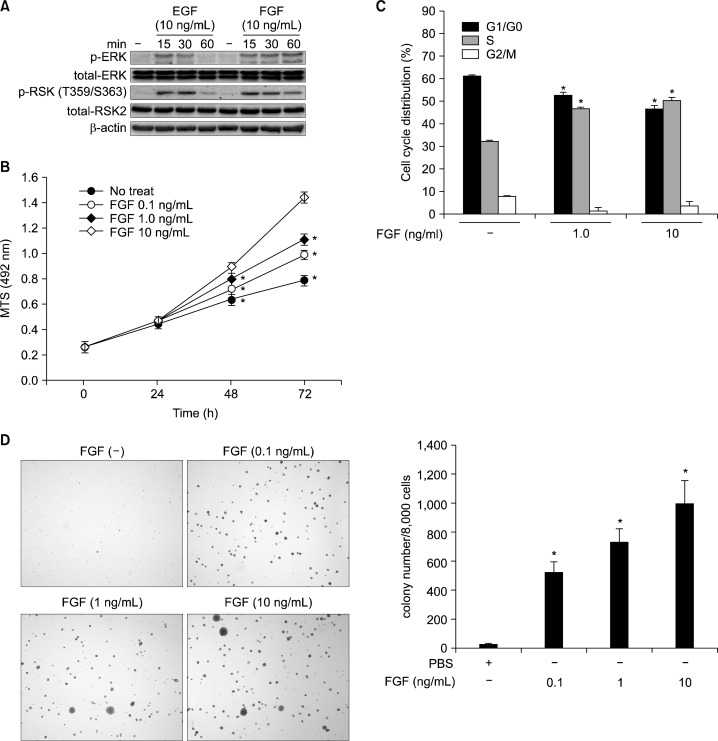
FGF is a growth factor enhancing the fibroblast proliferation through the activation of receptor tyrosine kinases in the cytoplasmic membrane as shown in EGF.2 However, the direct evidence for the roles of FGF as a tumor promoter has not been clearly understood. To examine the roles of FGF as a tumor promoter, we analyzed and compared the signaling protein profiles upon stimulation with EGF and FGF. We found that FGF stimulation induced ERKs phosphorylation (Fig. 1A). Interestingly, the highest level of ERKs phosphorylation at 15 minutes was decreased gradually to 30 minutes and disappeared at 60 minutes by EGF stimulation (Fig. 1A). In contrast, FGF stimulation continued to increase the phosphorylation of ERKs by 60 minutes (Fig. 1A). The phosphorylation pattern of p90RSKs (RSKs) at Thr359/Ser363 was correlated with that of ERKs (Fig. 1A). In a FGF-induced cell proliferation assay, we found that FGF induced the cell proliferation of JB6 Cl41 in a dose-dependent manner (Fig. 1B). Notably, FGF stimulation suppressed the G1/G0 cell cycle phase and induced the S cell cycle phase (Fig. 1C). Importantly, when JB6 Cl41 cells were stimulated with FGF in soft agar, the number of colony increased in a dose-dependent manner (Fig. 1D). These results demonstrated that FGF acted as a tumor promoter.
Figure 1.
FGF is a tumor promoter. (A) Protein profiles of the MAP kinase signaling pathway activated by FGF stimulation. JB6 Cl41 cells (1.5 × 106) were seeded into 100-mm cell culture dishes and stimulated with epidermal growth factor (EGF) or FGF as indicated. The proteins were extracted and visualized by Western blotting using antibodies as indicated. β-Actin was used as an internal control to verify the equal protein loading. (B) FGF induces cell proliferation. JB6 Cl41 cells (1 × 103) were seeded into 96-well plates and FGF-induced cell proliferation was analyzed by MTS assay. Data are presented as the mean ± S.D. of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.05). (C) FGF induces G1/S cell cycle transition. JB6 Cl41 cells (4 × 105) were seeded into 60-mm dishes, starved, and stimulated with FGF. The cells were harvested and fixed, and then cell cycle phases were analyzed by propidium iodide staining and flow cytometry using a FACSCalibur. Data are presented as the mean ± S.D. of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.05). (D) FGF induces anchorage-independent cell transformation in JB6 Cl41 cells. JB6 Cl41 cells (8 × 103) were mixed with indicated doses of FGF in top agar, and plated onto bottom agar of 6-well plates as described in “Materials and Methods”. The cells were cultured for 2 weeks, and colonies were observed and counted under an inverted microscope. Data are presented as the mean ± S.D. of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.05).
2. Kaempferol targeted and inhibited kinase activity of fibroblast growth factor receptor
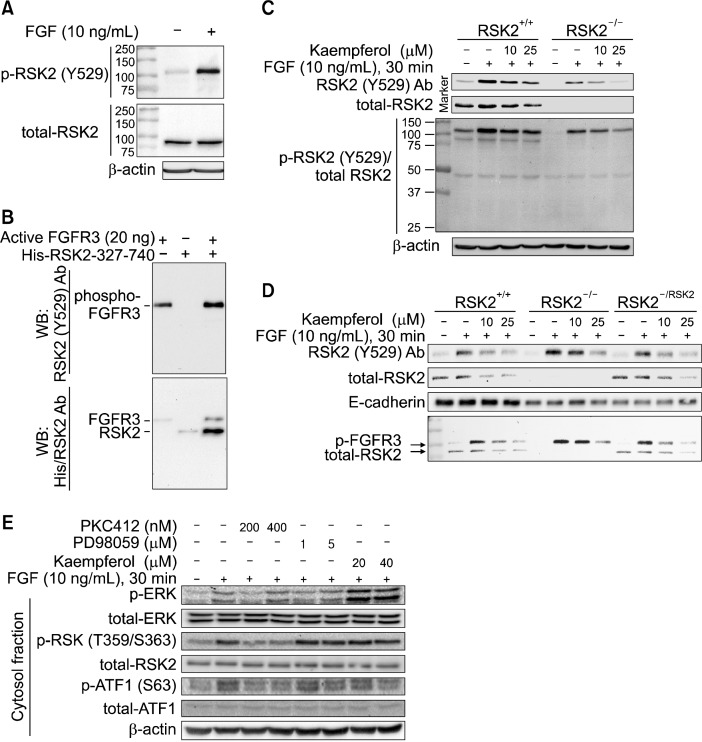
A recent study demonstrated that RSK2 Tyr529 is a target amino acid of FGFR3.23 To confirm, we conducted Western blotting with the FGF-stimulated membrane fraction proteins from JB6 Cl41 cells using a phopsho-RSK2 Tyr529 antibody as used in previous report.23 Surprisingly, we found that molecular weight of detected bands by phospho-RSK2 Tyr529 antibody was different from the RSK2 protein band which was detected at about 90 kDa (Fig. 2A). To confirm whether phospho-RSK2 Tyr529 antibody can recognize the RSK2 phosphorylation at Tyr529 or not, we conducted an in vitro kinase assay using an active FGFR3 kinase and purified His-RSK2 fusion protein harboring amino acid 327–740. We found that phospho-RSK2 Tyr529 antibody recognized the FGFR3 kinase domain, but not RSK2 (Fig. 2B, upper panel). Importantly, we further found that Western blotting using combination of His and phospho-RSK2 Tyr529 antibodies detected FGFR3 and His-RSK2 proteins at different molecular masses (Fig. 2B, bottom panel). These results demonstrated that the band detected by phospho-RSK2 Tyr529 antibody was phosphorylated FGFR3, but not RSK2. To confirm whether phospho-RSK2 Tyr529 antibody can recognize RSK2 in ex vivo or not, we conducted Western blotting using membrane fraction proteins extracted from RSK2+/+ and RSK2−/− mouse embryonic fibroblasts (MEFs). We found that phospho-RSK2 Tyr529 antibody detected a band in both RSK2+/+ and RSK2−/− MEFs (Fig. 2C, top panel). Notably, total-RSK2 antibody detected a single band in RSK2+/+ MEFs, but not RSK2−/− MEFs (Fig. 2C, 2nd panel). To compare the molecular masses between these two bands, we re-blotted the membrane with phospho-RSK2 Tyr529 and total-RSK2 antibodies. We found that phospho-RSK2 Tyr529 antibody detected 110–120 kDa bands which are the same size of FGFR3 in both RSK2+/+ and RSK2−/− MEFs (Fig. 2C, 3rd panel), while total-RSK2 antibody detected about 90 kDa protein in RSK2+/+ MEFs, but not in RSK2−/− MEFs (Fig. 2C, 3rd panel). To further confirm this finding, we used cells reintroduced RSK2 into RSK2−/− MEFs.16 We found that RSK2 protein re-appeared in RSK2−/−/RSK2 cells as shown in RSK2+/+ MEFs (Fig. 2D, 2nd panel). Interestingly, phospho-RSK2 Tyr529 antibody detected bands in RSK2+/+, RSK2−/− and RSK2−/−/ RSK2 cells (Fig. 2D, top panel). By the molecular weight comparison, we confirmed that the bands recognized by phospho-RSK2 Tyr529 and total-RSK2 antibodies harbored different molecular masses as indicated in Fig. 2C (Fig. 2D, bottom panel). Thus, we concluded that the band detected by phospho-RSK2 Tyr529 antibody was the phosphorylated FGFR3, but not phospho-RSK2 at Tyr529. Importantly, we found that the FGF-induced FGFR3 phosphorylation was suppressed by kaempferol treatment in a dose dependent manner (Fig. 2C and 2D). Our results suggest that EGF and FGF stimulation may use different signaling pathways (Fig. 1A). To test this speculation, we carried out the Western blotting by treatment of PKC412, a pan kinase inhibitor of receptor tyrosine kinases, PD98059, a MEK inhibitor, or kaempferol together with FGF. We found that phosphorylation of ERKs induced by FGF was slightly suppressed by PKC412, but not by PD98059, and was increased by kaempferol treatment (Fig. 2E). Interestingly, phosphorylation of RSKs at Thr359/Ser363 induced by FGF was inhibited by PKC412, and was not changeable by PD98059 and kaempferol (Fig. 2E). Notably, FGF-induced ATF-1 phosphorylation was correlated with the phosphorylation of RSKs at Thr359 and Ser363 (Fig. 2E). Taken together, these results demonstrated that the bands detected by phospho-RSK2 Tyr529 antibody were a phospho-FGFR3, and the FGF-induced signaling pathway was different from the canonical signaling pathway mediated by receptor tyrosine kinases such as EGF.
Figure 2.
Kaempferol inhibits FGFR 3 kinase activity. (A) phospho-RSK2 TyrY529 antibody recognizes a protein having different molecular weight from the RSK2. The JB6 Cl41 cells were stimulated with fibroblast growth factor (FGF) and membrane fraction proteins were extracted. The proteins were hybridized as indicated using phospho-RSK2 TyrY529 or total-RSK2 antibodies, and then horseradish peroxidase (HRP)-conjugated secondary antibody. β-Actin was used as an internal control to verify the equal protein loading. (B) phospho-RSK2 Tyr529 antibody recognizes FGFR3, but not RSK2. Purified His-RSK2-327-740 from Escherichia coli and active FGFR3 kinase were combined and carried out an in vitro kinase assay. The proteins were divided equally and indicated proteins were visualized by Western blotting using phospho-RSK2 Tyr529 and/or His-specific antibodies, and then HRP-conjugated secondary antibody. (C) Kaempferol inhibits FGFR3 phosphorylation. RSK2+/+ and RSK2−/− mouse embryonic fibroblasts (MEFs) (1.5 × 106) were seeded into 100-mm culture dishes, starved and stimulated with FGF and/or kaempferol as indicated. The membrane proteins were extracted, hybridized with phospho-RSK2 Tyr529 and total RSK2 antibodies as indicated, and visualized by Western blotting using HRP-conjugated secondary antibody. β-actin was used as an internal control to verify the equal protein loading. (D) Kaempferol inhibits FGFR3 phosphorylation and total RSK2 protein level. The pBabe-puro-RSK2 was infected into RSK2−/− MEFs and membrane proteins were extracted. The phospho-FGFR3 and total RSK2 proteins were visualized by Western blotting using specific antibodies as indicated. E-cardherin was used as an internal control to verify the equal protein loading of membrane fraction proteins. (E) Signaling profiles of FGFR3/RSK2 signaling axis by FGF stimulation. JB6 Cl41 cells were stimulated starved and stimulated with FGF with combination of chemical inhibitors as indicated. The membrane and cytosolic fraction proteins were extracted, and each protein was visualized by Western blotting using specific antibodies as indicated. β-Actin was used as an internal control to verify the equal protein loading.
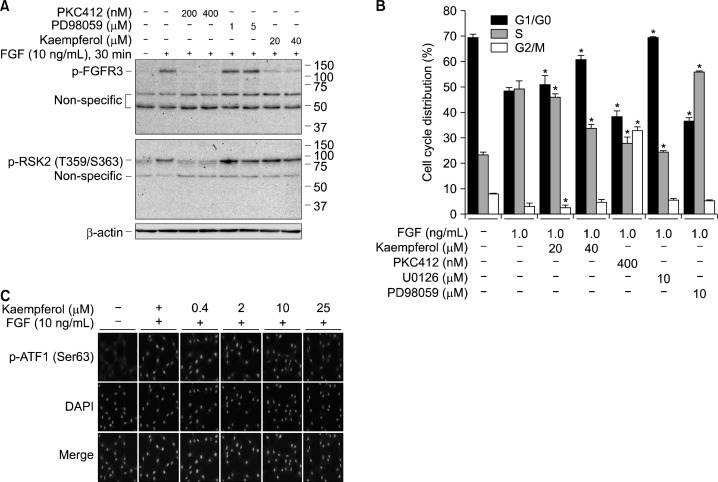
Figure 3.
Kaempferol inhibits FGFR3/RSK2 signaling-mediated ATF-1 nuclear accumulation. (A) Signaling profiles of FGFR3/RSK2 signaling axis by FGF stimulation. JB6 Cl41 cells were starved and stimulated with the combination of FGF and chemical inhibitors as indicated. The membrane fraction proteins were extracted and the phosphorylation of FGFR3 and RSK2 was visualized by Western blotting using specific antibodies as indicated. β-Actin was used as an internal control to verify the equal protein loading. (B) Effects on the cell cycle profiles by FGFR3/RSK2 signaling axis. JB6 Cl41 cells (4 × 105) were seeded into 60-mm dishes and starved. The cells were stimulated with the combination of FGF and chemical inhibitors as indicated. The cells were harvested, fixed, and analyzed the cell cycle phases by propidium iodide staining and flow cytometry analysis using FACSCalibur. Data are presented as the mean ± S.D. of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.05). (C) Inhibition of phospho-ATF-1 nuclear accumulation by kaempferol. JB6 Cl41 cells (2 × 104) were seeded into 4-chamber slides, starved stimulated with the combination of FGF and indicated doses of kaempferol, fixed, and permeabilized. The phospho-ATF-1 proteins were visualized by immunocytofluorescence assay using phos-pho-ATF-1 Ser63 and Alexa-568-conjugated secondary antibodies under a fluorescence microscope. 4,6-diamidino-2-phenylindole (DAPI) was used for nuclear staining.
3. Fibroblast growth factor signaling to RSK2 induced G1/S cell cycle transition and activating transcription factor-1 nuclear accumulation
To verify that kaempferol inhibits phosphorylation of FGFR3 induced by FGF, we conducted Western blotting by treatment of cells with PKC412, PD98059 or kaempferol together with FGF using membrane fraction proteins. We found that phosphorylation of FGFR3 was suppressed by PKC412, but not changed by PD98059 (Fig. 3A). Importantly, FGF-induced FGFR phosphorylation was dramatically suppressed by kaempferol treatment as PKC412 treatment (Fig. 3A). Furthermore, the cell cycle distribution analysis indicated that kaempferol and U0126 induced G1/G0 cell cycle accumulation and S-phase suppression (Fig. 3B), which were quite different from the patterns of cell cycle distribution shown in PKC412 or PD98059 treatment (Fig. 3B). Interestingly, kaempferol suppressed the nuclear accumulation of phosphorylated ATF-1 at Ser63 (Fig. 3C). These results suggested that kaempferol may target FGFR, and the FGF-induced signaling pathway may be different from the signaling pathway induced by EGF.
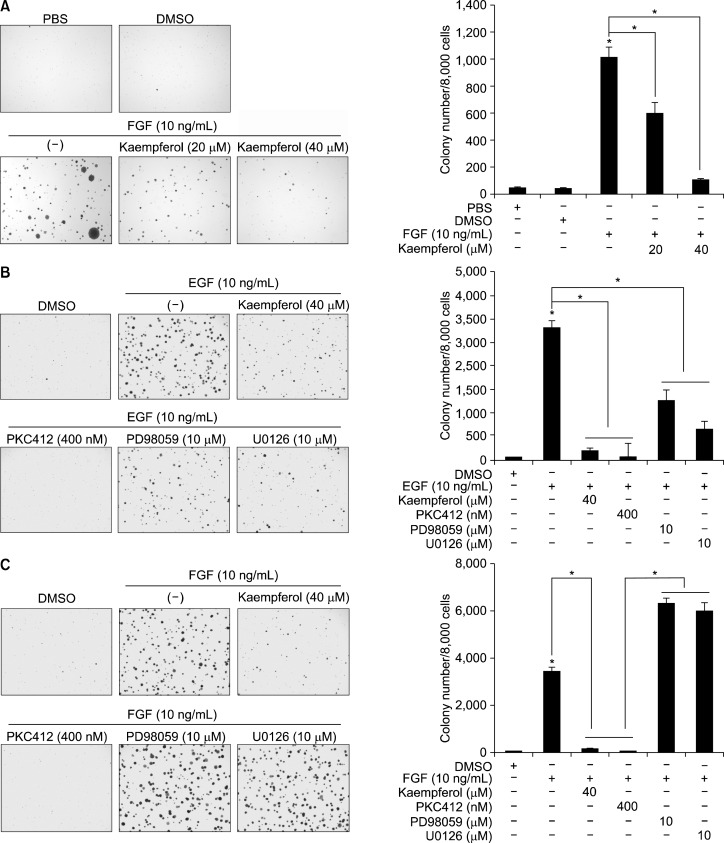
4. Kaempferol inhibited anchorage-independent cell transformation induced by fibroblast growth factor
To examine the effects of kaempferol on FGF-induced neoplastic cell transformation, we conducted a soft agar assay. Kaempferol inhibited FGF-induced anchorage-independent cell transformation in a dose-dependent manner (Fig. 4A). To compare the EGF- and FGF-induced signaling pathways involved in cell transformation, we treated cells with kaempferol, PKC412, PD98059 or U0126 together with EGF (Fig. 4B) or FGF (Fig. 4C). We found that EGF-induced anchorage-independent cell transformation was suppressed by treatment with kaempferol, PKC412, PD98059 and U0126 (Fig. 4B). Notably, FGF-induced cell transformation was suppressed by kaempferol or PKC412 treatment, but not by PD98059 or U0126 (Fig. 4C). Taken together, these results demonstrated that the FGF-mediated signaling pathway involved in cell proliferation and transformation utilized a non-canonical signaling pathway(s), which is not observed in the stimulation with growth factors such as EGF.
Figure 4.
EGF and FGF utilize the different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 cells. (A) Kaempferol suppresses FGF-induced anchorage-independent cell transformation in JB6 Cl41 cells. JB6 Cl41 cells (8 × 103) were mixed with indicated doses of kaempferol, FGF and top agar, and plated onto bottom agar of 6-well plates as described in “Materials and Methods”. The cells were cultured for 2 weeks, and colony growth was observed and counted under an inverted microscope. Data are presented as the mean ± S.D. of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.05). (B, C) EGF and FGF utilize the different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 cells. JB6 Cl41 cells (8 × 103) were mixed with chemical compounds as indicated together with EGF (B) or FGF (C), and plated onto bottom agar of 6-well plates as described in “Materials and Methods”. The cells were cultured for 2 weeks and colony growth was observed and counted under an inverted microscope. Data are presented as the mean ± S.D. of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.05).
DISCUSSION
FGF is a growth factor which regulates the proliferation of fibroblasts in human body.4 The signaling of FGF through the FGFRs, a receptor tyrosine kinase family harboring the single transmembrane domain, activates the mitogen-activated protein kinase signaling pathway.5 Because FGF acts as a mitogen, FGF might play an important role in fibroblast proliferation and transformation. However, there is little known about the roles of FGF as a tumor promoter. In this study, we provided concrete evidence that FGF induced cell proliferation and anchorage-independent cell transformation in JB6 Cl41 cells (Fig. 1). By comparison of the phosphorylated protein profiles stimulated by EGF,19 we confirmed that FGF also activates the MAP kinase signaling pathway including ERKs and RSKs (Fig. 1). However, the FGF-mediated signaling pathway might be different from the EGF-induced signaling pathway. The conclusion was obtained by use of the chemical inhibitors including PKC412, a pan inhibitor of receptor tyrosine kinase, PD98059, a MEKs inhibitor, and kaempferol, a RSK2 inhibitor (Fig. 2E). When the cells synchronized at G0/G1cell cycle phase by starvation were released by FGF, we found that U0126 and kaempferol inhibited G1/S cell cycle transition, resulting in the G1 cell cycle accumulation (Fig. 3B). Unexpectedly, when cells were treated with PD98059 or PKC412 in the presence of FGF, PD98059 showed the suppression of G1 phase cell population and induction of S phase cell population, and PKC412 showed the reduction of G1 phase cell population and induction of G2/M phase cell population (Fig. 3B). Interestingly, kaempferol reduced the FGF-induced FGFR phosphorylation and the total RSK2 protein level ex vivo (Fig. 2C). Previous report indicated that RSK2 is a substrate of FGFR3.23 Interestingly, we found that the phospho-RSK2 Tyr529 antibody recognized the phospho-FGFR3, but not the phosphorylated RSK2 at Tyr529 in vitro (Fig. 2B). These results were confirmed ex vivo that the phospho-RSK2 Tyr529 antibody recognized protein bands of same molecular weight to FGFR in both RSK2+/+ and RSK2−/− MEFs (Fig. 2A). Notably, RSK2 protein bands were observed at different molecular weight detected by phospho-RSK2 Tyr529 antibody in RSK2+/+ MEFs, but not in RSK2 −/− MEFs (Fig. 2C). Importantly, the band intensities increased by FGF stimulation were decreased by kaempferol treatment in a dose dependent manner (Fig. 2C and 2D). Taken together, these results demonstrated that the bands recognized by the phospho-RSK2 Tyr529 antibody were a phosphorylated FGFR3, but not phospho-RSK2 Tyr529. Unfortunately, the antibody is currently not available in market. Our previous results demonstrated that kaempferol, a natural compound contained in many dietary plants including green leaves of green onion, berries and endives, inhibited the N-terminal kinase activity of RSK2 by targeting the active pocket with about 7 μM of the half-maximal inhibitory concentration value.20 These results suggest that kaempferol might target the kinase domains of FGFR3 and RSK2. This hypothesis was supported by our results that phosphorylation of RSKs at Thr359/Ser363 was correlated with phosphorylation of ATF-1, a substrate of RSK2,24 induced by FGF (Fig. 2E) and nuclear accumulation of ATF-1 phosphorylated at Ser63 (Fig. 3C), which played an important role in neoplastic cell transformation.24 Another important finding was obtained by comparison of signaling pathway utilization between EGF and FGF involved in anchorage-independent cell transformation (Fig. 4). Our results clearly showed that EGF-induced neoplastic cell transformation was inhibited by the chemical inhibitors including kaempferol, PKC412, PD98059 and U0126, respectively (Fig. 4B). In contrast, FGF-induced neoplastic cell transformation in soft agar was inhibited by kaempferol and PKC412, but not by U0126 and PD98059 (Fig. 4C). Additionally, FGF-induced colony formation in soft agar was more increased by PD98059 or U0126 compared with FGF alone (Fig. 4C). Our results demonstrated that the FGF-mediated signaling pathway to induce cell proliferation and transformation is different from the canonical signaling pathway induced by EGF. Taken together, we conclude that dual targeting of RSK2 and FGFR3 is sufficient enough to inhibit FGF-induced cell proliferation and neoplastic cell transformation by kaempferol.
Acknowledgments
This study was supported by the Research Fund, M-2012-B0002-00028 of The Catholic University of Korea, by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2000961), and by the Ministry of Science, ICT and Future Planning (2012M3A9B6055466).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 2.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, et al. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–56. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Cao R, Hedlund EM. R regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86:785–9. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak P, Dvorakova D, Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett. 2006;580:2869–74. doi: 10.1016/j.febslet.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 6.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Jimeno A, Hidalgo M. Pharmacogenomics of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Biochim Biophys Acta. 2006;1766:217–29. doi: 10.1016/j.bbcan.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Ellerbroek SM, Halbleib JM, Benavidez M, Warmka JK, Wattenberg EV, Stack MS, et al. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metal-loproteinase-9 production and cell surface association. Cancer Res. 2001;61:1855–61. [PubMed] [Google Scholar]
- 9.Mosesson Y, Yarden Y. Oncogenic growth factor receptors: implications for signal transduction therapy. Semin Cancer Biol. 2004;14:262–70. doi: 10.1016/j.semcancer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Choi KC. Anti-cancer effect and underlying mechanism(s) of Kaempferol, a Phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol Res. 2013;29:229–34. doi: 10.5487/TR.2013.29.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 12.Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 13.Maron DJ. Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep. 2004;6:73–8. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- 14.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 15.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–11. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 16.Cho YY, Lee MH, Lee CJ, Yao K, Lee HS, Bode AM, et al. RSK2 as a key regulator in human skin cancer. Carcinogenesis. 2012;33:2529–37. doi: 10.1093/carcin/bgs271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HS, Cho HJ, Yu R, Lee KW, Chun HS, Park JH. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci. 2014;15:2722–37. doi: 10.3390/ijms15022722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J, et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol Carcinog. 2014 doi: 10.1002/mc.22154. [DOI] [PubMed] [Google Scholar]
- 19.Cho YY, Yao K, Kim HG, Kang BS, Zheng D, Bode AM, et al. Ribosomal S6 kinase 2 is a key regulator in tumor promoter induced cell transformation. Cancer Res. 2007;67:8104–12. doi: 10.1158/0008-5472.CAN-06-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho YY, Yao K, Pugliese A, Malakhova ML, Bode AM, Dong Z. A regulatory mechanism for RSK2 NH(2)-terminal kinase activity. Cancer Res. 2009;69:4398–406. doi: 10.1158/0008-5472.CAN-08-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao K, Chen H, Liu K, Langfald A, Yang G, Zhang Y, et al. Kaempferol targets RSK2 and MSK1 to suppress UV radiation-induced skin cancer. Cancer Prev Res. 2014 doi: 10.1158/1940-6207.CAPR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colburn NH, Wendel EJ, Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci USA. 1981;78:6912–6. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S, Dong S, Gu TL, Guo A, Cohen MS, Lonial S, et al. FGFR3 activates RSK2 to mediate hematopoietic transformation through tyrosine phosphorylation of RSK2 and activation of the MEK/ERK pathway. Cancer Cell. 2007;12:201–14. doi: 10.1016/j.ccr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K, Cho YY, Yao K, Nadas J, Kim DJ, Cho EJ, et al. Eriodictyol inhibits RSK2-ATF1 signaling and suppresses EGF-induced neoplastic cell transformation. J Biol Chem. 2011;286:2057–66. doi: 10.1074/jbc.M110.147306. [DOI] [PMC free article] [PubMed] [Google Scholar]