Abstract
In this review, we will summarize the current understanding of modulation of colitis-associated colon tumorigenesis by two natural products, baicalein and betaine, which have anti-inflammatory activities. Baicalein and betaine have been shown to provide various health benefits to organism in many ways. Baicalein is a phenolic flavonoid derived originally from the root of Scutellaria baicalensis Georgi. From ancient times, baicalein has widely been used in oriental medicines as an anti-inflammatory and anti-cancer therapy. Betaine, trimethylglycine, is an essential biochemical molecule of the methionine/homocysteine cycle and is synthesized by conversion of choline. Betaine is an important human nutrient obtained from various foods including sugar beet and lycium. Betaine has provided various health benefits including disease prevention. However, the action mechanisms of their activity remain poorly understood. Recent studies reported the effects of baicalein and betaine on cytotoxicity against colon cancer cells and chemically induced colitis-associated colon tumorigenesis in mice. Administrations of baicalein and betaine containing diets significantly inhibited the incidence of tumors and hyperplasia with down-regulation of inflammation. Therefore, baicalein and betaine might be applicable to the prevention of inflammation-associated colon carcinogenesis.
Keywords: Baicalein, Betaine, Inflammatory bowel disease, Colon cancer, Azoxymethane/dextran sodium sulfate model
INTRODUCTION
Cancer of colon and rectum [colorectal cancer (CRC)] is a malignant neoplasm arising from the lining of the large intestine. CRC is the third most common malignancy and one of the major causes of cancer-related death in the United States.1 Colitis-associated cancer is a type of colon malignancy which is preceded by clinically detectable inflammatory bowel disease (IBD), such as Crohn’s disease (CD) or ulcerative colitis (UC).2 IBD results from the inappropriate and ongoing activation of the mucosal immune system is driven by the presence of normal luminal flora. As many as 1.4 million people in the United States and 2.2 million people in Europe suffer from these diseases.3
The prevalence of IBD is much lower in Asian countries, including Japan and Korea than in Western countries, but it is rapidly increasing.4 The incidence of IBD in Korea has increased significantly over the past few decades. In case of prevalence of UC in Korea, it was quadrupled from 7.57/105 individuals in 1997 to 30.9/105 individuals in 2005. Adjusted prevalence rates of CD and UC per 105 individuals were 11.2 and 30.9, respectively.5 In regard to the incidence of IBD in Japan, the number of patients has been increasing over the time. The age-standardized prevalence of UC in Japan in 2005 was 63.6/105 individuals while that of CD was 21.2/105 individuals. The incidence in Japan of UC and CD is higher than in Korea.
IBD can occur in combination with defected immune response.6,7 An elevated level of tumor necrosis factor α (TNF-α) was especially found in the blood, intestinal mucosa and stools of patients with IBD. In addition to TNF-α, the increases of other pro-inflammatory mediators have been detected in stools and rectal dialysates from patients with IBD as well.6 The existing data revealed that TNF-α inhibitors are increasingly being used in the management of IBD-related disorders. The most commonly used biologics used for the treatment of IBD are TNF-α antibodies, such as infliximab, a chimeric immunoglobulin G (IgG) 1 monoclonal antibody; adalimumab, a human monoclonal IgG1 antibody; certolizumab pegol, a pegylated Fab fragment of a humanized IgG4 isotype monoclonal antibody.8 Even though these TNF inhibitors may increase the risk of tuberculosis, varicella, and other opportunistic infections, there is little evidence supporting that anti-TNF agents specifically increase the overall risk of serious infections. Similarly, there is little evidence that TNF antagonists raise the risk of developing malignancy.9 Therefore, for the use of TNF inhibitors in patients with IBD, more studies are needed.
In this review, we will describe the inflammation-induced colon carcinogenesis and the biological activities of baicalein and betaine focusing on their role as modulators of colitis-associated colon tumorigenesis. An introduction of the animal models for inflammation-induced colon carcinogenesis will be followed by discussion of the biochemical and cellular effects of baicalein and betaine.
INDUCTION OF COLON CARCINOGENESIS IN MICE BY AZOXYMETHANE (AOM) PLUS DEXTRAN SODIUM SULFATE (DSS)
A variety of available animal models of CRC have provided essential tools for investigating the complex development and pathogenesis of CRC. Among them, chemically induced CRC models are highly reliable due to their advantage in investigating CRC. This chemically- induced models are highly reproducible, can be readily tested in animals with different genetic backgrounds, and mimic the pathogenesis of human CRC.10 Therefore, these carcinogen- induced CRC rodent models are frequently used to evaluate the activity of chemopreventive or anticancer agents and also to identify risk factors.
Although there are a variety of chemical agents available to induce colon carcinogenesis in animals, AOM is widely used due to its practical advantage, such as reproducibility, high potency, simple application, excellent stability and a low price.10 AOM is a carcinogenic and neurotoxic chemical compound and a metabolite of 1,2-dimethylhydrazine. AOM is converted to methylazoxymethanol by cytochrome P450 2E1. Methylazoxymethanol induces genotoxicity in various tissues including colon.
The pro-inflammatory stimulus, such as DSS, is widely used to induce colitis in animals. Continuous exposure to DSS induces inflammatory conditions in colon. However, the incidence and the multiplicity of tumors with inflammation are low.11 When AOM was combined with the pro-inflammatory stimulus DSS, the tumor growth was accelerated with a significantly shorter latency time (2–3 months). To develop an efficient animal model for colitis-related carcinogenesis, male ICR mice were challenged with a single intraperitoneal administration (10 mg/kg body weight) of a genotoxic colonic carcinogen, AOM and an one week oral exposure (2% in drinking water) to a non-genotoxic carcinogen, DSS. After the treatment with AOM and DSS, all mice developed tumors.12 This can also be used in chemoprevention studies as well. Other chemicals including dimethylhydrazine and 2,4,6- trinitrobenzenesulfonic acid induce the formation of colon tumors. However, it takes longer than that induced by AOM plus DSS.
The most important molecular markers involved in the signal transduction of inflammation-induced colon carcinogenesis are TNF-α, interleukin (IL)-1β, IL-6, cyclooxygenase (COX)-2, inducible nitric oxide synthase (iNOS), 3-hydroxy-3-methylglutatyl coenzyme A reductase, retinoid X receptor-a, estrogen receptor-β, β-catenin, 5-lipoxygenase, signal transducers and activators of transcription 3, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and hemeoxygenase-1.13 These can also be considered as biomarkers and/or surrogate endpoints of colorectal carcinogenesis and as molecular targets in the management of colorectal malignancies.14
Over the last decade, various compounds have been tested for their oncogenic or chemopreventive activities in AOM/DSS-induced colon carcinogenesis model.15–20
EFFECTS OF BAICALEIN ON GROWTH OF HUMAN COLON CANCER CELLS AND AZOXYMETHANE PLUS DEXTRAN SODIUM SULFATE-INDUCED COLON TUMORIGENESIS
Baicalein (5,6,7-trihydroxyflavone, Fig. 1A), one of major flavonoids found in Scutellaria baicalensis Georgi, is widely used in Chinese herbal medicine for treating various inflammatory diseases and ischemia.21 Baicalein exhibits various biological effects, including anti-inflammatory22 and anti-tumor activity.23 So far, Scutellaria has been shown to have almost no or very low toxicity to animals and humans.24 It is also evident that this flavonoid exhibits selective cytotoxicity against malignant cells. In human myeloma cells, baicalein induced cell death, but did not so in normal myeloid cells or peripheral blood cells at the same plasma concentration indicating its selective cytotoxic effect of this agent.25
Figure 1.
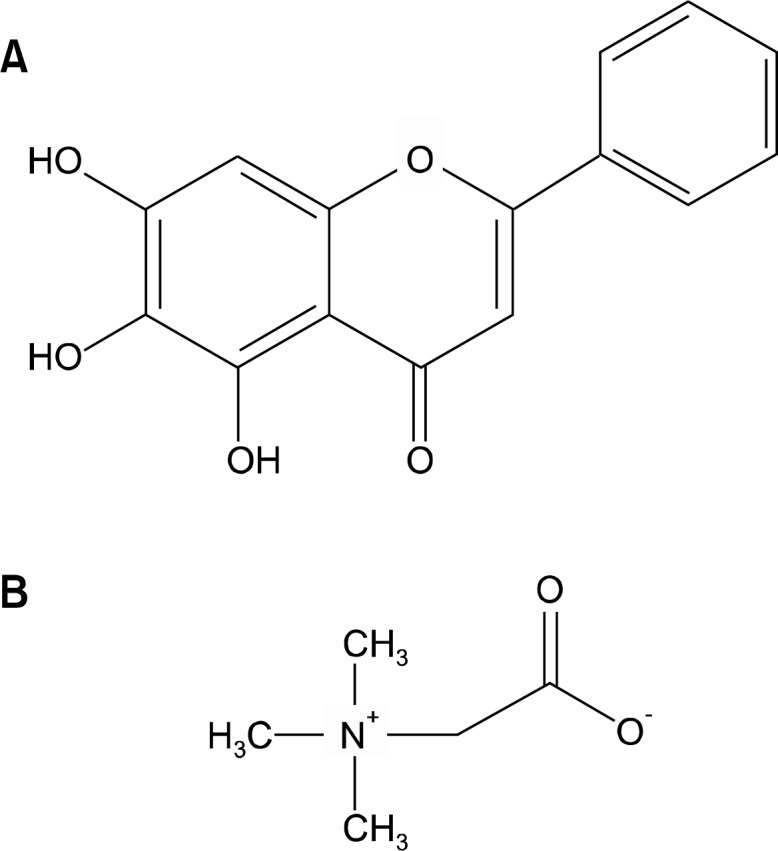
Structures of baicalein and betaine.
1. Modulation of anti-tumor activity
Several studies have demonstrated that baicalein inhibits growth of several human cancer cells.26,27 It possesses a direct cytotoxicity to a large panel of human malignant cell lines by inducing apoptotic cell death.26–31 The anti-cancer properties of baicalein were recently shown to be mediated through the inhibition of cell growth and induction of apoptosis in HCT116 human colon cancer cells.28 Cell viability was significantly decreased by treatment of baicalein in a concentration-dependent manner. The concentrations required for half-maximal inhibition of the cells were about 100 μM in HCT116 cells for 24 hour treatment and about 50 μM for 48 hour treatment. Cells treated with baicalein for 24 hours showed distinct morphological changes compared with that of the untreated control. Thus, they became rounded and more dispersed with aggregation.28 Apoptosis is an important process required for homeostasis and occurs through two broad pathways: the intrinsic pathway and the extrinsic pathway.32 Treatment with baicalein decreased the expression levels of procaspase-3 and -8, and induced cleavage of poly (ADP-ribose) polymerase.28
Bonham et al.33 reported anti-tumor activities of baicalein in various types of cancer cells and tumor models. Oral administering of 20 mg/kg baicalein inhibited growth of established prostate tumors by approximately 55%. Baicalein also exerts anticancer activity by inhibiting platelet-type 12-lipoxygenase, which has been shown to regulate growth, metastasis and angiogenesis in prostate cancer.34 Moreover, treatment with baicalein inhibited nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in A549 and H1299 human lung cancer cell lines.35 In hepatocellular carcinoma including H22, Bel-7404 and HepG2 cell lines, treatment with baicalein showed anticancer effects by regulating the transcription of cyclin D1 via a β-catenin-dependent mechanism.29
Baicalein also affects the invasion and expression of migration signaling molecules, such as matrix metalloproteinase (MMP)-2 and MMP- 9 in human hepatoma cells.36 According to recent studies, baicalein treatment showed inhibition of migration and invasion in gastric and cervical cancer cells via transforming growth factor-β and extracellular signal-regulated kinase signal pathways, respectively.37,38 Similarly, baicalein also exerted an inhibitory effect on cell migration in colon cancer cells.28 Therefore, baicalein has effective anti-metastatic activity for the treatment of colon cancer by inhibiting the expression of MMP-2 and MMP-9, thereby blocking cell migration and invasion.28 Based on previous studies, baicalein has therapeutic potential against several types of human cancers.
2. Modulation of the inflammation
NF-κB is the key transcriptional factor for synthesis of pro-inflammatory mediators, including iNOS, COX-2 and TNF-α. NF-κB also plays central roles in carcinogenesis and inflammation and thus it is considered as one of the molecular targets of cancer prevention and therapy.39 In fact, NF-κB activation has been reported to be involved in colon carcinogenesis and certain NF-κB inhibitors are able to suppress cancer development in these tissues.40 Numerous data suggest that baicalein exhibits anti-inflammatory activity through inhibition of NF-κB.40–43 Kim et al.28 reported the anti-inflammatory effects of baicalein in human colon cancer cells. They found that baicalein inhibited the activation of NF-κB subunits p50 and p65 through induction of peroxisome proliferator-activated receptor (PPAR)γ. As a result, baicalein suppressed the expression of the inflammatory mediator iNOS.28
The anti-inflammatory activity of baicalein is mediated not only by NF-κB but also by modulation of various signaling molecules. Baicalein has been reported to attenuate endothelium intimal hyperplasia by inhibiting inflammatory signaling molecules including extracellular signal-regulated kinase, protein kinase B or Akt and NF-κB in vascular smooth muscle cells.44 Baicalein attenuates the radiation-induced inflammatory process in mouse kidney by modulation of NF-κB and Forkhead family of transcription factors.41 In case of acute lung injury induced by lipopolysaccharide (LPS) in rat, baicalein inhibited NF-κB mediated inflammatory responses and upregulation of the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway.45 In the murine macrophage RAW 264.7 cell line, baicalein inhibited LPS-induced inflammation via upregulated estrogen receptor and inhibited NF-κB-dependent signaling.46
3. Modulation of colitis-associated colon tumorigenesis
As described earlier, baicalein has been reported to inhibit inflammation by modulation of PPARγ in human colon cancer cells.28 PPARγ agonists affect cell proliferation, differentiation, and apoptosis in a PPARγ-dependent and/or -independent manner and hence represent a potentially important family of therapeutic compounds for cancer treatment. Many studies show that PPARγ agonists, such as thiazolidinedione, have anti-tumorigenic properties in CRC by increasing the expression of tumor suppressor genes.47 Activation of PPARγ results in anti-inflammatory effects in several cell types, including smooth muscle cells and endothelial cells. PPARs act as anti-inflammatory agents by interfering with the transcriptional pathways involved in inflammatory responses, such as the modulation of NF-κB signaling. Baicalein is a potent PPARγ activator that inhibits NF-κB and mediates inflammatory responses in colon cancer cells.28
Recent studies have shown a significant association between deficiency of PPARγ and IBD.48 In addition, activation of PPARγ could attenuate the inflammation in the gut.49 These results suggest that colonic PPARγ may be a promising therapeutic target in patients suffering from IBD. In AOM plus DSS-induced colon carcinogenesis mouse model, baicalein recovered the colon length which was shortened by AOM/DSS, suppressed colonic inflammation and reduced the tumor incidence and hyperplasia induced by AOM/DSS.28 In addition, baicalein administration did not produce any observable toxicity or significant changes in body weight during experimental periods. The above accumulating evidence demonstrates that baicalein possesses potent anticancer and anti- inflammatory activities.28
EFFECTS OF BETAINE ON AZOXYMETHANE/DEXTRAN SODIUM SULFATE-INDUCED COLON TUMORIGENESIS
Betaine (trimethylglycine, Fig. 1B) is an essential biochemical molecule of the methionine/homocysteine cycle and is synthesized by conversion of choline.38 It was first discovered in the juice of sugar beets (Beta vulgaris) in the 19th century50 and since then has been found in various microorganisms, plant and animals.51 Betaine plays central roles in choline-mediated one-carbon metabolism, structural integrity and signaling functions of cell membranes and neurotransmitter synthesis.52 It also serves as an osmolyte that regulates cell volume and protects cells and proteins from environmental stresses including ionic stress.53
1. Modulation of inflammation and cancer by betaine
Methylation of homocysteine by betaine is confined to the liver and the kidney but the pathway involving folate exists in all body cells.54 Previous studies showed dietary choline and betaine intakes and associations with inflammatory markers in healthy free-eating adults enrolled in the ATTICA study.55 The subjects with higher dietary choline and betaine intakes showed significantly lower plasma C-reactive protein, IL-6 and TNF-α concentrations than those who lower the intakes.55 Moreover, betaine and choline may be involved in reducing inflammation as a source of one-carbon units for the metabolism of homocysteine.56
It has been reported that betaine may inhibit inflammatory processes by blocking the expression of pro- inflammatory genes as a consequence of suppressing NF-κB.57 Betaine has been reported to suppress pro-inflammatory NF-κB activation through modulation of reactive oxygen species (ROS) and thiol homeostasis during the aging process both in vitro and in vivo.57–59
Inflammation is an important tumor promoter and several cytokines, including TNF-α and IL-6 which are induced by inflammation, can promote tumor growth.12,14 Many protooncogenes and carcinogens cause activation of NF-κB, whereas chemicals with known chemopreventive properties can suppress NF-κB activation.60 So far, there are limited studies on association between intake of choline and betaine and cancer risks in humans, because food composition data have not been available until recently.38 Several epidemiologic studies have examined the association between dietary intake of choline and betaine and cancer risks. Interestingly, higher betaine intake may be protective against lung cancer through mitigating the adverse effect of smoking.61
Betaine inhibits tube formation, migration and invasion in human umbilical vein endothelial cells through suppression of NF-κB and Akt signaling pathways.62 In human adipocyte, treatment with betaine reduced hypoxia-induced inflammatory adipokines expression, which could have prevented low grade inflammation in obesity.63
2. Modulation of colitis-associated colon tumorigenesis
Recently, the results from a case-control study nested within the European Prospective Investigation into Cancer and Nutrition showed that plasma methionine, choline, and betaine status were modestly and inversely associated with the CRC risk.64 This study included 1367 incident CRC cases (965 colon and 40 rectum) and 2323 controls matched by gender, age group, and study center. Higher betaine concentration was associated with a reduced CRC risk among individuals with folate concentrations below the median of 11.3 nmol/l, but not among those with a higher folate status.64 This study suggests that individuals with high plasma methionine, choline, or betaine concentrations may be at a reduced risk of CRC. Although it was unclear that betaine could reduce inflammation-associated colon cancer development, it provided the possibility that these methyl group donors may play beneficial roles in colon cancer tumorigenesis. However, there are few reports about the role of betaine on the prevention of colon cancer tumorigenesis.
The inhibitory effects of betaine on AOM/DSS-induced colon tumorigenesis has recently been reported.65 Animals were given a single intraperitoneal injection of AOM (10 mg/kg body weight). Seven days after the AOM injection, animals received 2% DSS (weight/volume) in the drinking water for 7 days. Depending on the experimental goals and characteristics, DSS can be used in various ranges of concentration.66,67 Macroscopically, the AOM/DSS model resulted in 100% incidence of colonic tumors and hyperplasia, which were most frequently observed in the middle and distal colon. Notably, betaine administration did not show any adverse effect on the body weight changes as well as food intake during the experimental periods. Three doses of betaine administration (1, 5 and 10 mg/kg in diet) inhibited colitis-associated colon tumorigenesis in ICR male mice. The colon length was not different between the AOM/DSS group and the betaine-treated group. However, other factors, including gene expression of pro-inflammatory mediators, oxidative stress status, and hematoxylin and eosin staining results, were affected by betaine treatment.
Quantitative polymerase chain reaction data showed AOM/DSS-induced expression of inflammatory molecules including TNF-α, IL-6, COX-2 and iNOS, which was inhibited by betaine treatment in colonic mucosa. Furthermore, hematoxylin and eosin staining data showed that administration of betaine decreased AOM/DSS-induced inflammatory-related damages and hyperplasia in colonic mucosa compared with the AOM/DSS treated group.65 LPS is an endotoxin released by gram-negative bacteria that can be transferred to cluster of differentiation 14 by LPS-binding protein and recognized by Toll-like receptor 4 on the cellular surface of macrophages.68 LPS triggers the translocation of NF-κB leading to the expression of NF-κB-regulated genes, including TNF-α, IL-6, COX-2 and iNOS, in murine macrophage RAW 264.7 cells.69 These results demonstrated that betaine treatment inhibited the LPS-induced expression of TNF-α, IL-6, COX-2 and iNOS in RAW 264.7 cells.65 These results suggest that betaine might be a candidate for a potential anti-inflammatory or cancer chemopreventive agent.
3. Modulation of oxidative stress
Redox homeostasis plays a critical role in the protection of the cell from both internal and external oxidative and other forms of stresses, and it maintains the regulatory role of redox-sensitive transcription factors including NF-κB.70,71 Previous studies showed ROS plays an important role in cancer development, both in the initiation and promotion stages of carcinogenesis.72 In multi-step process of colon carcinogenesis, ROS was also found to be involved in all stages.73 It has been reported that carcinogenic metals, such as arsenic and/or chromium in drinking water promoted tumorigenesis in a murine AOM/DSS colitis- associated CRC model through modulation of redox status. Importantly ROS-mediated β-catenin activation by carcinogenic metals such as arsenic and/or chromium may play an important role in this promotion effect.74
Betaine has been reported to prevent lysophosphatidylcholine-triggered ROS generation and NF-κB activation in endothelial cells.59 In addition, a previous study reported that dietary betaine supplementation was capable of restoring redox balance by maintaining thiol homeostasis, thereby suppressing pro-inflammatory NF-κB activation during aging.58 Similarly betaine also exerted inhibitory effects on colitis-associated colon tumori-genesis by modulating redox status. In mice, AOM/DSS increased ROS formation. In contrast, betaine-treated groups showed decreased ROS generation in colonic mucosa.65
Glutathione (GSH) is a key intracellular thiol composed of glutamic acid, cysteine and glycine. GSH helps protect cells from free radical damage by acting as an antioxidant. GSH depletion can cause redox imbalance through increased oxidative stress.37 A recent study showed that the anti-tumorigenic effect of betaine was associated with GSH homeostasis in a AOM/DSS-induced CRC model.65 Mice in the AOM/DSS-treated group showed increased oxidized GSH (GSH disulfide [GSSG]) concentration compared to those in the control group. However, the betaine-treated group showed a decreased GSSG concentration compared to the AOM/DSS treated group. This result indicates that betaine can reduce oxidative stress, at least in part, by modulation of GSSG levels in various types of stress.65
SUMMARY AND IMPLICATIONS
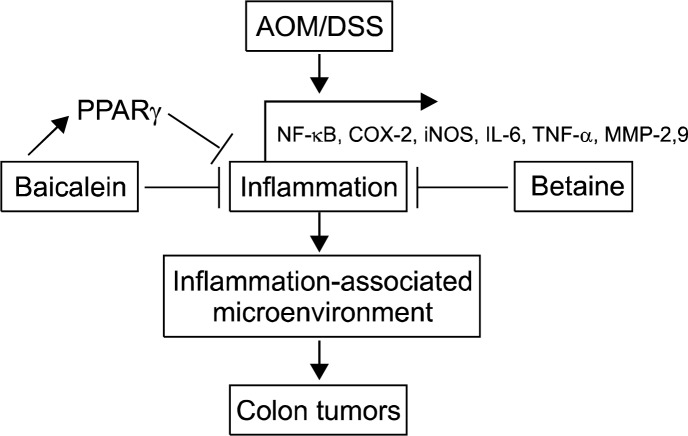
In this paper, we have outlined the current understanding of the mechanisms by which baicalein and betaine significantly decreased the incidence of colon tumor formation and hyperplasia with attenuation of inflammation. Much insight has been gained in recent years, particularly with respect to chemopreventive agents against inflammation-associated colon carcinogenesis. Baicalein and betaine suppressed inflammation and AOM/DSS-induced tumor formation and hyperplasia in colonic mucosa. Especially, betaine can possibly suppress ROS generation by modulation of GSH during colon tumorigenesis (Fig. 2). Therefore, baicalein and betaine may represent promising chemical entities which specifically target various aspects of inflammation and could be important leading compounds for the development of new chemopreventive agents against inflammation-associated colon tumorigenesis.
Figure 2.
Modulation of colitis-associated colon tumorigenesis by baicalein and betaine. Baicalein and betaine suppressed colon tumor formations by inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), cyclooxygenase (COX)-2, inducible nitric oxide synthase (iNOS), interleukin (IL)-6 and tumor necrosis factor (TNF)-α during azoxymethane/dextran sodium sulfate (AOM/DSS)-induced inflammation-associated colon tumorigenesis. MMP, matrix metalloproteinase; PPAR, peroxisome proliferator-activated receptor.
Acknowledgments
This work was supported by a 2-Year Research Grant of Pusan National University. We thank Aging Tissue Bank for providing research information.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Thiis-Evensen E, Kalager M, Bretthauer M, Hoff G. Long-term effectiveness of endoscopic screening on incidence and mortality of colorectal cancer: a randomized trial. United European Gastroenterol J. 2013;1:162–8. doi: 10.1177/2050640613483290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44:659–65. doi: 10.1007/s00535-009-0057-3. [DOI] [PubMed] [Google Scholar]
- 5.Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010;4:1–14. doi: 10.5009/gnl.2010.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64–77. doi: 10.3748/wjg.v20.i1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen OH, Seidelin JB, Munck LK, Rogler G. Use of biological molecules in the treatment of inflammatory bowel disease. J Intern Med. 2011;270:15–28. doi: 10.1111/j.1365-2796.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- 9.Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol. 2013;108:1835–42. doi: 10.1038/ajg.2013.294. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183–96. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J Gastroenterol Hepatol. 2002;17:1078–83. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin. 2008;29:1–20. doi: 10.1111/j.1745-7254.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichera A, Little N, Dougherty U, Mustafi R, Cerda S, Li YC, et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J Surg Res. 2007;142:239–45. doi: 10.1016/j.jss.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Miyamoto S, Sugie S, Yasui Y, Ishigamori-Suzuki R, Murakami A, et al. A tobacco-specific carcinogen, NNK, enhances AOM/DSS-induced colon carcinogenesis in male A/J mice. In Vivo. 2008;22:557–63. [PubMed] [Google Scholar]
- 17.Tanaka T, Yasui Y, Tanaka M, Tanaka T, Oyama T, Rahman KM. Melatonin suppresses AOM/DSS-induced large bowel oncogenesis in rats. Chem Biol Interact. 2009;177:128–36. doi: 10.1016/j.cbi.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 18.Barrett CW, Fingleton B, Williams A, Ning W, Fischer MA, Washington MK, et al. MTGR1 is required for tumorigenesis in the murine AOM/DSS colitis-associated carcinoma model. Cancer Res. 2011;71:1302–12. doi: 10.1158/0008-5472.CAN-10-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyoshi N, Nagasawa T, Mabuchi R, Yasui Y, Wakabayashi K, Tanaka T, et al. Chemoprevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by freeze-dried yam sanyaku and its constituent diosgenin. Cancer Prev Res. 2011;4:924–34. doi: 10.1158/1940-6207.CAPR-10-0279. [DOI] [PubMed] [Google Scholar]
- 20.Barrett CW, Singh K, Motley AK, Lintel MK, Matafonova E, Bradley AM, et al. Dietary selenium deficiency exacerbates DSS-induced epithelial injury and AOM/DSS-induced tumorigenesis. PLoS One. 2013;8:e67845. doi: 10.1371/journal.pone.0067845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS. Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin Mol Allergy. 2007;5:5. doi: 10.1186/1476-7961-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011;1813:1465–74. doi: 10.1016/j.bbamcr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnett BP, Silva S, Mesches MH, Wilson S, Jia Q. Safety evaluation of a combination, defined extract of Scutellaria baicalensis and Acacia catechu. J Food Biochem. 2007;31:797–825. [Google Scholar]
- 25.Ma Z, Otsuyama K, Liu S, Abroun S, Ishikawa H, Tsuyama N, et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105:3312–8. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HB, Lu P, Guo QY, Zhang ZH, Meng XY. Baicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathway. Oncol Lett. 2013;5:722–8. doi: 10.3892/ol.2012.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Song L, Cai L, Wei R, Hu H, Jin W. Effects of baicalein on apoptosis, cell cycle arrest, migration and invasion of osteosarcoma cells. Food Chem Toxicol. 2013;53:325–33. doi: 10.1016/j.fct.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Hossain MA, Kang YJ, Jang JY, Lee YJ, Im E, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int J Oncol. 2013;43:1652–8. doi: 10.3892/ijo.2013.2086. [DOI] [PubMed] [Google Scholar]
- 29.Zheng YH, Yin LH, Grahn TH, Ye AF, Zhao YR, Zhang QY. Anticancer effects of baicalein on hepatocellular carcinoma cells. Mar 4, 2014. Phytother Res Published Online First: [DOI] [PubMed]
- 30.Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25(2A):959–64. [PubMed] [Google Scholar]
- 31.Lin YT, Yang JS, Lin HJ, Tan TW, Tang NY, Chaing JH, et al. Baicalein induces apoptosis in SCC-4 human tongue cancer cells via a Ca2+-dependent mitochondrial pathway. In Vivo. 2007;21:1053–8. [PubMed] [Google Scholar]
- 32.Lorenzo HK, Susin SA. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist Updat. 2007;10:235–55. doi: 10.1016/j.drup.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Bonham M, Posakony J, Coleman I, Montgomery B, Simon J, Nelson PS. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin Cancer Res. 2005;11:3905–14. doi: 10.1158/1078-0432.CCR-04-1974. [DOI] [PubMed] [Google Scholar]
- 34.Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–7. [PubMed] [Google Scholar]
- 35.Gong WY, Wu JF, Liu BJ, Zhang HY, Cao YX, Sun J, et al. Flavonoid components in Scutellaria baicalensis inhibit nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in vitro. Int J Oncol. 2014;44:1561–70. doi: 10.3892/ijo.2014.2320. [DOI] [PubMed] [Google Scholar]
- 36.Chiu YW, Lin TH, Huang WS, Teng CY, Liou YS, Kuo WH, et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol Appl Pharmacol. 2011;255:316–26. doi: 10.1016/j.taap.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Brigelius R. Mixed disulfides: biological functions and increase in oxidative stress. In: Sies H, editor. Oxidative Stress. New York: Academic Press; 1985. pp. 243–72. [Google Scholar]
- 38.Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34:3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- 39.Surh YJ. NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr. 2008;17(suppl 1):269–72. [PubMed] [Google Scholar]
- 40.Rajakangas J, Misikangas M, Päivärinta E, Mutanen M. Chemoprevention by white currant is mediated by the reduction of nuclear beta-catenin and NF-kappaB levels in Min mice adenomas. Eur J Nutr. 2008;47:115–22. doi: 10.1007/s00394-008-0704-0. [DOI] [PubMed] [Google Scholar]
- 41.Lee EK, Kim JM, Choi J, Jung KJ, Kim DH, Chung SW, et al. Modulation of NF-kappaB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Free Radic Res. 2011;45:507–17. doi: 10.3109/10715762.2011.555479. [DOI] [PubMed] [Google Scholar]
- 42.Lee YM, Cheng PY, Chim LS, Kung CW, Ka SM, Chung MT, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, improves cardiac contractile function in endotoxaemic rats via induction of heme oxygenase-1 and suppression of inflammatory responses. J Ethnopharmacol. 2011;135:179–85. doi: 10.1016/j.jep.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Suk K, Lee H, Kang SS, Cho GJ, Choi WS. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. J Pharmacol Exp Ther. 2003;305:638–45. doi: 10.1124/jpet.102.047373. [DOI] [PubMed] [Google Scholar]
- 44.Peng CY, Pan SL, Huang YW, Guh JH, Chang YL, Teng CM. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-kappaB activity in vascular smooth-muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:579–88. doi: 10.1007/s00210-008-0328-1. [DOI] [PubMed] [Google Scholar]
- 45.Tsai CL, Lin YC, Wang HM, Chou TC. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014;153:197–206. doi: 10.1016/j.jep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Fan GW, Zhang Y, Jiang X, Zhu Y, Wang B, Su L, et al. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-kappaB-dependent pathways. Inflammation. 2013;36:1584–91. doi: 10.1007/s10753-013-9703-2. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi K, Cekanova M, McEntee MF, Yoon JH, Fischer SM, Renes IB, et al. Peroxisome proliferator-activated receptor ligand MCC-555 suppresses intestinal polyps in ApcMin/+ mice via extracellular signal-regulated kinase and peroxisome proliferator-activated receptor-dependent pathways. Mol Cancer Ther. 2008;7:2779–87. doi: 10.1158/1535-7163.MCT-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoyagi Y, Nagata S, Kudo T, Fujii T, Wada M, Chiba Y, et al. Peroxisome proliferator-activated receptor gamma 2 mutation may cause a subset of ulcerative colitis. Pediatr Int. 2010;52:729–34. doi: 10.1111/j.1442-200X.2010.03195.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto-Furusho JK, Peñaloza-Coronel A, Sánchez-Muñoz F, Barreto-Zuñiga R, Dominguez-Lopez A. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) expression is down-regulated in patients with active ulcerative colitis. Inflamm Bowel Dis. 2011;17:680–1. doi: 10.1002/ibd.21322. [DOI] [PubMed] [Google Scholar]
- 50.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–49. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 51.Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291–5. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 52.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 53.Teixidó N, Cañamás TP, Usall J, Torres R, Magan N, Viñas I. Accumulation of the compatible solutes, glycine-betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett Appl Microbiol. 2005;41:248–52. doi: 10.1111/j.1472-765X.2005.01757.x. [DOI] [PubMed] [Google Scholar]
- 54.Olthof MR, Verhoef P. Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab. 2005;6:15–22. doi: 10.2174/1389200052997366. [DOI] [PubMed] [Google Scholar]
- 55.Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–30. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 56.Slow S, Elmslie J, Lever M. Dietary betaine and inflammation. Am J Clin Nutr. 2008;88:247–248. doi: 10.1093/ajcn/88.1.247. author reply 248. [DOI] [PubMed] [Google Scholar]
- 57.Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci. 2005;60:1252–64. doi: 10.1093/gerona/60.10.1252. [DOI] [PubMed] [Google Scholar]
- 58.Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, et al. Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol Pharm Bull. 2007;30:2244–9. doi: 10.1248/bpb.30.2244. [DOI] [PubMed] [Google Scholar]
- 59.Lee EK, Jang EJ, Jung KJ, Kim DH, Yu BP, Chung HY. Betaine attenuates lysophosphatidylcholine-mediated adhesion molecules in aged rat aorta: modulation of the nuclear factor-kappaB pathway. Exp Gerontol. 2013;48:517–24. doi: 10.1016/j.exger.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 60.Bharti AC, Aggarwal BB. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann N Y Acad Sci. 2002;973:392–5. doi: 10.1111/j.1749-6632.2002.tb04671.x. [DOI] [PubMed] [Google Scholar]
- 61.Ying J, Rahbar MH, Hallman DM, Hernandez LM, Spitz MR, Forman MR, et al. Associations between dietary intake of choline and betaine and lung cancer risk. PLoS One. 2013;8:e54561. doi: 10.1371/journal.pone.0054561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yi EY, Kim YJ. Betaine inhibits in vitro and in vivo angiogenesis through suppression of the NF-kappaB and Akt signaling pathways. Int J Oncol. 2012;41:1879–85. doi: 10.3892/ijo.2012.1616. [DOI] [PubMed] [Google Scholar]
- 63.Olli K, Lahtinen S, Rautonen N, Tiihonen K. Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. Br J Nutr. 2013;109:43–9. doi: 10.1017/S0007114512000888. [DOI] [PubMed] [Google Scholar]
- 64.Nitter M, Norgard B, de Vogel S, Eussen SJ, Meyer K, Ulvik A, et al. Plasma methionine, choline, betaine, and dimethylglycine in relation to colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Ann Oncol. 2014;25:1609–15. doi: 10.1093/annonc/mdu185. [DOI] [PubMed] [Google Scholar]
- 65.Kim DH, Sung B, Kang YJ, Jang JY, Hwang SY, Lee Y, et al. Anti-inflammatory effects of betaine on AOM/DSSinduced colon tumorigenesis in ICR male mice. Int J Oncol. 2014;45:1250–6. doi: 10.3892/ijo.2014.2515. [DOI] [PubMed] [Google Scholar]
- 66.Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res. 2010;3:1314–33. doi: 10.1158/1940-6207.CAPR-09-0272. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Wu WK, Li ZJ, Chan KM, Wong CC, Ye CG, et al. 2,3′, 4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br J Pharmacol. 2010;160:1352–61. doi: 10.1111/j.1476-5381.2010.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 69.Ci X, Ren R, Xu K, Li H, Yu Q, Song Y, et al. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation. 2010;33:126–36. doi: 10.1007/s10753-009-9166-7. [DOI] [PubMed] [Google Scholar]
- 70.Palozza P, Serini S, Torsello A, Di Nicuolo F, Piccioni E, Ubaldi V, et al. Beta-carotene regulates NF-kappaB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. J Nutr. 2003;133:381–8. doi: 10.1093/jn/133.2.381. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–74. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 72.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 73.Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, et al. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 2009;139:2072–8. doi: 10.3945/jn.108.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Mandal AK, Saito H, Pulliam JF, Lee EY, Ke ZJ, et al. Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/beta-catenin signaling pathway. Toxicol Appl Pharmacol. 2012;262:11–21. doi: 10.1016/j.taap.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]