Abstract
Background:
Health beneficial effects of blueberry have been well documented. Obesity is health hazard that is associated with metabolic abnormalities. We investigated the effect of blueberry leaf extract (BBLE) on high-fat diet (HFD)-induced obesity in C57BL/6J mice.
Methods:
C57BL/6 mice were fed HFD with or without BBLE for 10 weeks. Body weight, serum parameter, and adipose tissues morphology were assessed. The expression of mRNA associated with adipogenesis was measured using real-time polymerase chain reaction (RT-PCR) analysis.
Results:
Administration of BBLE to mice challenged with HFD significantly decreased the body weight gain, the levels of plasma triglyceride (TG) and liver lipid peroxidation, and reduced the adipocyte size and improved hepatic status compared with the group treated with HFD only. BBLE treatment significantly improved glucose control compared with the HFD group. Moreover, BBLE showed an inhibitory effect on adipocyte differentiation in obese mice together with significant decrease in the lipid accumulation by downregulating gene expression of adipocyte-specific transcription factors, such as peroxisome proliferation-activity receptor and acetyl coenzyme A carboxylase and upregulating the mRNA expression of adiponectin, which are critical for adipogenesis.
Conclusion:
BBLE suppressed the body weight gain in the HFD-fed C57BL/6 mice. Intake of BBLE reduced body weight in HFD-fed mice by 20%. Furthermore, BBLE supplementation significantly decreased the TG level in the liver and inhibited leptin secretion. BBLE supplementation also improved insulin resistance. Therefore, BBLE is a possible agent to prevent obesity.
Keywords: Blueberry leaf extracts, High-fat diet-induced obesity, Adipocytes
INTRODUCTION
Obesity is a multifactorial condition posing major health problems worldwide because it is considered to be a risk factor associated with the development of various chronic diseases, including cardiovascular diseases, insulin resistance, dyslipidemia, inflammation, fatty liver, heart disease, hypertension, and diabetes.1,2 It has been recognized as a killer disease because approximately 200,000 individuals throughout the world die every year due to obesity.3 Adipocytes play a critical role in regulating lipid metabolism and energy balance, and they are associated with body mass gain and obesity. As lipid accumulation causes the process of adipogenesis and the programmed differentiation of pre-adipocytes, which is involved in several stages related to obesity, many studies have aimed to reduce obesity by focusing on decreasing pre-adipocyte differentiation and proliferation, inhibiting lipogenesis and increasing lipolysis.4 Currently, there are some therapeutic approaches for treating obesity, such as appetite suppression, self-control and decrease in gastrointestinal absorption.5
Natural products, especially edible medicinal plants as a whole or their different parts, are popular traditional remedies because of the proven health benefits associated with their secondary metabolites, such as flavonoids, terpenoids, chalcones, alkaloids, anthocyanins, etc. Berries are particularly rich sources of anthocyanins, which are important plant pigments. Multiple lines of evidence suggest that anthocyanins are effective in preventing various chronic diseases.6–8 Previous studies have demonstrated the anti-lipidemic and anti-obesity effects of blueberry fruit juice9 and peel extract.10 However, the anti-obesity potential of blueberry leaf extracts (BBLE) has not been examined yet. The present study has been aimed at investigating the effects of BBLE on high-fat diet (HFD)-induced obesity in C57BL/6J mice with particular focus on the possible modulation of adipogenesis- and/or obesity-related gene expression by BBLE.
MATERIALS AND METHODS
1. Preparation of blueberry leaf extracts
Blueberry leaves were collected in January 2013 in Dea-ya farm (Gimcheon, Korea) and were generously provided by Agricultural Development & Technology center in Tea-nam holdings (Seoul, Korea). The dried leaves were extracted with 70% ethanol. The extract was filtered and evaporated in a rotary evaporator and lyophilized. The dried extract (95% yield) was stored at −20°C until subsequent use.
2. Animal experiment
All the experimental procedures were conducted by protocol which was approved by the Committee on the Ethics of Animal Experiments and according to the National Institutes of Health Guide for Care and Use of Laboratory Animals. Twenty-six male C57BL/6 mice (6 weeks old) were purchased from the Samtako Bio Korea (Osan, Korea) and kept in a specific pathogen free facility. They were permanently kept in individual cage under 12h-light/12h-dark cycle and fed ad libitum during the overall experiment. After one week of adaptation, seven mice were fed on normal diet and twenty-one mice were given HFD (provided 45 kcal% fat in Research Diets inc., New Brunswick, NJ, USA) for 4 weeks to induce obesity. And then they were split into four groups and were fed on specific diets for a period of 5 weeks. The groups are following as: first group mice were fed on normal diet (provided 10 kcal% fat in Research Diets, inc.) and permitted ad libitum consumption of water (N + DW), second group mice were fed on HFD and permitted ad libitum consumption of water (HFD + DW), third group mice were fed on HFD and permitted ad libitum consumption of catechin (5%) (HFD +PC), fourth group were fed on HFD and permitted ad libitum consumption of blueberry leaf (2%) drinking (HFD+B). After 5 weeks, blood samples, heart, liver, kidney and adipose tissue were collected. Tissue samples were weighed and fixed in 10% formalin solution or stored at 80°C for further experiments. Body weight and food consumption were measured every week. Food consumption was determined for each of the four groups by weighing the total amount of food given at the start of each week and then subtracting by the amount of food remaining at the end of week. The average food consumed per mouse was then obtained by dividing the number of the mice.
3. Quantitative real-time polymerase chain reaction
Total RNA from liver and white adipose tissue were extracted with Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The single-stranded cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche Ltd., Basel, Switzerland). Quantitative real-time polymerase chain reaction (PCR) was performed using CFX96 real time detection system (Bio-Rad, life Science Research, Hercules, CA, USA). The 20 μl reaction mixture was prepared as follows: 10 μl of SYBR Green Quantitative PCR Super Mix-UDG (Invitrogen), 4 μl of forward primer (10 μM), 0.4 μl of reverse primer (10 μM), and 2 μl of cDNA. The real-time PCR conditions were as follows: 95°C for 10 minutes followed by forty five cycles at 95°C for 15 seconds, 60°C for 5 seconds, and 72°C for 15 seconds. The sequences of primers for PCR analysis were as follow: peroxisome proliferator-activated receptor (PPAR)-γ (sence) ATGGAACGTAACGGTCAAG-TC, (antisence) GCACTGCCGCTG CTAAGACGT; Acetyl coenzyme A carboxylase (ACC) (sence) AGATGTAGTTGCA GCAATTCGTGA, (antisence) CCGTAGGGTGCTACCGATGCAGAC; Adiponectin (sence) GATAGGTGCA-ACTGTCCTGAC, (antisence) GCACATGTA-CTACTGGG ACAT; GAPDH (sence) ATTC-CATGGCACCGTC-AAGGC, (antisence) TCAGGTC CACCACTGACACGT.
4. Histological analysis
Liver and epididymal white adipose tissue samples were fixed with 10% formalin and then stained with oil red O and hematoxylin and eosin, respectively.
5. Biochemical assays
After 5 weeks of experimental diets, the rats were euthanized, and the tissues were dissected out and analyzed. The body and fatty tissue weights were measured with sensitivity limits of 0.1 g and 0.01 g, respectively. The body mass index was calculated by dividing the weight (g) by the square of body length (cm2). Blood was collected from each rat, stored at 37°C for 30 minutes, and centrifuged at 4,000 g at 4°C for 10 minutes to obtain the plasma. The epididymis fat pad and perirenal fat pad were excised, weighed and stored at −70°C until being assayed. The concentrations of plasma triglyceride (TG), total cholesterol, and high-density lipoprotein-cholesterol were assayed enzymatically using commercial kits (Asan Phamaceutical Co., Ltd., Seoul, Korea).
RESULTS
1. Effects of BBLE or catechin on body weight, food intake and liquid consumption in HFD-fed mice
Mice treated with catechin or BBLE in drinking for 10 weeks remained healthy. The average initial body weight of mice receiving HFD was 33 g, which was not significantly different from the other treatment groups. After 5 weeks, the mice had gained higher body weight compared with the mice fed on normal or low-fat diet (LFD) (Table 1). Administration of catechin or BBLE to HFD-treated mice markedly reduced HFD-induced body weight by 6.9% and 13.9%, respectively, but the body weight was still higher than that of mice receiving normal (LFD) group. Liquid consumption by mice in the LFD group (3.12 ± 0.93 mL day per mouse) was the highest and the group of mice fed on a HFD (1.96 ± 0.12 mL day per mouse) without receiving a BBLE or catechin supplement was the lowest (Table 1).
Table 1.
Effect of BBLE treatment on body weight in HFD-induced obesity mouse model C57BL/6J mice
| Group
|
||||
|---|---|---|---|---|
| N1) | C2) | PC3) | B4) | |
| Water intake (ml) | 3.12 ± 0.93 | 1.96 ± 0.12 | 2.07 ± 0.18 | 3.07 ± 1.17 |
| Food intake (g) | 3.08 ± 0.93 | 2.68 ± 0.24 | 2.76 ± 0.21 | 2.74 ± 0.33 |
| Initial weight (g) | 24.44 ± 1.21 | 33.34 ± 2.35 | 32.88 ± 2.47* | 33.08 ± 1.34* |
| Body weight gain (g) | 3.61 ± 1.18 | 11.48 ± 1.57 | 8.83 ± 2.02* | 5.53 ± 1.41* |
Data are expressed as means ± s.d. (n = 6). ANOVA with duncan’s test:
P < 0.01, significantly from the value of the HFD group.
N, negative control mice;
C, high-fat diet (HFD)-fed mice;
PC, administration of catechin in HFD-fed mice;
B, administration of Blueberry leaves extract (BBLE) in HFD-fed mice.
2. Effects of BBLE or catechin on the serum parameters of HFD-fed mice
Counting of blood cells in different treatment groups revealed that the white blood cells, red blood cells, and platelets counts were higher in the HFD-fed mice compared to LFD-fed mice. Intake of BBLE or catechin slightly decreased HFD-induced increment in blood cells count (Table 2). Mice fed HFD showed elevation in serum glucose, TG and cholesterol levels compared to LFD group. BBLE or catechin consumption significantly decreased HFD-induced serum glucose and cholesterol levels (Table 2). Comorbidities with obesity such as type 2 diabetes and cardiovascular disease are associated with chronic inflammation, which is able to be determined systemically in related organs. In this study, inflammatory cells were increased in the high-fat diet group compared to the control and decreased in the catechin or the BBLE treated group.
Table 2.
Effect of BBLE treatment on serum parameter in HFD-induced obesity mouse model C57BL/6J mice
| Group
|
||||
|---|---|---|---|---|
| N | C | PC | B | |
| WBC (k/μl) | 10.158 ± 0.429 | 14.652 ± 0.329 | 9.9475 ± 0.517 | 10.599 ± 0.126 |
| Neutrophil (k/μl) | 4.635 ± 0.288 | 6.192 ± 0.313 | 4.0675 ± 0.227 | 4.493 ± 0.155 |
| Lymphocyte (k/μl) | 5.375 ± 0.228 | 8.198 ± 0.314 | 5.6125 ± 0.447 | 5.803 ± 0.078 |
| Monocyte (k/μl) | 0.1325 ± 0.127 | 0.216 ± 0.123 | 0.24 ± 0.093 | 0.266 ± 0.058 |
| Eosinophil (k/μl) | 0.0175 ± 0.005 | 0.036 ± 0.015 | 0.0175 ± 0.01 | 0.03 ± 0.017 |
| Basophil (k/μl) | 0.0025 ± 0.005 | 0.01 ± 0.012 | 0.005 ± 0.006 | 0.0033 ± 0.006 |
| RBC (M/μl) | 683 ± 14.468 | 843.6 ± 8.385 | 675.75 ± 10.436 | 712 ± 8.544 |
| Hb (g/dl) | 17.375 ± 0.206 | 21.56 ± 0.688 | 17.15 ± 0.404 | 17.9 ± 0.265 |
| HCT (%) | 49.55 ± 0.624 | 54.34 ± 0.627 | 47.175 ± 0.299 | 47.666 ± 0.513 |
| Platelet (k/μl) | 700 ± 19.494 | 855.8 ± 22.521 | 704.25 ± 14.408 | 717.333 ± 23.072 |
| AST (U/L) | 74.60 ± 3.13 | 122.60 ± 3.85 | 75.00 ± 3.37 | 88.67 ± 3.06 |
| ALT (U/L) | 57.00 ± 2.12 | 87.00 ± 2.24 | 57.50 ± 2.38 | 69.33 ± 0.58 |
| ALP (U/L) | 172.80 ± 3.42 | 276.20 ± 6.14 | 183.50 ± 3.42 | 189.33 ± 2.52 |
| LDH (mmol/mL) | 332.00 ± 13.04* | 548.00 ± 11.77 | 390.50 ± 4.20* | 400.67 ± 8.33* |
| HDL-C (mmol/mL) | 118.00 ± 3.39 | 78.60 ± 2.97 | 99.50 ± 6.03 | 102.67 ± 6.03 |
| LDL-C (mmol/mL) | 42.60 ± 3.51* | 133.60 ± 3.78 | 90.25 ± 3.77* | 97.67 ± 4.04* |
| Glucose (mmol/mL) | 80.4 ± 4.22 | 126.40 ± 6.27 | 90.0 ± 3.65 | 94.67 ± 4.16 |
| Insulin (μU/mL) | 21.34 ± 1.75 | 41.98 ± 1.33 | 24.50 ± 1.00 | 24.33 ± 1.15 |
| TG (mg/dL) | 43.00 ± 5.70* | 174.40 ± 4.28 | 94.00 ± 1.83* | 88.33 ± 6.11* |
| T-CHOL (mg/dL) | 59.00 ± 1.58 | 117.00 ± 4.69 | 83.50 ± 2.65 | 100.00 ± 6.00 |
K/μL: ×103/mm3, M/μL: ×106/mm3. Data are expressed as means ± s.d. (n = 6). Duncan’s test:
P < 0.01 significantly from the value of the HFD group. WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; HCT, hematocrit; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lip-oprotein-cholesterol; TG, triglycerides; T-CHOL, total cholesterol.
Data are expressed as means ± s.d. (n = 6). ANOVA with Duncan’s test:
*P < 0.01 significantly from the value of the HFD group. DW, distilled water.
To examine the liver function, aspartate transaminase and alanine transaminase were monitored. As a result, aspartate transaminase and alanine transaminase were increased in the high-fat diet group but were decreased in the catechin or BBLE treated group. Because hyperlipidemia is known to induce metabolic disease such as hypertension, diabetes, or obesity, blood lipid control by food digest is considered to be important.11 Visceral fat which is increased by obesity is sensitive to the lipolysis signals and is known to be well resolved.12 In this study, a blood concentration of lipid was analyzed. In catechin and BBLE treated groups, the plasma low-density lipoprotein was decreased to 32.5% and 26.9%, respectively compared to that in the high-fat diet control group, while neutral fat was 45.9% and 49.4%, respectively.
3. Impact of BBLE or catechin on HFD-induced alteration of liver and adipose tissue morphology
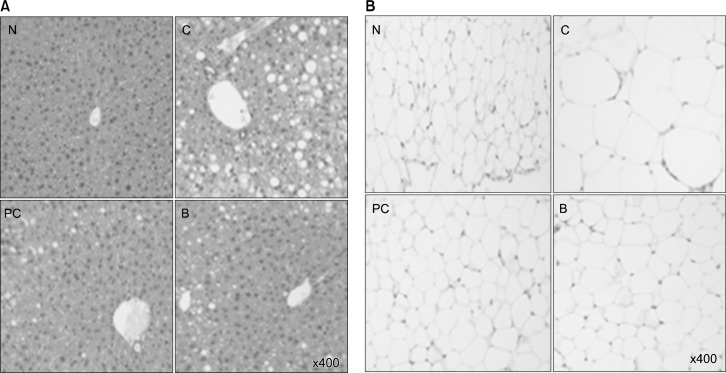
Mice fed HFD showed intense lipid accumulation in the liver (Fig. 1A). In contrast, drinking of BBLE or catechin significantly alleviated HFD-induced lipid accumulation. Figure 2 displays the histology of epididymis of white adipose tissue of mice. The mice fed HFD showed hypertrophy of the adipocytes in the adipose tissue, which was markedly attenuated by treatment with catechin or BBLE (Fig. 1B).
Figure 1.
Effect of BBLE on tissue morphology. (A) Liver morphology (magnification ×400). (B) Epididymal adipose tissue morphology (magnification ×400). N, negative control mice; C, high-fat diet (HFD)-fed mice; PC, HFD-fed mice + catechin; B, HFD-fed mice + BBLE.
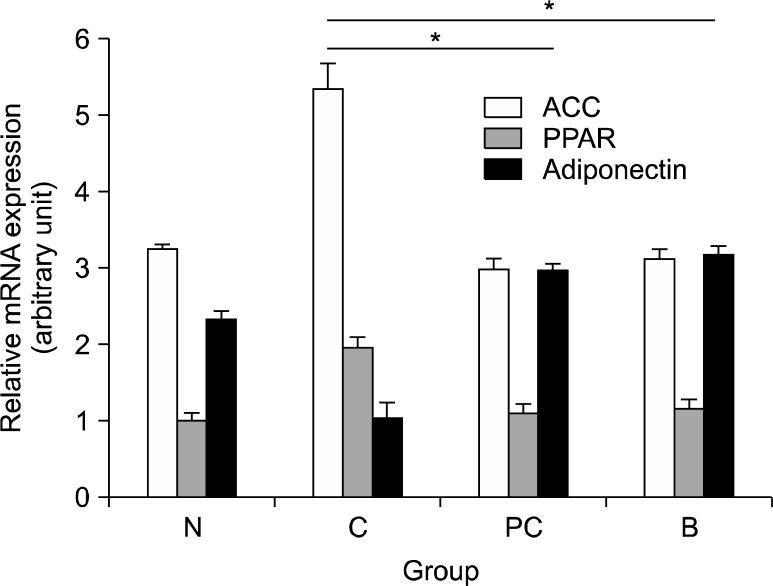
Figure 2.
Gene expression was determined by quantitative real-time polymerase chain reaction. ACC, acetyl coenzyme A carbox-ylase; PPAR-γ, peroxisome proliferator-activated receptor-γ; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; N, negative control mice; C, HFD-fed mice; PC, administration of catechin in HFD-fed mice; B, administration of BBLE in HFD-fed mice. Data are expressed as means ± s.d. (n = 6). Duncan’s test: *P < 0.01 significantly from the value of the HFD group.
4. Effects of BBLE or catechin on antioxidant status
To determine the antioxidant potential of BBLE, we examined the effect of BBLE or the reference compound catechin on the lipid peroxidation as well as the gene expression of several antioxidant enzymes. As shown in Table 3, treatment with HFD depleted the hepatic gene expression of the two representative antioxidant enzymes superoxide dismutase and catalase, which was restored by treatment with BBLE or catechin. Moreover, drinking of BBLE or catechin protected mice from HFD-induced oxidative stress as revealed by reduced xanthine/xanthine oxidase activity and decreased thiobarbituric acid reactive substances in comparison with the mice fed HFD only.
Table 3.
Effect of antioxidant in HFD-induced obesity mouse model C57BL/6J mice
| Group
|
||||
|---|---|---|---|---|
| N | C | PC | B | |
| SOD1) (U/mL) | 34.27 ± 1.04** | 14.07 ± 0.86 | 37.47 ± 0.76** | 32.05 ± 0.77** |
| CAT2) (U/mL) | 6.40 ± 0.42** | 3.83 ± 0.40 | 8.05 ± 0.26** | 6.11 ± 0.25** |
| XO3) (U/mL) | 1.63 ± 0.06* | 2.37 ± 0.12 | 1.33 ± 0.06* | 1.59 ± 0.08* |
| TBARS4) (nmol/mg) | 2.31 ± 0.11** | 4.95 ± 0.30 | 1.80 ± 0.08** | 2.46 ± 0.10** |
Data are expressed as means ± s.d. (n = 6). ANOVA with duncan’s test:
P < 0.05,
P < 0.01 significantly from the value of the HFD group. N, negative control mice; C, HFD-fed mice; PC, administration of catechin in HFD-fed mice; B, administration of BBLE in HFD-fed mice;
SOD, superoxide dismutase;
CAT, catalase;
XO, xanthine oxidase;
TBARS, thiobarbituric acid reactive substances.
5. Molecular biological observation of liver and white adipose tissue
The mRNA expression levels of PPAR-γ, adiponectin and ACC were determined in liver and adipose tissue. Quantitative real-time PCR analysis was also performed to evaluate the expression of PPAR-γ, adiponectin and ACC. Compared to the control group, mice fed HFD showed an up-regulation of PPAR-γ and ACC genes, and a down-regulation of adiponectin gene. Catechin or BBLE treatment groups showed markedly reduced expression levels of PPAR-γ and ACC compared with the HFD-fed mice group, while the adiponectin expression levels were increased (Fig. 2). The ACC mRNA expression level was significantly increased in high-fat diet group compared with the normal control, while its level was significantly decreased in the catechin or BBLE treated group. ACC is one of the enzymes upregulated when lipometabolism is activated, and ACC converts acetyl coenzyme A into malonyl-coenzyme A contributing to fatty acid synthesis and oxidation in mitochondria.
Adiponectin was negatively correlated with inflammatory parameters and fasting insulin concentrations,13 and hypo-adiponectinemia is associated with type II diabetes. Therefore, significant increase of adiponectin, an anti-inflammatory cytokine, by BBLE suggested that this formulation has an inhibitory effect on insulin resistance caused by obesity-induced inflammation.
DISCUSSION
Blueberry is known to be particularly rich in anthocyanins, which have been reported to elicit beneficial effects in regulating metabolic syndrome.14 Anthocyanins are natural components of the human diet, as they are present in many vegetables and fruits, especially in berries. They have attracted attention because of their health related functions such as anti-obesity properties.15 Obesity is a major characteristic of metabolic syndrome; it is closely correlated with dyslipidemia, hyperglycemia and hypertension and is associated with an increased propensity for the development of cardiovascular diseases.15,16 Among various types of berries, blueberry is the rich source of anthocyanins. The protective effects of blueberry fruit juice or peel extracts have been shown to alleviate insulin resistance, reduce obesity and dyslipidemia which have prompted us to investigate the anti-obesity effect of BBLE on HFD-induced obesity in C57BL/6J mice. Our result demonstrated that BBLE treatment led to significant decreases in body weight gain, the extents of the crown-like structure in adipose tissue and the adipocyte size, and improved glucose control, plasma insulin, plasma TG and fatty liver in HFD-fed mice. Drinking of BBLE decreased the epididymal fat and the size of adipocytes, indicating that BBLE may directly affect both the number and the size of adipocytes in adipose tissues. The present investigation confirmed that HFD-fed mice gained body weight, showed increased serum and liver lipids, as well as elevated insulin and leptin levels.17,18 In this respect, Lim et al. showed that mulberry fruit extract treatment has potential anti-obesity and anti-diabetic effects through modulation of obesity-induced inflammation and oxidative stress in HFD-induced obesity.19 Similarly, Titta et al. found blood orange juice inhibited fat accumulation in C57/BL6 mice.20 In this study, we found that HFD induced obesity and increased TG in the mouse liver, which was attenuated by BBLE treatment.
Chronic inflammation associated with obesity may affect every tissue systemically, which can be recognized by the immune system as well as other cell types, thereby inducing cytokines or chemokines. Chronic and endogenous inflammation associated with obesity shows different characteristics such as being painless or nonfebrile compared with typical inflammation. In obesity, the number of peripheral mononuclear cells are increased as well as its activity and proinflammatory cytokines is increased. Hotamisligil et al. showed that when inflammatory factors were increased, the incidence of the type II diabetes was increased.21
Although the effects of the body fat level of mice on the response to BBLE remain unknown, BBLE consumption may regulate lipid metabolism by suppressing the fatty acid synthesis via downregulation of PPAR-γ and ACC gene expression. Prior et al. also demonstrated that supplementation with anthocyanins exhibited improved insulin resistance in HFD-induced obese mice (C57BL/6). Adiponectin is an adipocyte secretory protein hormone that modulates metabolic processes, such as fatty acid oxidation and glucose regulation.22,23 The circulating level of adiponectin is decreased in obese subjects, while the increased concentration of adiponectin hormone reduces bodyweight of obese animals.24 Thus, the increased adiponectin gene expression appears as a biochemical basis of anti-obesity effects of BBLE. In this study, the enzyme activity of superoxide dismutase or catalase, the anti-oxidant enzymes, was significantly decreased in the HFD group compared to the normal control, supporting results of the previous study by Carantoni et al. which showed that obesity caused imbalance of the anti-oxidant system.25 On the other hands, BBLE treatment increased the antioxidant enzyme activity. A recent study suggested that the mechanism of obesity and oxidative stress is similar on the basis of the large amount of macrophages present in fat tissue which generate reactive oxygen species to cause oxidative stress.26 Therefore, obesity is considered to be a key factor inducing cardiovascular disease, inflammatory disease or cancer because reactive oxygen species produced by macrophases in fat tissue will cause the oxidative modification of DNA, protein or lipid.27
In summary, BBLE suppresses the body weight gain of the HFD-fed C57BL/6 mice. Intake of BBLE reduced body weight in HFD-induced obese mice by 20%. Furthermore, BBLE supplementation significantly decreased the TG levels in the liver, and inhibited leptin secretion. BBLE supplementation also improved insulin resistance. Therefore, BBLE is a possible agent for the prevention of obesity.
Acknowledgments
This project was supported by the Small and Medium Business Administration R & D Program (C0138155), Korea.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Nieves JW, Komar L, Cosman F, Lindsay R. Calcium potentiates the effect of estrogen and calcitonin on bone mass: review and analysis. Am J Clin Nutr. 1998;67:18–24. doi: 10.1093/ajcn/67.1.18. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Kwon CS, Sohn HY, Kim SH, Kim JH, Son KH, Lee JS, et al. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem. 2003;67:1451–6. doi: 10.1271/bbb.67.1451. [DOI] [PubMed] [Google Scholar]
- 4.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–14. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Yun JW. Possible anti-obesity therapeutics from nature--a review. Phytochemistry. 2010;71:1625–41. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem. 2008;56:647–53. doi: 10.1021/jf071993o. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Pittman HE, 3rd, Prior RL. Fate of anthocyanins and anti-oxidant capacity in contents of the gastrointestinal tract of weanling pigs following black raspberry consumption. J Agric Food Chem. 2006;54:583–9. doi: 10.1021/jf052108+. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Cao G, Prior RL. Absorption and metabolism of anthocya-nins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132:1865–71. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- 9.Wu T, Tang Q, Gao Z, Yu Z, Song H, Zheng X, et al. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS One. 2013;8:e77585. doi: 10.1371/journal.pone.0077585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Park HJ, Kang SN, Jang SH, Lee SJ, Ko YG, et al. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS One. 2013;8:e69925. doi: 10.1371/journal.pone.0069925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon BS, Park JW, Kim BK, Kim HK, Jung TS, Hahm JR, et al. Fermented mushroom milk-supplemented dietary fibre prevents the onset of obesity and hypertriglyceridaemia in Otsuka Long-Evans Tokushima fatty rats. Diabetes Obes Metab. 2005;7:709–15. doi: 10.1111/j.1463-1326.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 12.Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–6. [PubMed] [Google Scholar]
- 13.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 14.Prior RL, S EW, T RR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58:3970–6. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 15.He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010;1:163–87. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 16.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speakman J, Hambly C, Mitchell S, Król E. Animal models of obesity. Obes Rev. 2007;8(suppl 1):55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 18.List EO, Berryman DE, Wright-Piekarski J, Jara A, Funk K, Kopchick JJ. The effects of weight cycling on lifespan in male C57BL/6J mice. Int J Obes. 2013;37:1088–94. doi: 10.1038/ijo.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim HH, Lee SO, Kim SY, Yang SJ, Lim Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp Biol Med. 2013;238:1160–9. doi: 10.1177/1535370213498982. [DOI] [PubMed] [Google Scholar]
- 20.Titta L, Trinei M, Stendardo M, Berniakovich I, Petroni K, Tonelli C, et al. Blood orange juice inhibits fat accumulation in mice. Int J Obes. 2010;34:578–88. doi: 10.1038/ijo.2009.266. [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–34. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arcari DP, Bartchewsky W, dos Santos TW, Oliveira KA, Funck A, Pedrazzoli J, et al. Antiobesity effects of yerba mate extract (Ilex paraguariensis) in high-fat diet-induced obese mice. Obesity. 2009;17:2127–33. doi: 10.1038/oby.2009.158. [DOI] [PubMed] [Google Scholar]
- 23.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–34. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegrist M, Rank M, Wolfarth B, Langhof H, Haller B, Koenig W, et al. Leptin, adiponectin, and short-term and long-term weight loss after a lifestyle intervention in obese children. Nutrition. 2013;29:851–7. doi: 10.1016/j.nut.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Carantoni M, Abbasi F, Warmerdam F, Klebanov M, Wang PW, Chen YD, et al. Relationship between insulin resistance and partially oxidized LDL particles in healthy, nondiabetic volunteers. Arterioscler Thromb Vasc Biol. 1998;18:762–7. doi: 10.1161/01.atv.18.5.762. [DOI] [PubMed] [Google Scholar]
- 26.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–41. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]