Abstract
Background:
The Helicobacter felis (H. felis) mouse model has been developed for the research regarding pathogenesis of chronic gastritis and gastric cancer. The aim of this study was to investigate long-term H. felis colonization in the stomachs of C57BL/6 mice and subsequent histologic findings and inflammatory reactions including pro-inflammatory cytokines.
Methods:
Twenty-three female C57BL/6 mice at 4 weeks of age were gavaged with H. felis, and 13 control mice served as vehicle only. The mice were sacrificed at 4, 24, and 52 weeks after inoculation. The infection status and degree of inflammation were determined by culture and histopathology. The level of gastric mucosal myeloperoxidase (MPO), tumor necrosis factor alpha (TNF-α), and interleukin-1beta (IL-1β) were measured by ELISA.
Results:
The overall infection rate was 100%, as determined by the culture and histology. At 4, 24, and 52 weeks, the neutrophil and monocyte scores were significantly higher in infected mice than in control mice. At 24 weeks after inoculation, most of the infected mice showed mucosal atrophy with or without metaplasia, and a few showed focal dysplasia. Adenocarcinoma was observed in one mouse at 52 week post-infection. Gastric mucosal MPO and IL-1β levels were significantly higher in infected mice than those in control mice at 24 and 52 weeks. However, the expression of gastric mucosal TNF-α was not significantly different between the infected and control mice at any time-point.
Conclusions:
Long-term H. felis-infection in C57BL/6 mice provoked a severe inflammatory reaction and it progressed into atrophy, metaplasia, dysplasia and cancer. IL-1β might play an important role in the inflammatory response of mice to Helicobacter species.
Keywords: Helicobacter felis, Inflammation, Interleukin-1beta, Tumor necrosis factor-alpha
INTRODUCTION
Helicobacter pylori (H. pylori) infection is a major etiologic factor associated with gastric cancer,1 and the bacterial pathogen was classified as a “definite biological carcinogen” by the World Health Organization in 1994.2 Various experimental animal models have been used to elucidate the underlying pathological and biochemical mechanisms of Helicobacter-associated gastric cancer. A limited number of H. pylori strains have successfully colonized the mouse stomach. The most common organisms that colonize the mouse stomach are H. pylori Sydney strain 1 (SS1) and Helicobacter felis (H. felis).3 H. felis, first isolated from the gastric tissue of the cat, causes natural infections in mice and is analogous to H. pylori in humans.4 Infection of the C57BL/6 strain of mice with H. pylori SS1, a mouse-adapted H. pylori strain, results in chronic colonization and hypertrophy, but these mice do not always develop dysplasia and carcinoma.5 Chronic H. felis infection induces severe inflammation, atrophy, metaplasia, dysplasia, and gastric cancer in C57BL/6 mice.6
H. pylori-associated gastric inflammation is mediated by pro-inflammatory cytokines such as the tumor necrosis factor (TNF)-α, interferon-γ, interleukin (IL)-1β, IL-6, IL-8, and IL-18.7,8 IL-1β is an important inflammatory mediator and may be involved in carcinogenesis.9,10 TNF-α is also associated with inflammation, immune regulation, and tissue repair and is an import factor in the development of digestive diseases.11
The aim of this study was to investigate chronic H. felis colonization in the stomachs of C57BL/6 mice and inflammatory reactions, including the production of pro-inflammatory cytokines. We report the results of these investigations and the subsequent histologic findings.
MATERIALS AND METHODS
1. Animals and H. felis infection
Four-week-old female C57BL/6 mice (Orient Co., Ltd., Seoul, Korea) weighing 10–15 g were used for the experiments. All mice were housed in a cage maintained at 23oC with a 12/12-hour light/dark cycle under specific pathogen-free conditions. The mice were administered orogastrically with vehicle only (0.25 mL, for controls) or vehicle containing more than 1 × 107 colony-forming units (CFU)/mL of H. felis (ATCC 49179), 5 times every other day. The mice were sacrificed by CO2 asphyxiation at 4, 24, and 52 weeks after inoculation. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital (IACUC number: BA1004-059/016-01).
2. Histopathology
At necropsy, stomach tissue was taken from the greater curvature beginning at the squamocolumnar junction and ending at the gastroduodenal junction. Linear gastric strips were fixed in 10% formalin solution, processed by standard methods, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. The stomach mucosa was histologically examined for inflammatory and epithelial changes and for the presence of H. pylori. The degree of neutrophil infiltration, mononuclear cell infiltration, atrophy, and metaplasia was assessed according to the updated Sydney classification as follows: 0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked.
3. Measurement of mucosal myeloperoxidase, tumor necrosis factor alpha, and interleukin-1 beta
Ten milligrams of scraped mucosa were homogenized for 30 seconds with a Polytron homogenizer in 200 μL of ice-cold lysis buffer (200 mM NaCl, 5 mM EDTA, 10 mM Tris [pH 7.4], 10% glycerin, 1 mM PMSF, 1 μg/mL leupeptin, and 28 μg/mL aprotinin). The cell suspensions were centrifuged at 13,000 rpm for 15 minutes, and the resulting supernatant was assayed using a myeloperoxidase (MPO) ELISA kit (HyCult Biotechnology, Uden, The Netherlands). For TNF-α and IL-1β, the appropriate kits from R&D Systems (Minneapolis, MN, USA) were used following the manufacturer’s instructions. Protein concentration was measured using a Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). The concentration of each cytokine was measured in picograms per milligram of protein. All assays were performed in triplicate.
4. Statistical analysis
Data are expressed as means ± SEMs. Comparison of between the 2 groups (experimental and control) was performed using the Mann-Whitney U test or Fisher’s exact test. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA).
RESULTS
1. Helicobacter felis colonization
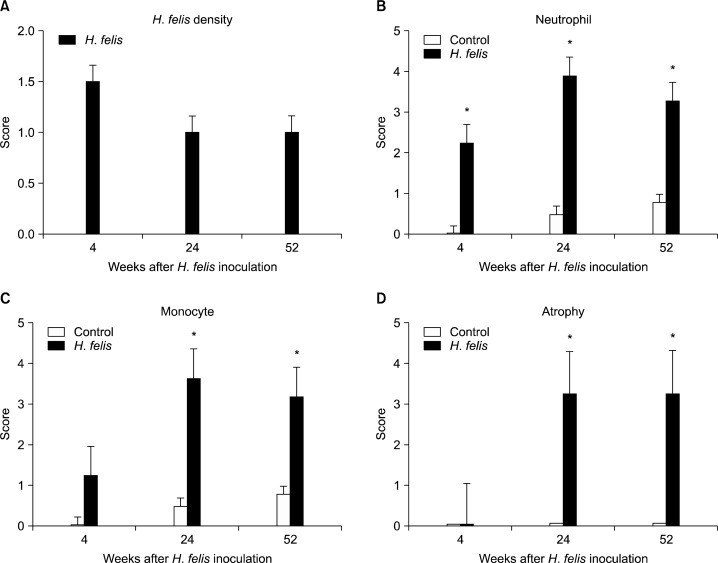
The H. felis infection status was evaluated by culture or histology. Of the 23 infected mice, 14 (60.8%) showed positive results in culture. However, all infected mice (100%) tested positive for infection by histopathology (Table) and had H. felis density scores of 1.5 at week 4, and 1.0 at week 24 and 52 (Fig. 1A). No H. felis were isolated in the stomach of the control mice.
Table.
Histologic findings in mice infected with H. felis over 1 year
| Histologic findings | Control | Weeks after inoculation
|
||
|---|---|---|---|---|
| 4 | 24 | 52 | ||
| H. felis (+) | 0/13 | 4/4 | 8/8 | 11/11 |
| Neutrophil | 0/13 | 4/4 | 8/8 | 11/11 |
| Monocyte | 0/13 | 3/4 | 8/8 | 11/11 |
| Mucosal atrophy | 0/13 | 0/4 | 8/8 | 11/11 |
| Intestinal metaplasia | 0/13 | 0/4 | 5/8 | 9/11 |
| Lymphoid aggregation | 0/13 | 1/4 | 7/8 | 11/11 |
| Hyperplasia | 0/13 | 0/4 | 5/8 | 0/11 |
| Dysplasia | 0/13 | 1/4 | 2/8 | 3/11 |
| Gastric cancer | 0/13 | 0/4 | 0/8 | 1/11 |
Figure 1.
Gastric histopathology scores of Helicobacter felis (H. felis) density (A), Neutrophil (B), Monocyte (C), Atrophy (D) at 4, 24, and 52 weeks after H. felis inoculation. The neutrophil and monocyte grades of infected mice peaked at week 24 and were significantly higher at all of time points compared with the control mice. In case of atrophy significantly higher atrophic scores appeared at 24 weeks and it continued up to 52 weeks. Data are presented as means ± SEMs. *P < 0.05 compared with controls at the same time-point.
2. Histopathology
The neutrophil and monocyte grades of infected mice peaked at week 24 and were significantly higher at all of time points compared with the control mice (Fig. 1B–C). No atrophy occurred up to week 4, however, all infected mice had significantly higher atrophic scores than control mice after 24 weeks. The mean atrophy scores were 3.25 in week 24 and 3.27 in week 52 (Fig. 1D).
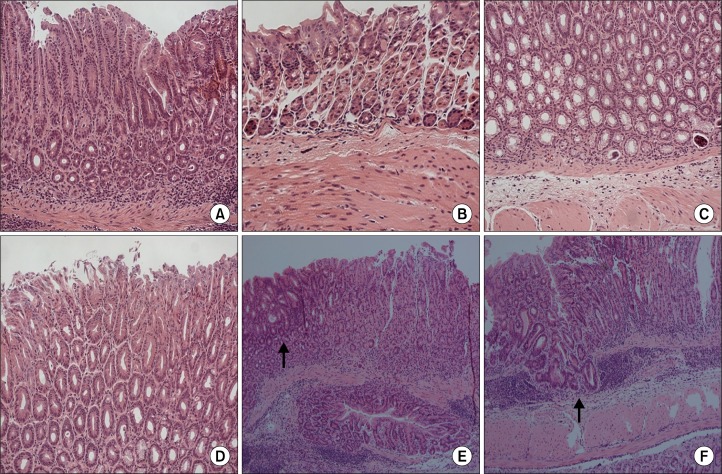
All control mice demonstrated normal histopathology (Fig. 2A). At 4 weeks after inoculation, neutrophil and monocyte infiltration occurred in all infected mice, suggesting chronic active gastritis (Fig. 2B). In particular, one mouse at week 4 demonstrated lymphoid aggregations, erosion, and high grade dysplasia. At 24 weeks after inoculation, mucosal atrophy and mucous metaplasia appeared in all infected mice. The normal architecture was completely lost and replaced by pseudo-pyloric metaplasia (Fig. 2C) and a hyperplastic change of the mucosa was observed (Fig. 2D). Focal low-grade dysplasia was also noted in some mice. At 52 weeks after inoculation, dysplastic change (Fig. 2E) and adenocarcinoma with focal submucosal invasion (Fig. 2F) was noted in a few mice (Table).
Figure 2.
Histopathologic findings of the gastric mucosa at each time-point. Hematoxylin and eosin (H&E) staining; ×200 (A–D), ×100 (E, F). Normal gastric mucosa (A), Marked neutrophil infiltration at 4 weeks after inoculation (B), pseudopyloric metaplasia (C) and hyperplasia (D) at 24 weeks after inoculation, low-grade dysplasia (arrow) (E) and adenocarcinoma with focal submucosal invasion (arrow) (F) at 52 weeks after inoculation.
3. Expression of pro-inflammatory cytokines
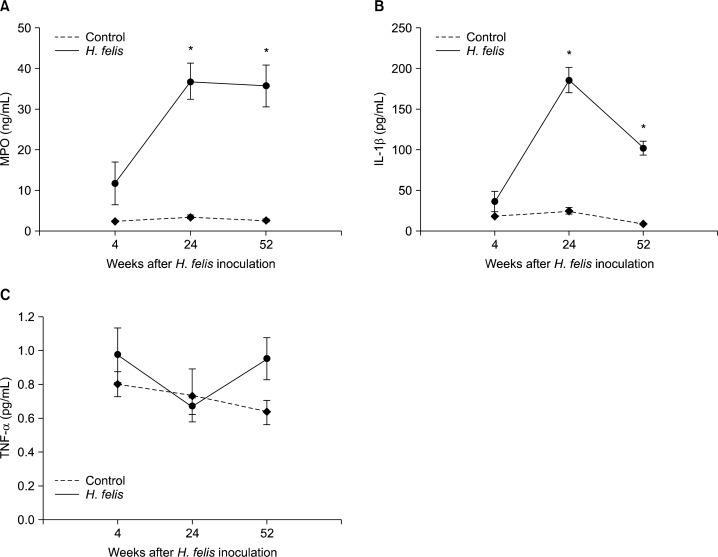
At 24 and 52 weeks, the gastric mucosal MPO level (ng/mL) in H. felis-infected mice was significantly higher (week 4, 11.8 ± 5.2; week 24, 36.9 ± 4.4; week 52, 35.8 ± 5.1; P < 0.05) than that in control mice (Fig. 3A). The gastric mucosal IL-1β expression (pg/mL) in H. felis-infected mice peaked at week 24 and decreased trend (week 4, 36.4 ± 12.5; week 24, 186.0 ± 15.8; week 52, 102.2 ± 8.5). At weeks 24 and 52, the level of IL-1β expression was significantly higher for infected mice than that for control mice (P < 0.05) (Fig. 3B). The gastric mucosal TNF-α expression (pg/mL) in H. felis-infected mice (week 4, 11.8 ± 5.2; week 24, 36.9 ± 4.4; week 52, 35.8 ± 5.1) did not show any significance compared with control mice and there was no correlation between the weeks and gastric mucosal TNF-α level (Fig. 3C).
Figure 3.
Expression of gastric mucosal myeloperoxidase (MPO) (A), interleukin-1beta (IL-1β) (B), and tumor necrosis factor-alpha (TNF-α) (C) by ELISA. Data are presented as means ± SEMs. *P < 0.05 compared with controls at the same time-point.
DISCUSSION
In this study, we observed severe inflammation, glandular atrophy, metaplasia, dysplastic change, and adenocarcinoma in mice stomachs at 4, 24, and 52 weeks after H. felis inoculation and successfully established the chronic H. felis infection model in C57BL/6 mice. In addition, we analyzed the levels of gastric mucosal pro-inflammatory cytokines such as IL-1β and TNF-α.
A reliable animal model of H. pylori infection is necessary for evaluating vaccine efficacy and for understanding the pathological mechanism of the organism. After discovery of H. pylori by Marshall and Warren in 1984,12 many attempts had been made to establish animal models. In early 1990, H. felis, a close relative of H. pylori that was isolated from the cat stomach, and it was used because human clinical strains of H. pylori were not able to colonize mice stomachs.13 H. felis-infected C57BL/6 mice showed the classic sequence of histologic changes consistent with human infection, including gastritis, oxyntic gland atrophy, surface epithelial proliferation, metaplasia, dysplasia and adenocarcinoma.14 Sakagami et al.15 reported that C57BL/6 mice infected with H. felis showed moderate to severe chronic active gastritis, which increased in severity over time and atrophic changes after 6 months. In other studies of a longer duration, H. felis-infected mice showed gastric metaplasia, dysplasia and invasive cancer.6,14,16 In 1997, Lee et al.17 first introduced mouse adopted H. pylori strain, “the Sydney strain of H. pylori (SS1)”. H. pylori SS1 shows high levels of colonization in C57BL/6 mice. However, previous studies showed that chronic H. pylori SS1 infection in C57BL/6 mice results in chronic colonization and hypertrophy, but these mice do not always develop dysplasia and carcinoma.18,19 These differences may be associated with differences in the colonization pattern between H. pylori SS1 and H. felis. H. pylori SS1 tends to localize in the upper foveolae, whereas H. felis tends to colonize the deep foveolae and antral glands.17,20 In addition, H. pylori SS1 adheres to the epithelium at the top of the pits, as observed by electron microscopy. In contrast, H. felis has only been observed free in the gastric mucus and does not adhere to the epithelium.17,21 We previously reported about H. pylori SS1 infection in C57BL/6 mice with minimal to mild (score 1–2) neutrophil infiltration 4 weeks after inoculation.22 Furthermore, the mean gastric mucosal MPO level was 3.4 ± 0.6 ng/mL at 4 weeks after H. pylori infection.22 In the present study, H. felis infection resulted in mild to moderate neutrophil infiltration (score 2–3) during the same period, and one infected mice showed high-grade dysplasia 4 weeks after inoculation. The mean gastric mucosal MPO level during same period was 11.8 ± 5.2 ng/mL, and the level then markedly increased more than 30 ng/mL at weeks 24 and 52. Therefore, H. felis apparently provoked more severe inflammation than did the H. pylori SS1 strain and ultimately led to adenocarcinoma.
Gastric mucosal pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α increase in H. pylori infected animal. IL-1β physiologically induced by H. pylori infection enhanced gastric carcinogenesis by affecting both inflammatory and epithelial cells.23 Sun et al.24 reported that there was an increased IL-1β expression at all time-points during long-term H. pylori infection. TNF-α, produced mainly by activated macrophages, is a cytokine with pleiotropic function; editing injured cells, effete immune cells and repairing inflammatory damage.25 The role of TNF-α in experimental mice is not clear; results are inconsistent. Several studies reported no detectable difference in the grade or activity of gastritis in TNF receptor deficient mice compared with wild-type mice after H. pylori26,27 or H. felis28 infection. Our results agreed with those of these studies; the gastric mucosal TNF-α expression in H. felis-infected mice was not significantly different from that in control mice. This observation supports the results of our previous H. pylori study.22 Therefore, TNF-α does not appear to participate in the development of an inflammatory response caused by Helicobacter species. However, in their study on chronic H. pylori infection-induced gastritis using Mongolian gerbils, Sun et al.24 reported that the expression of TNF-α increased significantly at 6, 32 and 56 weeks after inoculation. Hasegawa et al.29 reported that the induction of gastritis by H. felis infection was clearly depressed in TNF-α knockout mice compared with C57BL/6 mice and suggested that TNF-α is critical for the induction of gastritis by H. felis infection. Further animal experiments are needed to clarity the role of TNF-α in the inflammatory responses of H. pylori infection.
In conclusion, H. felis well colonized the stomachs of C57BL/6 mice, and provoke severe inflammation, and eventually induced mucosal atrophy, metaplasia, dysplasia and cancer. IL-1β plays an important role in H. felis gastric inflammation; however, TNF-α is not involved in the inflammatory processes. Further trials are needed to clarify these inflammatory mechanisms.
Acknowledgments
This work was supported by a National Research Foundation (NRF) of Korea grant for the Global Core Research Center (GCRC) funded by the Korea government (MSIP) (No. 2011-0030001).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 3.Hayakawa Y, Fox JG, Gonda T, Worthley DL, Muthupalani S, Wang TC. Mouse models of gastric cancer. Cancers. 2013;5:92–130. doi: 10.3390/cancers5010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee A, Hazell SL, O’Rourke J, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–50. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai X, Carlson J, Stoicov C, Li H, Wang TC, Houghton J. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology. 2005;128:1937–52. doi: 10.1053/j.gastro.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 6.Fox JG, Sheppard BJ, Dangler CA, Whary MT, Ihrig M, Wang TC. Germ-line p53-targeted disruption inhibits Helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer Res. 2002;62:696–702. [PubMed] [Google Scholar]
- 7.Tomita T, Jackson AM, Hida N, Hayat M, Dixon MF, Shimoyama T, et al. Expression of Interleukin-18, a Th1 cytokine, in human gastric mucosa is increased in Helicobacter pylori infection. J Infect Dis. 2001;183:620–7. doi: 10.1086/318541. [DOI] [PubMed] [Google Scholar]
- 8.Genta RM. The immunobiology of Helicobacter pylori gastritis. Semin Gastrointest Dis. 1997;8:2–11. [PubMed] [Google Scholar]
- 9.Ellis MK, Zhao ZZ, Chen HG, Montgomery GW, Li YS, McManus DP. Analysis of the 5q31 33 locus shows an association between single nucleotide polymorphism variants in the IL-5 gene and symptomatic infection with the human blood fluke, Schistosoma japonicum. J Immunol. 2007;179:8366–71. doi: 10.4049/jimmunol.179.12.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis. 2000;59(suppl 1):i60–4. doi: 10.1136/ard.59.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubacek JA, Rothe G, Pit’ha J, Skodová Z, Stanĕk V, Poledne R, et al. C(−260)-->T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–20. doi: 10.1161/01.cir.99.25.3218. [DOI] [PubMed] [Google Scholar]
- 12.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna MT, Balish E. Inability of human clinical strains of Helicobacter pylori to colonize the alimentary tract of germfree rodents. Can J Microbiol. 1990;36:237–41. doi: 10.1139/m90-041. [DOI] [PubMed] [Google Scholar]
- 14.Rogers AB, Fox JG. Inflammation and Cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol. 2004;286:G361–6. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- 15.Sakagami T, Dixon M, O’Rourke J, Howlett R, Alderuccio F, Vella J, et al. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–48. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–89. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–97. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 18.Thompson LJ, Danon SJ, Wilson JE, O’Rourke JL, Salama NR, Falkow S, et al. Chronic Helicobacter pylori infection with Sydney strain 1 and a newly identified mouse-adapted strain (Sydney strain 2000) in C57BL/6 and BALB/c mice. Infect Immun. 2004;72:4668–79. doi: 10.1128/IAI.72.8.4668-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intra-epithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 20.Danon SJ, O’Rourke JL, Moss ND, Lee A. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995;108:1386–95. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- 21.Lee A, O’Rourke J. Gastric bacteria other than Helicobacter pylori. Gastroenterol Clin North Am. 1993;22:21–42. [PubMed] [Google Scholar]
- 22.Lee JY, Kim N, Nam RH, Choi YJ, Seo JH, Lee HS, et al. No correlation of inflammation with colonization of Helicobacter pylori in the stomach of mice fed high-salt diet. J Cancer Prev. 2014;19:144–51. doi: 10.15430/JCP.2014.19.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shigematsu Y, Niwa T, Rehnberg E, Toyoda T, Yoshida S, Mori A, et al. Interleukin-1beta induced by Helicobacter pylori infection enhances mouse gastric carcinogenesis. Cancer Lett. 2013;340:141–7. doi: 10.1016/j.canlet.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Sun YQ, Petersson F, Monstein HJ, Söderholm JD, Rehfeld JF, Borch K. Long-term morpho-functional development of Helicobacter pylori-induced gastritis in Mongolian gerbils. Scand J Gastroenterol. 2005;40:1157–67. doi: 10.1080/00365520510023378. [DOI] [PubMed] [Google Scholar]
- 25.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thalmaier U, Lehn N, Pfeffer K, Stolte M, Vieth M, Schneider-Brachert W. Role of tumor necrosis factor alpha in Helicobacter pylori gastritis in tumor necrosis factor receptor 1-deficient mice. Infect Immun. 2002;70:3149–55. doi: 10.1128/IAI.70.6.3149-3155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panthel K, Faller G, Haas R. Colonization of C57BL/6J and BALB/c wild-type and knockout mice with Helicobacter pylori: effect of vaccination and implications for innate and acquired immunity. Infect Immun. 2003;71:794–800. doi: 10.1128/IAI.71.2.794-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto T, Kita M, Ohno T, Iwakura Y, Sekikawa K, Imanishi J. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol Immunol. 2004;48:647–54. doi: 10.1111/j.1348-0421.2004.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa S, Nishikawa S, Miura T, Saito Y, Madarame H, Sekikawa K, et al. Tumor necrosis factor-alpha is required for gastritis induced by Helicobacter felis infection in mice. Microb Pathog. 2004;37:119–24. doi: 10.1016/j.micpath.2004.06.004. [DOI] [PubMed] [Google Scholar]