Abstract
Thrombin, which has the leading role in the blood coagulation cascade, is an important biomarker in hemostasis and cardiovascular disease (CVD) development. In this study, a measurement system capable of continuously monitoring individual thrombin generation using droplet microfluidic technology is manipulated. The thrombin generation assay based on fluogenic substrate is performed within the droplets and the thrombin generation curve of plasma sample activated by tissue factor is measured in real-time to reflect the sample conditions dynamically. The injection of the inhibitor of thrombin generation is developed to assay the inhibited curve which relates to thrombin self-inhibition in biological systems. This microfluidic system is integrated with the microdialysis probe, which is useful to connect to the living animals for future in vivo real time thrombin measurements for rapid CVD diagnosis.
I. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide. The majority of CVD patients suffer from blood coagulation problems that are not clinically identified by standard coagulation assays.1–3 Thrombin is the central enzyme in the blood coagulation cascade. Thrombin levels are closely correlated with the coagulation phenotype of an individual,4 which is affected by the combination of procoagulant and anticoagulant factors and is a reflection of the sum of developmental, environmental, genetic, nutritional, and pharmacological influences.5 In concert with platelet activation, thrombin cleaves fibrinogen to generate fibrin, the protein scaffolding of the clot, and converts factor fXIII into an active transglutaminase fXIIIa which cross-links to stabilize the noncovalently associated fibrin strands, as shown in supplementary Fig. S1.6 Recent technical advances have led to significant interest in the development of tools for measuring thrombin generation to detect rapid blood coagulation, particularly assays using fluorogenic substrates. However, most of these assays involve highly complex, costly, and time-consuming procedures7,8 so are not easy to be applied for rapid detections. Thus, the measurement of real-time blood coagulation for CVD diagnosis remains challenging.
Microfluidics permits miniaturization, integration, and reconfiguration and is a burgeoning technology for various applications in biological assays.9,10 Microfluidic devices have been used to facilitate the analysis of platelet function and coagulation biology in series for blood-related research and can be a useful tool for clinical CVD diagnostics.11 Several previous studies have demonstrated the possibility of combining microfluidics technology with thrombin detection assays. The measurement of clotting times for comparison with thrombin generation in whole blood or plasma samples in a small volume using a plug-based microfluidic system was investigated by Ismagilov et al.12 This concept was further developed by conducting a thrombin generation assay in a microfluidic device to produce a time-saving, accurate, easy-to-operate, and cost-efficient test.13 The advantages of microfluidic devices for different applications in CVD diagnosis include high throughput,14,15 high accuracy,16 a well-controlled experimental environment,17 the possibility of integration with other detection techniques,18 and the ability to mimic the real microenvironment of the bloodstream.19 A real-time assay using a nanomaterial sensor for bisulfide in microdialysis effluents was reported by Shaorong Liu et al.20 However, an integrated microfluidic system that enables not only a reduction in sample volume but also continuous, real-time, in vivo monitoring of thrombin molecular generation is still lacking for integration with clinical, personalized thrombin studies.
In this study, we describe the development and application of a continuous microfluidic system for performing real-time measurements of thrombin generation in various types of samples. The amount of thrombin generated was detected using a fluorogenic substrate in a well-mixed microdroplet carrier. The droplet-based microfluidic chip was also integrated with a microdialysis probe, enabling potential in vivo studies with live animals. The physiological sample was continuously tested using droplet encapsulation. The order of the droplets corresponded to a time series for the real-time measurement of biomarker activities. Compare with the conventional thrombin generation assay, the required volume of the plasma sample for the assay was significantly scaled down by using a microfluidic system. Accordingly thrombin generation could be measured in real time in human platelet-poor plasma and dialysis samples collected using a specific molecular weight cut-off from human platelet-poor plasma. In addition, an inhibitor of thrombin generation was injected, and normal and inhibited thrombin generation curves were assayed simultaneously.
II. EXPERIMENTAL METHODS
The fluorogenic thrombin substrate Z-Gly-Gly-Arg-AMC·HCl (I-1140) was obtained from Bachem, and a stock solution was prepared at a concentration of 100 mM in pure DMSO. Pure thrombin in powder form containing 1000 units (based on the information provided by the supplier, it was calculated that there was 1000/112 = 8.93 mg of thrombin in 1 ml) was purchased from Sigma (T8885). The thrombin powder was dissolved in 2.48 ml of thrombin activation buffer (50 mM Tris-Cl, pH 7.3, 140 mM NaCl) to prepare a thrombin solution at a stock concentration of 100 μM. Human platelet-poor plasma was obtained from Sigma (P2918). Innovin powder prepared from purified recombinant human tissue factor produced in E. coli and combined with synthetic phospholipids (thromboplastin), calcium, buffers, and stabilizers was purchased from Siemens. Because thromboplastin was present in the Siemens Innovin powder, no platelets were required in the plasma sample. To prepare the buffer solutions, 10 ml of DI water was added to the Innovin powder. GPRP peptide powder was purchased from Chinapeptides Co. Ltd. Human tissue factor was purchased from Sigma-Aldrich and was added to the vial at a concentration of 10 mg/ml to prepare the thrombin activation solution. The thrombin inhibitor Pefabloc SC (AEBSF; 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride) was purchased from Roche and was dissolved in DI water.
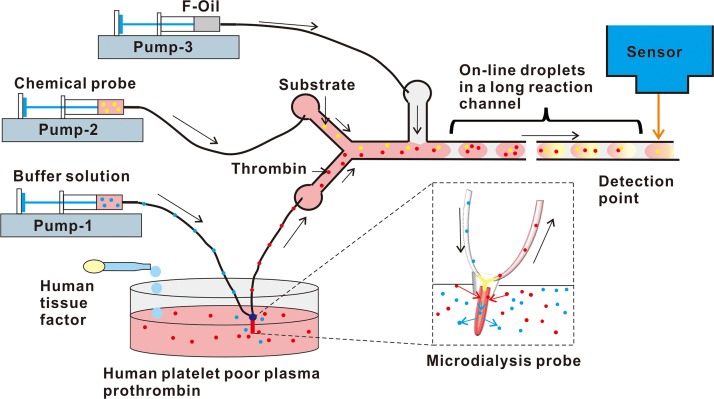
The microfluidic system for measuring thrombin generation in real time is shown in Fig. 1. The microfluidic chip was fabricated from polydimethylsiloxane (PDMS) using a standard soft lithography process. The PDMS slab was bonded to a glass slide by exposing it to air plasma for 15 s using a handheld corona treater (BD-25, Electro-Technic Products). The microfluidic network consisted of a mixing component, the droplet formation site, an exterior incubation tube, and the signal detection point. For continuous sampling for real-time measurements, one of the inlets of the microfluidic chip was connected to the sample generating thrombin, and the fluorogenic substrate was injected at another inlet. Aqueous phase microdroplets were formed, and the reagents mixed spontaneously in the droplets to initiate the reactions for thrombin sensing. For the thrombin generation assay, the injected reagent was the inhibitor of the generation pathway. The droplets formed at the T-junction were passed through the detection area. These droplets were aligned in the order of the time series and passed through an exterior incubation tube to achieve a longer, controlled incubation time (∼1 min) for signal read out. These droplets then passed through a CCD camera on-line to screen the thrombin activities in the solutions dynamically over time. The exposure time of the CCD camera is 50 ms to fit for the high throughput microdroplet screening. The short exposure time also ensures no observation delay for the real-time assay.
FIG. 1.
Design of the continuous microfluidic analysis system for real-time thrombin generation.
Depending on the stimulation of the prepared solutions, the thrombin generation level in the solution changed dynamically. To setup the microfluidic device, a microdialysis probe was placed into the solutions. Then, a physiological buffer medium was slowly pumped through the microdialysis probe. At the tip area of the probe, a membrane with a known cut-off size was used to allow the buffer medium to equilibrate with the surrounding solution. Molecules with a size smaller than the membrane cut-off value diffused from the solutions into the buffer medium in the probe tip. After fluidic exchange, the buffer medium containing molecules of the desired size from the solutions was collected at the outlet. The collected buffer medium was then injected into the microfluidic chip for continuous measurements of thrombin activity. The microdialysis probe and the microfludic chip was connected through an adapter fit for the diameter of the probe tube and the microfluidic chip. The flow rates of the whole system were adjusted for microdialysis process and for performing the droplet generations in a microfluidic chip. The exchange recovery rate between the buffer medium inside the probe and the outside bloodstream was determined by the cut-off size of the membrane and the flow rate of the buffer medium. By controlling these two parameters, biomarkers, such as thrombin, were selected from the fluid flow in the sample solutions based on their size.
HFE-7500 fluorocarbon oil (3M Novec™, Singapore) with 2% Krytox (modified with a PEG head) surfactant21,22 was used as the continuous phase. Syringes supplied by Terumo were connected to the microfluidic device with 0.38 mm (ID) medical grade polyethylene microtubing and were operated using Harvard Apparatus syringe pumps. The flow rates of the continuous (oil) phase were varied from 0.5 μl/min to 0.1 μl/min, whereas the flow rates for the reagent and fluorogenic substrate were maintained at 0.1 μl/min. The device was placed on a Nikon Ti-Eclipse fluorescent microscope, and an automated excitation light source, CoolLED, was connected to the optical set along with a multiple band pass filter to detect the signals in the droplets in real time over different periods. Images from the recorded movie were analyzed using the ImageJ software.
III. RESULT AND DISCUSSION
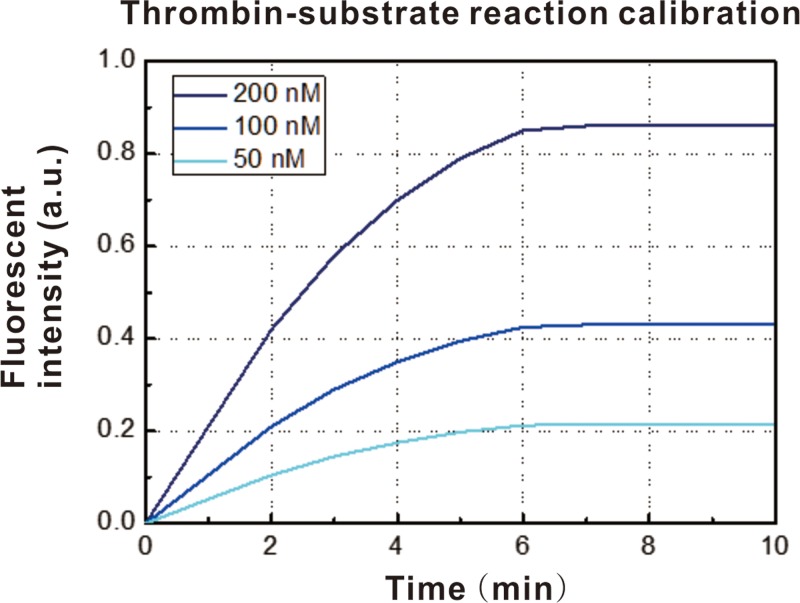
To continuously test the sample stream for real-time measurements of thrombin generation, a droplet-based microfluidic device was developed. The first step was to detect the performance of the fluorescent probe for thrombin in the microfluidic system. One of the inlets was connected to a sample solution of pure thrombin in thrombin activation buffer at a final concentration of 100 nM. The other inlet was connected to a solution of the thrombin fluorogenic substrate I-1140 at a concentration of 50 nM, 100 nM, or 200 nM. The two aqueous phases were mixed in the microchannel, and the droplets formed were passed through a reaction channel outside of the microchip for ∼1 min to permit fluorescence signal generation. Finally, the droplets passed the detection point, and the fluorescent signal was captured by a high-speed CCD camera, as shown in Fig. 2. The thrombin molecules that were generated cleaved the fluorogenic substrate, resulting in an increase in the fluorescent intensity corresponding to thrombin activity. The intensity curves obtained at the three different concentrations of the fluorogenic substrate exhibited a similar profile, which could be divided into two regions. When the thrombin molecules reacted with the substrate, the fluorescence intensity increased, which corresponded to the increasing region of the plot. Once the substrate was consumed by the generated thrombin, the fluorescent intensity plateaued and did not change with time, corresponding to the saturated region of the plot. In our experiment, the real-time measurement of the thrombin generation process was dependent on the time lapse used. Thus, the incubation time of the microdroplet carrier was fixed at 1 min to ensure the accuracy of the measurements, based on the results of the calibration of the reaction between thrombin and the substrate.
FIG. 2.
Measurement of fluorescent intensity of the pure thrombin sample mixed with fluorescent probe.
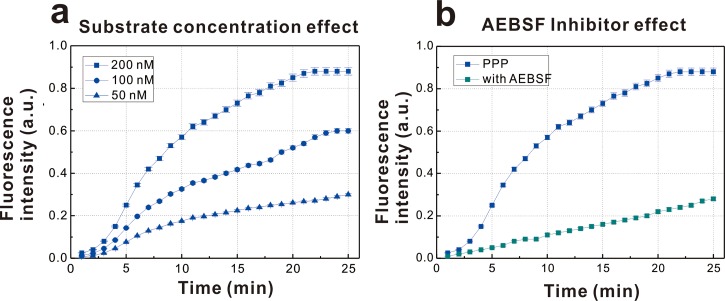
Thrombin generation in human platelet-poor plasma was detected using the same microfluidic system. A reaction pool containing a human plasma sample was mixed with the Siemens Innovin solution, which was prepared from purified recombinant human tissue factor produced in E. coli. Concomitantly with platelet activation, thrombin cleaves fibrinogen to generate fibrin, the protein scaffold for clot formation, and converts factor XIII into the active form of transglutaminase XIIIa, which cross-links and stabilizes the noncovalently associated fibrin strands.23 In addition, in association with thrombomodulin, thrombin can be suppressed by thrombin activatable fibrinolysis inhibitor, which delays plasmin-mediated dissolution of the fibrin clot.24,25 After the addition of the Innovin solution, GPRP peptide solution was added; GPRP slows clot formation without affecting thrombin generation.26 Then, the activated plasma sample was mixed with different concentrations of the thrombin fluorogenic substrate I-1140 in the water-in-oil droplets. The droplets were passed through a long reaction channel for ∼5 min outside the microchip to permit fluorescence generation by the enzymatic reactions. Finally, the droplets passed through the detection chip, and the fluorescent signal was captured by a high-speed CCD camera. The fluorescence detected represented thrombin generation induced by human tissue factor using three different fluorogenic substrate concentrations, as shown in Fig. 3(a). The fluorescent intensity increased over time, and the thrombin generation curve was more stable at higher fluorogenic substrate concentrations. The thrombin generation curve for 200 nM substrate exhibited a lag phase, a generation phase, and an inactivation phase. To study the self-inhibition of thrombin generation, inhibitor analysis was conducted using the microfluidic system. The volume of inhibitor injected was ∼30% of the volume of the droplets. The inhibitor AEBSF was mixed at a concentration of 100 nM with a microdroplet containing plasma sample with GPRP activated by human tissue factor and the fluorogenic substrate. The inhibition of thrombin generation by AEBSF is shown in Fig. 3(b). The rate and level of thrombin generation were significantly lower than those of the whole plasma sample. AEBSF is a water-soluble, irreversible serine protease inhibitor that irreversibly inactivates thrombin and other serine proteases. Its sulfonyl functional group binds to the active center of the thrombin molecule, inhibiting cleavage of the fluorogenic substrate and resulting in smaller increases in fluorescence intensity.
FIG. 3.
(a) Measurement of thrombin generation curve of human platelet poor plasma activated by human tissue factor and (b) the inhibition of AEBSF to the thrombin generation curve of human platelet poor plasma activated by human tissue factor.
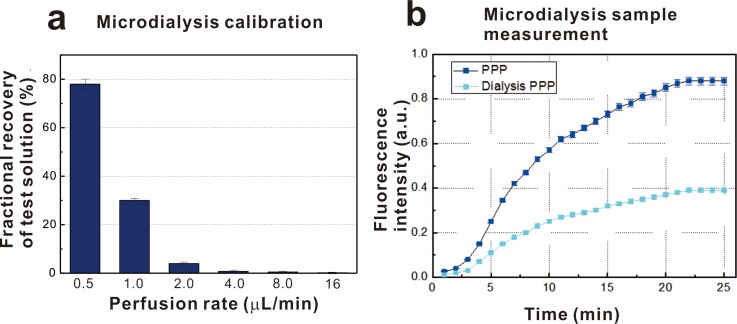
A microdialysis probe was implanted to enable the analysis of physiological sample solutions. The exchange recovery rate between the buffer medium inside the probe and the outside bloodstream was determined by the cut-off of the membrane and the flow rate of the buffer medium, as shown in Fig. 4(a). By controlling these two parameters, biomolecules of specific sizes can be selected from the bloods. A 20 kDa fluorescently labelled dextran-FITC was used as a model biomolecule, and a high recovery rate and reproducibility were demonstrated at flow rates ranging from 0.5 to 16 μl/min. The dextran-FITC recovery was measured; and the concentration of dextran-FITC was determined by the perfusion rate. The molecular weight of active thrombin is 36 kDa. Thus, a probe with a larger molecular weight cut-off was used to sample thrombin using the current dialysis setup. Real-time thrombin measurements were achieved by integrating the continuous microfluidic chip and the dialysis probe. A dialysis probe with a cut-off of 1000 kD was used to separate the prothrombin molecule from the human plasma sample. The sample was dialyzed for 12 h, and the collected sample was mixed with the GPRP peptide and human tissue factor responsible for thrombin generation in vivo. The results for thrombin generation are shown in Fig. 4(b). The speed of thrombin generation was much lower than was observed for the plasma sample. Thus, the growing tendency was justified. The level of loss during the dialysis process was observed to be approximately 50%. This integrated microfluidic system could be further developed to detect real-time thrombin signals by implanting the microdialysis probe in a blood vessel.
FIG. 4.
(a) Dextran-FITC concentration measurement by fluorescence intensity of different perfusion rate and (b) Measurement of thrombin generation of sample collected through microdialysis probe.
IV. CONCLUSIONS
In this paper, a microfluidic system that enables continuous sampling of thrombin generation was developed and integrated with a microdialysis probe for potential implantation in the bloodstream of live animals. The microfluidic chip used enabled microdroplet formation and the subsequent measurement of a fluorescent signal. A fluorogenic substrate for thrombin was used, and a thrombin generation assay based on microdroplet fluorescent signal measurement was performed. The performance of the microdialysis probe for pro-thrombin molecule dialysis was determined, and the thrombin generation curve of a sample was measured and found to be comparable with that of the original sample, although with a lower intensity of fluorescence. The continuous microfluidic system ensures real-time detection of thrombin generation with high throughput, accuracy, and flexibility. The system could be further integrated with multiple molecule detection systems based on a picoinjector array or used for in vivo studies by implanting the microdialysis probe into an animal. Various biomarkers, such as thrombin can be measured using this system, and we expect that the proposed microfluidic system will enable the elucidation of individual and variable patient pharmacokinetics and true personalized CVD diagnosis and drug dosing for individual patients to support optimal clinical therapy.
ACKNOWLEDGMENTS
We gratefully acknowledge the funding provided by the NUS start-up Grant No. R-397-000-137-133, the Singapore MIT Alliance for Research and Technology (SMART) research Grant No. R-397-000-146-592, the National Medical Research Council clinician scientist funding scheme (NMRC/CSA/035/2012), and the facilities provided by Singapore Institute for Neurotechnology (SINAPSE).
References
- 1.Brummel-Ziedins K. E., J. Thromb. Haemostasis 11(1), 212 (2013). 10.1111/jth.12256 [DOI] [PubMed] [Google Scholar]
- 2.Raskob G. E., Silverstein R., Bratzler D. W., Heit J. A., and White R. H., Am. J. Prev. Med. 38(4), S502 (2010) 10.1016/j.amepre.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 3.Brummel-Ziedins K. E., Orfeo T., Rosendaal F. R., Undas A., Rivard G. E., Butenas S., and Mann K. G., J. Thromb. Haemostasis 7(1), 181 (2009). 10.1111/j.1538-7836.2009.03426.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Veen J. J., Gatt A., and Makris M., Br. J. Haematol. 142(6), 889 (2008). 10.1111/j.1365-2141.2008.07267.x [DOI] [PubMed] [Google Scholar]
- 5.Brummel-Ziedins K. E., Everse S. J., Mann K. G., and Orfeo T., J. Thromb. Thrombolysis 37(1), 32 (2014) 10.1007/s11239-013-1006-9 [DOI] [PubMed] [Google Scholar]
- 6.See supplementary material at http://dx.doi.org/10.1063/1.4894747E-BIOMGB-8-012492 for thrombin generation pathway and regulation factors.
- 7.Morita T., Kato H., Iwanaga S., Takada K., and Kimura T., J. Biochem. 82(5), 1495 (1977). [DOI] [PubMed] [Google Scholar]
- 8.van Berkel S. S., van der Lee B., van Delft F. L., Wagenvoord R., Hemker H. C., and Rutjes F. P., ChemMedChem 7(4), 606 (2012). 10.1002/cmdc.201100560 [DOI] [PubMed] [Google Scholar]
- 9.Chen C. H., Miller M. A., Sarkar A., Beste M. T., Isaacson K. B., Lauffenburger D. A., Griffith L. G., and Han J., J. Am. Chem. Soc. 135(5), 1645 (2013). 10.1021/ja307866z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C. H., Sarkar A., Song Y., Miller M. A., Kim S. J., Griffith L. G., Lauffenburer D. A., and Han J., J. Am. Chem. Soc. 133(27), 10368 (2011). 10.1021/ja2036628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colace T. V., Tormoen G. W., McCarty O. J. T., and Diamond S. L., Annu. Rev. Biomed. Eng. 15, 283 (2013). 10.1146/annurev-bioeng-071812-152406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H., Li H. W., Munson M. S., Van Ha T. G., and Ismagilov R. F., Anal. Chem. 78(14), 4839 (2006). 10.1021/ac0601718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch K., van Berkel S. S., van de Wal M. M. E. B., Nieuwland P. J., van Hest J. C. M., and Rutjes F. P. J. T., J. Appl. Phys. 105, 102012 (2009). 10.1063/1.3116634 [DOI] [Google Scholar]
- 14.Wang H., Liu Y., Liu C. C., Huang J. Y., Yang P. Y., and Liu B. H., Electrochem. Commun. 12, 258 (2010). 10.1016/j.elecom.2009.12.008 [DOI] [Google Scholar]
- 15.Chen F., Wang Z., Li P., Lian H. Z., and Chen H. Y., Electrophoresis 34(24), 4839 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Welsh J. D., Colace T. V., Muthard R. W., Stalker T. J., Brass L. F., and Diamond S. L., J. Thromb. Haemostasis 10(11), 2344 (2012). 10.1111/j.1538-7836.2012.04928.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos S. Meyer Dos, Zorn A., Guttenberg Z., Picard-Willems B., Kläffling C., Nelson K., Klinkhardt U., and Harder S., Biomicrofluidics 7(5), 56502 (2013). 10.1063/1.4824043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheldof D., Hardij J., Cecchet F., Chatelain B., Dogné J. M., and Mullier F., J. Extracell. Vesicles 18(2), 19728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onasoga-Jarvis A. A., Puls T. J., O'Brien S. K., Kuang L., Liang H. J., and Neeves K. B., J. Thromb. Haemostasis 12(3), 373(2014). 10.1111/jth.12491 [DOI] [PubMed] [Google Scholar]
- 20.Zhu X. C., Xu L., Wu T. B., Xu A. Q., Zhao M. P., and Liu S. R., Biosens. Bioelectron. 15(55), 438 (2014) 10.1016/j.bios.2013.12.056 [DOI] [PubMed] [Google Scholar]
- 21.Ramji R., Wang M., Bhagat A. A. S., Weng D. T. S., Thakor N. V., Lim C. T., and Chen C. H., Biomicrofluidics 8, 034104 (2014). 10.1063/1.4878635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo R. C., Ranjan S., Zhang Y., and Chen C. H., Chem. Commun. 49, 7887–7889 (2013). 10.1039/c3cc44111c [DOI] [PubMed] [Google Scholar]
- 23.Coughlin S. R., Proc. Natl. Acad. Sci. U.S.A. 96(20), 11023 (1999). 10.1073/pnas.96.20.11023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahlan A. and Ahluwalia J., Arch. Pathol. Lab. Med. 138(2), 278 (2014). 10.5858/arpa.2012-0639-RS [DOI] [PubMed] [Google Scholar]
- 25.Carlson M. A., Calcaterra J., Johanning J. M., Pipinos I. I., Cordes C. M., and Velander W. H., J. Surg. Res. 187(1), 334 (2014). 10.1016/j.jss.2013.09.039 [DOI] [PubMed] [Google Scholar]
- 26.Michelson A. D., Blood Coagulation Fibrinolysis 5(1), 121 (1994). 10.1097/00001721-199402000-00014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4894747E-BIOMGB-8-012492 for thrombin generation pathway and regulation factors.