Abstract
Background
Whether screening mammography programs should include women in their 40s is controversial. In Canada, screening of women aged 40–49 years has not been shown to reduce mortality from breast cancer. Given that screening mammography reduces mean tumour size and that tumour size is inversely associated with survival, the lack of benefit seen with screening is puzzling and suggests a possible adverse effect on mortality of mammography or subsequent treatment (or both) that counterbalances the expected benefit derived from downstaging.
Methods
We followed 50,436 women 40–49 years of age until age 60 for mortality from breast cancer. Of those women, one half had been randomly assigned to annual mammography and one half to no mammography. The impact of mammography on breast cancer mortality was estimated using a left-censored Cox proportional hazards model.
Results
Of 256 deaths from breast cancer recorded in the study cohort, 134 occurred in women allocated to mammography, and 122 occurred in those receiving usual care and not allocated to mammography. The cumulative risk of death from breast cancer to age 60 was 0.53% for women assigned to mammography and 0.48% for women not so assigned. The hazard ratio for breast cancer–specific death associated with 1 or more screening mammograms before age 50 was 1.10 (95% confidence interval: 0.86 to 1.40).
Conclusions
Mammography in women 40–49 years of age is associated with a small but nonsignificant increase in the risk of dying of breast cancer before age 60. Caution should be exercised when recommending mammographic screening to women before age 50.
Keywords: Breast cancer, screening, mammography, mortality
1. INTRODUCTION
Whether screening mammography programs should include women as young as 40 is controversial. Guidelines vary, but in 2009, the U.S. Preventive Services Task Force recommended against routine mammograms for women in their 40s 1. Recently, guidelines in British Columbia changed to reverse the prior position that mammography be offered annually to all women between the ages of 40 and 50 2. In Switzerland, it has been proposed that routine screening mammography be discontinued 3. The motivating factor was a lack of clear evidence that screening young women results in a decrease in their mortality from breast cancer. Secondary factors related to the expense (namely, the cost of saving a single life) and the potential harms (anxiety, false-positive results, negative biopsies, and overdiagnosis) 4. Our group has reported that up to 50% of nonpalpable mammographically-detected invasive breast cancers represent examples of overdiagnosis 3.
In theory, there are several possible reasons why screening mammography might be harmful in terms of breast cancer mortality, including the effect of radiation on cancer initiation and progression, and the possible adverse effects of treatment on the natural history of breast cancer. Retsky and colleagues argued that screening, followed by the active treatment of otherwise indolent breast cancers, has the potential to reactivate dormant metastases and to accelerate their growth 4. In their model, a cancer that has spread beyond the breast at the time of diagnosis (albeit microscopically) cannot be managed entirely by local therapy, and cancer cells that remain after surgery have the potential to disappear (through chemotherapy or regression) or to grow (through resistance to therapy and natural growth). There is relatively little evidence to support that hypothesis, but if advancing the date of breast cancer diagnosis were to have an adverse impact on survival by perturbing the putative balance, that impact should be apparent in a randomized trial of screening.
In evaluating the impact of mammography on cancer mortality, two design issues are paramount. First, is mammography assigned randomly? If not, women with a high a priori risk of breast cancer might be more diligent than women at average or low risk in seeking screening. Second, is the comparison considering mortality rates? Shifts in stage at diagnosis? Or survival after diagnosis?
In the 25-year follow-up to the Canadian National Breast Screening Study, we recently reported that mammography can result in downstaging of tumour size and nodal status, with a commensurate improvement in 10-year survival, and yet have no effect on mortality 3. Reasons include lead-time bias and overdiagnosis.
Traditionally, to evaluate data from a randomized screening trial, women in exposed and unexposed cohorts are followed from the time of randomization until death from cancer or the end of the follow-up period. When that conventional analytic approach is used in the context of a 5-year screening period, women 40–49 years of age at study entry could potentially be 40–53 years of age at the time of diagnosis, and (given a 25-year follow-up period) could potentially be 40–74 years of age at the time of death from breast cancer. The women under follow-up are thus synchronized for date from study initiation, but not for specific age at first screening. That approach is robust for evaluating the global benefit of screening per se, but might not be ideal for determining whether initiation of screening in women in a particular age group has a beneficial or a detrimental impact on early death from breast cancer. Obtaining an estimate of the absolute risk of dying from breast cancer by age 60 in Canadian women with and without prior exposure to mammography is also of interest.
In the present analysis of the National Breast Screening Study dataset, we estimated the effect on death from breast cancer before age 60 of a woman’s undergoing screening mammography one or more times before age 50. In particular, we were interested in the possibility that the number of deaths from breast cancer transiently increases in the immediate aftermath of entering a screening program.
2. METHODS
In 1980, a randomized controlled trial of screening mammography and breast physical examination was initiated in 89,835 women 40–59 years of age in Canada 3,5–8. Participants were recruited to the study through a general publicity campaign and a review of population lists, with personal invitation by letter, group mailings, and invitation by treating physicians. The present analysis focuses on women who were 40–49 years of age at study entry. Women were eligible for the randomized trial if they were between the ages of 40 and 49, had not undergone mammography in the preceding 12 months, had no history of breast cancer, and were not pregnant. Participants signed an informed consent form approved by the University of Toronto’s Research Ethics Board.
Fifteen screening centres were located in six Canadian provinces. Before randomization, women underwent a physical breast examination and were randomly assigned to receive annual mammography and physical examination, or usual care in the community. Women with abnormal findings either on physical examination or on mammography were referred to a special review clinic directed by the surgeon affiliated with the study centre. If necessary, diagnostic mammography was performed. If further diagnostic investigation was recommended, the woman was referred to a specialist chosen by her family physician. Cancer treatment was arranged through the patient’s physician and was not influenced by the study team. Follow-up was conducted actively until June 1996 and then passively through record linkage until December 31, 2005. All dates of breast cancer diagnoses and all dates of death from breast cancer were ascertained by linkage with the Canadian Cancer Registry and the Canadian Vital Statistics Death Database, maintained by Statistics Canada in Ottawa. Reports on all deaths were provided to the study investigators with the certified underlying cause of death.
2.1. Analysis
The present analysis was performed on the cohort of 50,436 women who were followed from age 40 until age 60. They were censored at death from breast cancer or from another cause. All subjects had reached their 60th birthday before completion of follow-up in December 2005. Because study subjects entered the study at or after their 40th birthday, a left-censored survival analysis was conducted, whereby survival curves were constructed from age 40 using summary data from individual subjects who were left-censored to their age at study entry.
The primary outcome was death from invasive breast cancer before age 60. The hazard ratio for death associated with mammography was calculated by considering mammography exposure as a time-dependent covariate—that is, the exposure status changed from unexposed to exposed at the time of the first mammogram. Given that mammography status was not dictated by the patient or her physician (that is, it was assigned in the context of a randomized trial), covariates were equally distributed in the groups with respect to mammography (Table i); we therefore did not adjust for other predictors in the model. A p value of 0.05 was used as the cut-off for statistical significance.
TABLE I.
Characteristics of the women who were and were not allocated to screening mammography
| Variable |
Prior mammogram?
|
p Value | |
|---|---|---|---|
| No | Yes | ||
| Participants | 25,216 | 25,220 | — |
| Mean year of birth | 1938.5 | 1938.5 | 0.76 |
| Mean age (years) | |||
| At entry | 44.74 | 44.73 | 0.63 |
| At menarche | 12.8 | 12.8 | 0.32 |
| At first birth | 24.0 | 24.0 | 0.89 |
| Mean parity | 2.33 | 2.32 | 0.50 |
| Use oral contraceptives [ever (%)] | 71.1 | 70.7 | 0.25 |
3. RESULTS
Mean age at study entry in the study cohort was 44.7 years (range: 40–49 years), and mean duration of follow-up was 15.2 years. Of the 50,436 subjects in the cohort, 25,216 (50.0%) were exposed to one or more mammograms during the study period (that is, they were assigned to the mammography group). For women who underwent mammography, the mean number of mammograms was 4.2.
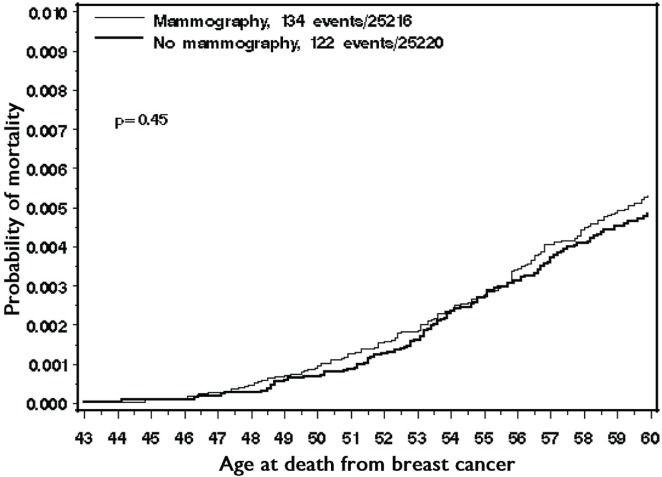
In the cohort, 1424 women died before age 60, with 256 of the deaths (18.0%) being attributable to breast cancer. Among the women who died of breast cancer, 52.3% had been assigned to screening mammography. Among the women who died of another cause, 50.9% had been assigned to screening mammography. The overall cumulative incidence of death from breast cancer to age 60 was 0.50%. The cumulative incidence of death from breast cancer was 0.48% for women who had never undergone mammography and 0.53% for women who had undergone mammography (Figure 1). The hazard ratio for death from breast cancer, given the use of screening mammography, was 1.10 (95% confidence interval: 0.86 to 1.40; p = 0.45).
FIGURE 1.
Cumulative incidence of death from breast cancer to age 60, by mammography group.
In the cohort, 1878 women were diagnosed with breast cancer before age 60 (including cancers diagnosed during the 4-year study period and cancers diagnosed between the end of the study period and age 60). Mean age at diagnosis was 52.7 years in the 982 women who were assigned to screening mammography and 53.1 years in the 896 women who were not assigned to screening mammography (p = 0.11). The average time from study entry to diagnosis of breast cancer was 8.4 years for the women who were assigned to screening mammography and 8.8 years for the women who were not so assigned. The average time from diagnosis to death for the women who died of breast cancer was 4.5 years among those assigned to mammography and 4.6 years among those not assigned to mammography.
Table ii presents the distribution, by age of death, of women with one or more prior mammograms. Table iii presents the distribution, by time since study entry, of deaths from breast cancer among women with one or more prior mammograms. These data do not suggest that mammography might be associated with an increase in deaths at an early age or in deaths occurring shortly after diagnosis.
TABLE II.
Proportion of patients with a prior mammogram who died of breast cancer, by age at death
| Age at death (years) | Deaths (n) |
Prior mammogram
|
|
|---|---|---|---|
| (n) | (%) | ||
| 40 | 0 | 0 | — |
| 41 | 0 | 0 | — |
| 42 | 0 | 0 | — |
| 43 | 2 | 1 | 50 |
| 44 | 2 | 1 | 50 |
| 45 | 1 | 1 | 100 |
| 46 | 7 | 4 | 57.1 |
| 47 | 7 | 5 | 71.4 |
| 48 | 12 | 5 | 41.7 |
| 49 | 9 | 6 | 66.7 |
| 50 | 14 | 9 | 64.3 |
| 51 | 17 | 7 | 42.2 |
| 52 | 16 | 7 | 43.8 |
| 53 | 30 | 13 | 43.3 |
| 54 | 20 | 10 | 50 |
| 55 | 28 | 17 | 60.7 |
| 56 | 28 | 16 | 57.1 |
| 57 | 22 | 10 | 45.5 |
| 58 | 22 | 11 | 50 |
| 59 | 19 | 11 | 57.9 |
TABLE III.
Proportion of patients with a prior mammogram who died of breast cancer before age 60, by years elapsed since study entry
| Time since study entry (years) | Deaths (n) |
Prior mammogram
|
|
|---|---|---|---|
| (n) | (%) | ||
| 0–1 | 0 | 0 | — |
| 1–2 | 0 | 0 | — |
| 2–3 | 6 | 4 | 66.7 |
| 3–4 | 10 | 5 | 50.0 |
| 4–5 | 16 | 9 | 56.3 |
| 5–6 | 11 | 9 | 81.8 |
| 6–7 | 16 | 8 | 50.0 |
| 7–8 | 22 | 13 | 59.1 |
| 8–9 | 19 | 9 | 47.4 |
| 9–10 | 22 | 13 | 59.1 |
| 10–11 | 26 | 13 | 50.0 |
| 11–12 | 25 | 7 | 28.0 |
| 12–13 | 18 | 5 | 27.8 |
| 13–14 | 22 | 12 | 54.6 |
| 14–15 | 15 | 9 | 60.0 |
| 15–16 | 10 | 8 | 80.0 |
| 16–17 | 9 | 7 | 77.8 |
| 17–18 | 7 | 2 | 28.6 |
| 18–19 | 1 | 0 | 0 |
| 19–20 | 1 | 1 | 100 |
4. DISCUSSION
In the National Breast Screening Study, no reduction in breast cancer mortality was seen with assignment to mammography or to usual care 3. It is not clear why a mortality reduction failed to occur (an observation that is extensively discussed within the primary report). A small and nonsignificant excess of breast cancer deaths before age 60 was found among women assigned at random to receive mammography between the ages of 40 and 49. A similar excess in early breast cancer mortality was seen for women 40–49 years of age at initial assignment in the Swedish two-county study 9. After a mean of 7 years, the hazard ratio for death associated with mammography in this young group was 1.26 (95% confidence interval: 0.56 to 2.84) 9. In the hip trial 10, a small and nonsignificant excess of breast cancer deaths was also associated with mammography in the first 9 years among women 40–49 years of age at diagnosis (30 deaths vs. 27 deaths). On average, mammography advanced the diagnosis of breast cancer by 4 months in that study.
Retsky and colleagues from the Demicheli group have proposed that advancing the diagnosis of breast cancer might be deleterious in a proportion of cases because, during the course of diagnosis and subsequent treatment, a latent cancer could be reactivated and induced to spread 4. Their argument is based on the reproducible observation that the annual breast cancer mortality rate peaks at about 2–3 years after diagnosis. Furthermore, if overdiagnosis is accepted to be a common phenomenon 11 (that is, a number of breast cancers are dormant or will regress), then it is prudent to ask if internal or external factors might be influencing growth.
In the present study, the probability of dying of breast cancer before age 60 was approximately equal for women who did and did not initiate screening mammography in their 40s. The number of deaths from breast cancer was 134 for women who underwent mammography and 122 for women who did not. Our study used data from a randomized trial that set out to evaluate the potential harmful effect of mammography on breast cancer mortality. Approaching the study question using another design—for example, observational study—is difficult. Historical comparisons are fraught with difficulties relating to temporal trends in treatment and mortality rates, and outside of clinical trials, mammography is not performed randomly with respect to cancer risk.
All the women in the study who were assigned to screening mammography received one or more screening mammograms (mean number: 4.2). For the purposes of the present study, we assumed that none of the women assigned to the control arm underwent mammography before age 50; however, it is possible that some of those women underwent mammography off-study before the age of 50—in particular, after the study period was over. Crossover might, to some extent, have reduced the effect of mammography on the true mortality difference; however, we expect that the size of any such effect would be small (and in the event that the first mammogram occurred after age 50, the results of the central analysis would not be affected).
5. CONCLUSIONS
Our data do not support the hypothesis of Retsky et al. that screening can be harmful 4; however, we cannot rule out a small deleterious effect of mammography on subsequent breast cancer mortality. It is also possible that countervailing influences are at work—that is, some lives will be saved through early detection, but they will be balanced by a similar number of iatrogenic deaths attributable to screening and treatment.
6. ACKNOWLEDGMENTS
This study was supported by funding from the Canadian Breast Cancer Research Alliance, the Canadian Breast Cancer Initiative, the Canadian Cancer Society, Health Canada, and the National Cancer Institute of Canada.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 2.BC Cancer Agency (bcca) Vancouver, BC: BCCA; Home > Breast > For Health Professionals > Breast Screening Policy [Web page] n.d. [Available at: http://www.screeningbc.ca/Breast/ForHealthProfessionals/BreastScreeningPolicy.htm; cited January 21, 2014] [Google Scholar]
- 3.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retsky M, Demicheli R, Hrushesky W, Baum M, Gukas I. Surgery triggers outgrowth of latent distant disease in breast cancer: an inconvenient truth? Cancers (Basel) 2010;2:305–37. doi: 10.3390/cancers2020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ. 1992;147:1459–76. [Erratum in: CMAJ 1993;148:718] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ. 1992;147:1477–88. [Erratum in: CMAJ 1993;148:718] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women age 50–59 years. J Natl Cancer Inst. 2000;92:1490–9. doi: 10.1093/jnci/92.18.1490. [DOI] [PubMed] [Google Scholar]
- 8.Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med. 2002;137:305–12. doi: 10.7326/0003-4819-137-5_Part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tabár L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829–32. doi: 10.1016/S0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro S. Evidence on screening for breast cancer from a randomized trial. Cancer. 1977;39(suppl):2772–82. doi: 10.1002/1097-0142(197706)39:6<2772::AID-CNCR2820390665>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Narod SA. Disappearing breast cancers [Countercurrents series] Curr Oncol. 2012;19:59–60. doi: 10.3747/co.19.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]