Abstract
The use of all-trans-retinoic acid (atra) and anthracyclines (with or without cytarabine) in the treatment of acute promyelocytic leukemia (apl) has dramatically changed the management and outcome of the disease over the past few decades. The addition of arsenic trioxide (ato) in the relapsed setting—and, more recently, in reduced-chemotherapy or chemotherapy-free approaches in the first-line setting—continues to improve treatment outcomes by reducing some of the toxicities associated with anthracycline-based approaches. Despite those successes, a high rate of early death from complications of coagulopathy remains the primary cause of treatment failure before treatment begins. In addition to that pressing issue, clarity is needed about the use of ato in the first-line setting and the role of hematopoietic stem-cell transplantation (hsct) in the relapsed setting. The aim for the present consensus was to provide guidance to health care professionals about strategies to reduce the early death rate, information on the indications for hsct and on the use of ato in induction and consolidation in low-to-intermediate–risk and high-risk apl patients.
Keywords: Acute promyelocytic leukemia, apl, management, supportive care, prophylaxis, infusions, arsenic trioxide, ato, Trisenox, first-line treatment, transplantation, allogeneic transplantation, autologous transplantation
1. INTRODUCTION
Acute promyelocytic leukemia (apl) is a relatively rare form of leukemia, accounting for about 10%– 15% of adults diagnosed with acute myeloid leukemia in the United States each year1. Cells from 92% of patients diagnosed with apl are characterized by a balanced reciprocal translocation between chromosomes 15 and 17, resulting in a fusion of the PML (promyelocytic leukemia) and RARA (retinoic acid receptor alpha) genes2. In most of the remaining apl cases, the variant translocations are characterized by fusions of RARA to other genes. Cases in which RARA translocations are lacking occur very rarely.
The PML–RARA translocation is not only a distinguishing feature of apl, it also drives the disease and is a key target for successful treatment. The presence of pml–rar-α leads to a differentiation block at a retinoic acid–dependent stage of myeloid differentiation and also to increased self-renewal of cells at an earlier stage in differentiation. However, those defects can specifically be overcome by pharmacologic amounts of the retinoid all-trans retinoic acid (atra) and by arsenic trioxide (ato), either alone or in combination3.
Another distinguishing feature of apl is prominent coagulopathy, which is often found at presentation or shortly thereafter. Coagulopathy is associated with a high risk of hemorrhagic death, or thrombosis, or both1. Without early initiation of treatment, apl can be rapidly lethal. Even if definitive therapy is started early, mortality from hemorrhage (and thrombosis) occurs at a high rate during the first few days after presentation.
Since the recognition of apl as a distinct disease in 1957, several key developments have changed its prognosis from highly fatal to very curable4. Those developments include the discovery of the apl cell’s heightened response to atra, to anthracyclines (with or without cytarabine) and to ato, the adoption of risk-adapted treatments, and the recognition that immediate aggressive supportive care is required to reduce the incidence of early death from the complications of coagulopathy and thrombosis3,5. The use of atra in combination with anthracyclines (with or without cytarabine) as first-line treatment has made it possible to attain complete remission rates exceeding 90% and cure rates close to 80%3. In patients with low- and intermediate-risk disease, further improvements in outcome have recently been described with the use of non-chemotherapeutic approaches in which the atra–ato combination has resulted in a lower frequency of hematologic toxicities while still maintaining the high and durable response rates seen with conventional chemotherapy-based regimens6. Similar improvements in outcome have also been observed using reduced-chemotherapy ato-containing approaches in high-risk apl7.
Despite those successes, several unmet needs in apl treatment remain. The most significant challenge continues to be the high incidence of early death. In addition, although chemotherapy-based approaches have led to excellent response rates, they are associated with significant early hematologic toxicities and also with delayed long-term adverse effects, including cardiomyopathy and secondary malignancies such as therapy-related myelodysplasia and acute myeloid leukemia. In addition, for the small number of patients with relapsed apl, uncertainty remains about the best treatment options, especially the roles of autologous or allogeneic hematopoietic stem-cell transplantation (hsct) in that setting. Finally, there is a need to improve outcomes in older patients, who might not be able to tolerate regimens based on conventional chemotherapy.
2. PURPOSE
The present review provides health care professionals with guidance about strategies to reduce the early death rate and information concerning the indications for hsct and the use of ato in induction and consolidation therapies for low-to-intermediate–risk and high-risk apl patients. The recommendations presented here were developed by a panel of apl experts from across Canada and focus on the treatment and management of adult patients with newly diagnosed and relapsed apl with the classical t(15;17) translocation.
3. METHODS
3.1. Identification and Selection of Studies
A systematic search of published papers in medline and of abstracts submitted to the annual meetings of the American Society of Hematology, the American Society of Clinical Oncology, and the European Hematology Association used these search terms: apl management, apl supportive, apl prophylaxis, apl infusion, ato first-line treatment, arsenic trioxide apl, Trisenox apl, transplant, apl transplant, apl allogeneic, and apl autologous. Publications were excluded if they were published before January 2009, if they included treatment with ato as a single agent in induction and consolidation, or if they examined the role of ato in maintenance.
3.2. Formulation of Recommendations
After review and discussion of the evidence, the panel formulated recommendations with the aim of updating recommendations published in 2009 by the European LeukemiaNet (eln)8. The panel considered both the level of evidence and the quality of the studies.
4. RECOMMENDATIONS
4.1. Question 1
How should patients with suspected apl be managed at first presentation?
Background:
The high incidence of early death before and during induction treatment remains the most significant cause of treatment failure in apl9,10. Despite the use of atra therapy for almost two decades, early mortality has been reduced only modestly, as recently noted in a 2011 population-based study of the Surveillance, Epidemiology, and End Results database11. Contrary to reported incidence rates of early death from cooperative clinical trials (which are in the 5%–10% range)11, recent population-based studies have shown those rates to be much higher, ranging from 17% (in a study of the Surveillance, Epidemiology, and End Results database) to 29% in a study of the Swedish Adult Acute Leukemia Registry11–13. Consistent with those findings, the results of a Canadian study by Paulson et al. in 2014 showed that the incidence of early death was 22%14. Factors contributing to the discrepancy between clinical trials and population-based studies might be a failure to refer patients with poor performance status (including those with cranial and pulmonary hemorrhage) to a leukemia centre in a timely manner and exclusion of such patients from clinical trials15.
Overall, these recent studies identify a major unresolved issue in the management of an otherwise highly curable malignancy and should instruct health care providers to highlight the main cause of early death with an aim to identify treatment and management strategies that complement atra therapy. Given that early death occurs mainly as a result of complications from coagulopathy (bleeding and thrombosis)11,16, which develops early and can progress rapidly, early consideration of the possibility of apl and the immediate initiation of treatment once apl is suspected (and before it is proven) is critical.
Current guidelines recommend that first-line treatment of potential complications be initiated immediately based on clinical presentation and the morphology of apl cells in an analysis of a peripheral blood smear or bone aspirate and before genetic confirmation8,17. Later genetic confirmation of PML– RARA is still mandatory, because a positive response to induction treatment with atra or ato depends on the presence of the pml–rar-α fusion protein17.
The high risk of hemorrhagic early death in apl has prompted the authors of both the European and the North American guidelines to strongly recommend implementation of three measures at the earliest suspicion of apl and before genetic confirmation8,17:
Immediate atra therapy to help resolve the coagulopathy
Aggressive replacement of cryoprecipitate, platelets, and fresh-frozen plasma
Frequent, ongoing clinical monitoring of the patient
The eln guidelines also highlight several factors associated with an increased likelihood of fatal hemorrhage: higher counts of white blood cells (wbcs) or peripheral blasts, abnormal levels of creatinine, poor performance status, active bleeding, hypofibrinogenemia (<100 mg/dL) or higher levels of fibrin degradation products or D-dimers in combination with an increase in prothrombin time or activated partial thromboplastin time8. Immediate atra treatment is critical because atra is known to rapidly reduce the biologic drivers of apl-associated coagulopathy, and therefore is likely to reduce the risk of early death from severe bleeding8. To accompany immediate atra therapy initiation, the recommendations call for aggressive and ongoing transfusions with fresh-frozen plasma, cryoprecipitate, and platelet concentrates to maintain fibrinogen levels above 1.5 g/L and platelet levels at 30–50×109/L8,17. The initial workup for coagulopathy should include, at a minimum, platelet count, prothrombin time, activated partial thromboplastin time, and fibrinogen level8.
Patients presenting with a wbc count exceeding 10×109/L also have a higher risk of differentiation syndrome than do patients with a count of 10×109/L or fewer8,17. In a study by Kelaidi et al. examining high-risk patients in the apl 93 and apl 2000 trials, prophylactic dexamethasone was shown to reduce early induction deaths attributable to differentiation syndrome18. Based on that study, the eln and U.S. National Comprehensive Cancer Network (nccn) guidelines recommend considering prophylactic dexamethasone for patients with more than 10×109/L wbcs or those showing the first signs of differentiation syndrome8,17. The use of anticoagulant or antifibrinolytic therapy such as heparin or tranexamic acid to reduce the risk of bleeding is not recommended, and invasive procedures such as lumbar puncture and central venous catheterization are to be avoided before and during the early stages of remission induction because of the high risk of bleeding in apl8.
Evidence:
We identified only three studies that focused on supportive care (Table i). The study by Ikezoe et al.21 examined the effects of recombinant human thrombomodulin on the clinical outcomes of patients with coagulopathy. Rescue from disseminated intravascular coagulation was shown to occur earlier in patients treated with recombinant human thrombomodulin than in historical control patients (log-rank p = 0.019). In the study by Barreto et al.19, the effect of the prophylactic antifungal agent voriconazole on the incidence of differentiation syndrome in patients receiving atra plus chemo-therapy was examined. A trend toward an increased incidence of differentiation syndrome in patients receiving voriconazole was observed, although the difference was not statistically significant because of the small sample size (hazard ratio: 1.96; 95% confidence interval: 0.65 to 5.94; p = 0.23). A study by Chang et al.20 identified a higher wbc count (26.73 ± 6.18/ μL vs. 13.03 ± 3.03/μL, p = 0.026) and prolonged prothrombin time (4.85 ± 0.70 s vs. 2.59 ± 0.28 s, p = 0.002) and activated partial thromboplastin time (3.98 ± 1.68 s vs. 0.96 ± 0.93 s, p = 0.017) as risk factors for bleeding in patients with apl.
TABLE I.
Clinical trial data for supportive care treatments in acute promyelocytic leukemia (apl)
| Reference | Complication and treatment | Pts (n) | Median age (years) | Efficacy results and safety |
|---|---|---|---|---|
| Barreto et al., 201219 (apl patients at the Mayo Clinic during 2000–2011) | ||||
| atra plus chemotherapy plus voriconazole | 31 | 56 | Only body mass index differed between study arms | |
| atra plus chemotherapy (no fungal prophylaxis) | 15 | (range: 18–80) | [higher in patients receiving voriconazole (hr: 1.04; 95% ci: 1.001 to 1.078; p=0.0427)]. | |
| The overall incidence of ds was 35% (n=16), with patients receiving voriconazole being more likely to experience ds (hr: 2.31; 95% ci: 0.78 to 6.874; p=0.1308). After adjusting for body mass index, patients receiving voriconazole had a higher tendency to experience ds [especially severe ds (13 of 16 cases, 81%)]; however, because of small numbers, the trend was not statistically significant (hr: 1.96; 95% ci: 0.65 to 5.94; p=0.23). Admission to the intensive care unit was needed for management of severe ds in 7 patients (44%), 5 of whom had received voriconazole. Mean length of those stays was 4 days (range: 1–7 days), with no patients requiring intubation, but 29% receiving vasopressor support. No deaths were attributable to ds. | ||||
| Chang et al., 201220 | ||||
| Correlation of clinical bleeding events with lab coagulation profiles in apl | 116 | — | Overt dic occurred in 77.6% of patients. | |
In patients with bleeding,
| ||||
| Fibrinogen levels, platelet counts, and leukemia cell percentages were nonsignificantly different between bleeding and non-bleeding patients. | ||||
| Before initiation of atra, 7 patients experienced severe bleeding. | ||||
| Ikezoe et al., 201221 | ||||
| dic caused by apl; treated with rtm plus atra plus chemotherapy versus historical controls | 9 8 |
— — |
Intracranial vascular incidents developed in 2 control patients. No bleeding-related mortality was noted in patients treated with rtm. Rescue from dic occurred earlier in patients treated with rtm than in historical controls (log-rank p=0.019). |
|
Pts = patients; atra = all-trans-retinoic acid; hr = hazard ratio; ci = confidence interval; ds = differentiation syndrome; dic = disseminated intravascular coagulation; wbc = white blood cell; rtm = recombinant human thrombomodulin.
Recommendations:
Owing to a lack of high-level evidence on supportive care in the medical literature, the panel made no changes or new recommendations beyond those made by the eln. The panel strongly recommends prompt institution of supportive care measures as a critical strategy to reduce the early death rate in apl (Table ii). A provisional diagnosis of apl (based on clinical presentation and morphology of the leukemic cells in a blood smear or bone marrow aspirate) is routinely available before genetic confirmation. Therefore, if a patient is suspected of having apl, atra should be started immediately. Based on laboratory tests, cryoprecipitate, fresh-frozen plasma, and platelets should be infused immediately, with the goal of maintaining fibrinogen levels above 1.5 g/L and platelets at 30×109/L. To consistently maintain those target levels, the panel recommends repeat monitoring at least every 6 hours, because daily monitoring can be inadequate in the presence of ongoing consumptive coagulopathy. Referral to a leukemia centre should occur promptly. Molecular genetic confirmation of the PML–RARA translocation should be obtained as quickly as possible to warrant initiation of full induction therapy (per the recommendations in response to question 2). The panel also recommends administering prophylactic steroids to all patients with high-risk disease17.
TABLE II.
Recommendations for supportive care in newly diagnosed or suspected acute promyelocytic leukemia (apl)
| Supportive care | Implementation | Target | |
|---|---|---|---|
| 1 | Frequent, aggressive transfusions | Cryoprecipitate | Fibrinogen levels should be greater than 1.5 g/L |
| Platelets | Platelet counts should be at least 30×109/L | ||
| Fresh-frozen plasma | |||
| 2 | Therapy with atra | Should be started immediately | Should be administered in divided doses |
| Purpose is to treat coagulopathy and to initiate induction | |||
| 3 | Frequent monitoring | Immediate | Every 6 hours |
atra = all-trans-retinoic acid.
4.2. Question 2
How should ato be used in induction and consolidation for newly diagnosed apl patients?
Background:
In combination with an anthracycline, and with or without cytarabine, atra has been the standard backbone in induction and consolidation treatment for newly diagnosed apl patients for more than a decade. Induction therapy using such a regimen yields a complete response (cr) rate in the 90%–95% range3. The role of ato became clearer after several studies showed efficacy and safety for ato with or without atra in the relapsed setting22,23 and, more recently, in first-line induction and consolidation6,7,24–26. In 1998, Soignet et al.22 were the first to corroborate reports from China about the efficacy of ato in treating apl by showing that ato is highly efficacious at inducing remission in relapsed disease. Those results were confirmed in a larger multicentre trial in 2001 by the same group, who reported a cr rate of 85%, and 18-month overall survival (os) and relapse-free survival rates of 66% and 56% respectively in relapsed apl23. Those studies formed the basis for the approval of ato for the treatment of relapsed or refractory apl in Europe, the United States, and Canada. Several subsequent studies demonstrated similar results, with cr rates of 80%–100% and 2-year os rates ranging from 56% to 82%27–29.
Overall, ato has been shown to be well tolerated, with most patients experiencing only mild toxic effects. In some patients, ato is associated with differentiation syndrome and QTc prolongation, both of which are effectively counteracted when specific preventive measures are implemented30. The published studies also show that hematologic toxicity with ato is lower than it is with chemotherapeutic regimens for re-induction of remission3.
Currently, apl treatment is based on risk stratification by wbc count17. Patients with a count of 10×109/L or less are classified as low risk (platelets > 40×109/L) or intermediate risk (platelets ≤ 40×109/L) for relapse, and patients with a count exceeding 10×109/L are considered at high risk of relapse.
In 2009 and 2011 respectively, the eln and the nccn recommended 1 cycle of atra plus anthracycline-based chemotherapy (idarubicin alone, or daunorubicin plus cytarabine) as the standard first-line induction treatment for newly diagnosed patients with low-to-intermediate–risk and high-risk apl8,17. In 2013, the nccn guidelines were updated to include ato plus atra (without chemotherapy) as an option for first-line induction and consolidation treatment in newly diagnosed patients with low-to-intermediate–risk apl17. Consolidation treatment after induction has been more controversial and varied. However, the eln recommends atra plus 2–3 cycles of anthracycline-based chemotherapy as the standard approach for consolidation therapy, and at least 1 dose of cytarabine is recommended in high-risk patients less than 60 years of age8. For consolidation in high-risk patients, the nccn recommends three options: ato in combination with atra and daunorubicin, daunorubicin plus cytarabine with 5 doses of intrathecal chemotherapy, or atra in combination with idarubicin, cytarabine, and mitoxantrone. For low-to-intermediate–risk patients, the nccn recommends four options: ato plus atra, atra in combination with idarubicin and mitoxantrone, daunorubicin plus cytarabine, and ato in combination with atra and daunorubicin.
Evidence:
Our literature search revealed nine studies that examined the efficacy and safety of ato in combination with chemotherapy in first-line treatment, and another five studies that examined the efficacy and safety of the chemotherapy-free approach of ato plus atra in first-line treatment of newly diagnosed apl patients (Table iii).
TABLE III.
Clinical trial data for first-line arsenic trioxide (ato) treatments in acute promyelocytic leukemia (apl)
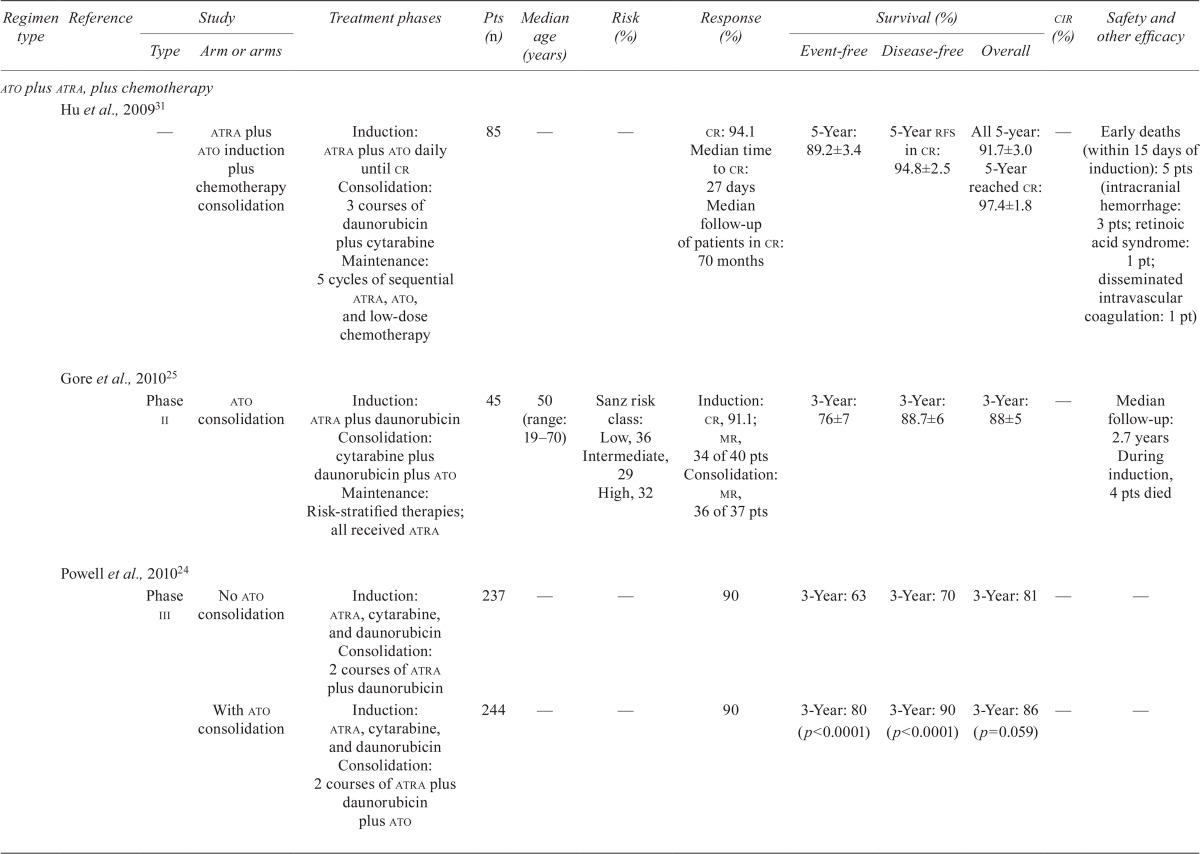

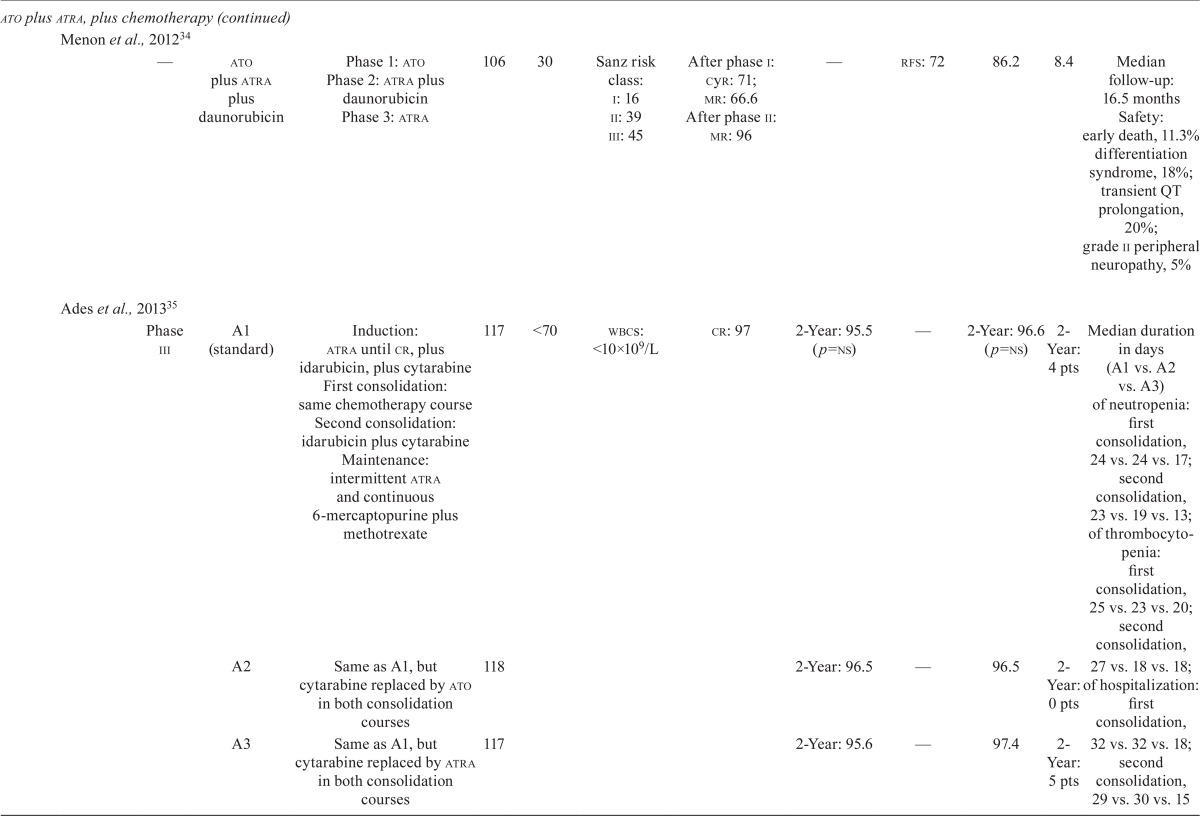
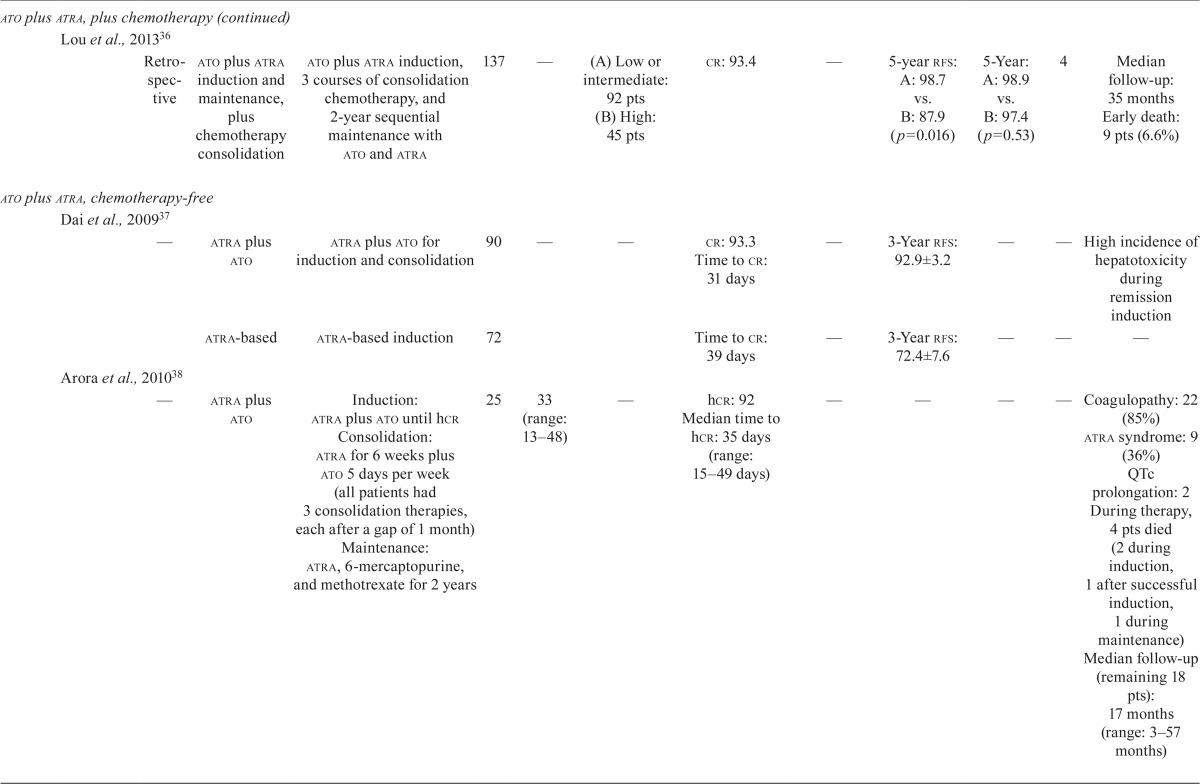
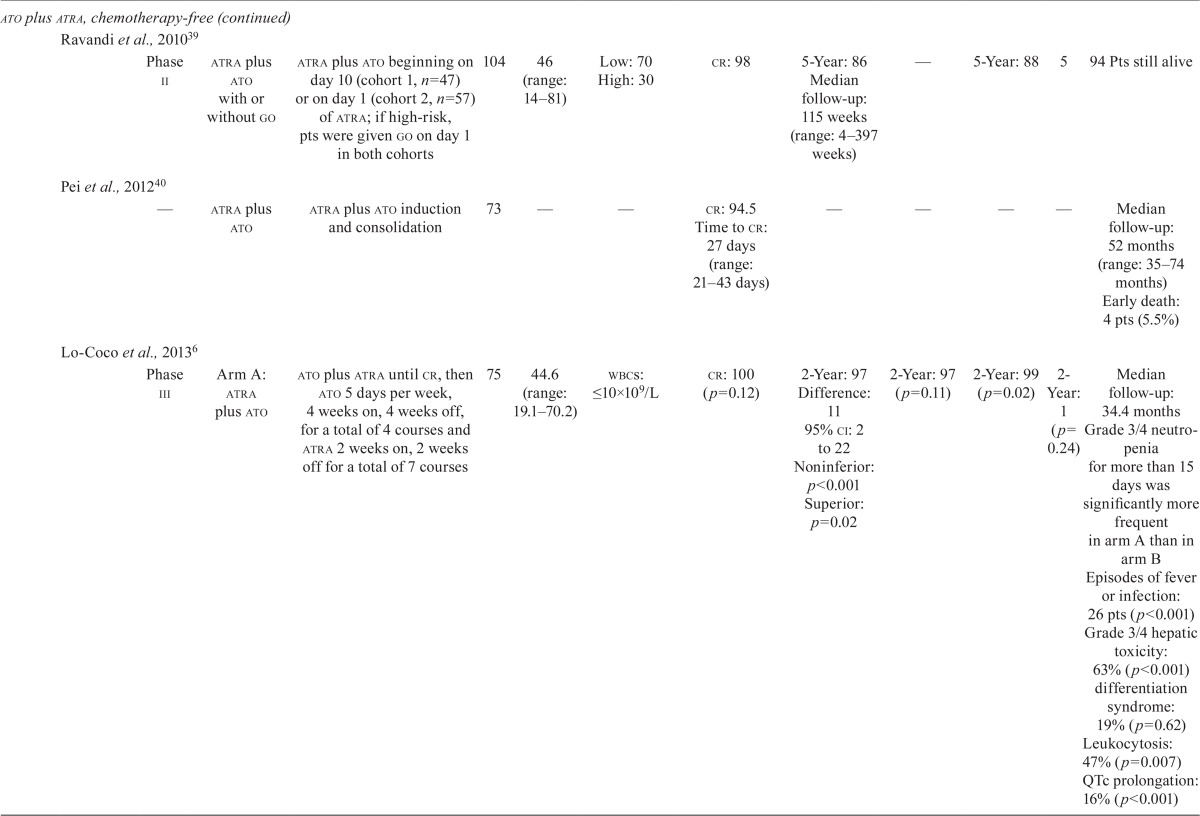
| Regimen type | Reference | Study | Treatment phases | Pts (n) | Median age (years) | Risk (%) | Response (%) | Survival (%) | cir (%) | Safety and other efficacy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Type | Arm or arms | Event-free | Disease-free | Overall | ||||||||||
| ato plus atra, plus chemotherapy | ||||||||||||||
| Hu et al., 200931 | ||||||||||||||
| — | atra plus ato induction plus chemotherapy consolidation | Induction: atra plus ato daily until cr Consolidation: 3 courses of daunorubicin plus cytarabine Maintenance: 5 cycles of sequential atra, ato, and low-dose chemotherapy | 85 | — | — | cr: 94.1 Median time to cr: 27 days Median follow-up of patients in cr: 70 months | 5-Year: 89.2±3.4 | 5-Year rfs in cr: 94.8±2.5 | All 5-year: 91.7±3.0 5-Year reached cr: 97.4±1.8 | — | Early deaths (within 15 days of induction): 5 pts (intracranial hemorrhage: 3 pts; retinoic acid syndrome: 1 pt; disseminated intravascular coagulation: 1 pt) | |||
| Gore et al., 201025 | ||||||||||||||
| Phase ii | ato consolidation | Induction: atra plus daunorubicin Consolidation: cytarabine plus daunorubicin plus ato Maintenance: Risk-stratified therapies; all received atra |
45 | 50 (range: 19–70) | Sanz risk class: Low, 36 Intermediate, 29 High, 32 | Induction: cr, 91.1; mr, 34 of 40 pts Consolidation: mr, 36 of 37 pts | 3-Year: 76±7 | 3-Year: 88.7±6 | 3-Year: 88±5 | — | Median follow-up: 2.7 years During induction, 4 pts died | |||
| Powell et al., 201024 | ||||||||||||||
| Phase iii | No ato consolidation | Induction: atra, cytarabine, and daunorubicin Consolidation: 2 courses of atra plus daunorubicin | 237 | — | — | 90 | 3-Year: 63 | 3-Year: 70 | 3-Year: 81 | — | — | |||
| With ato consolidation | Induction: atra, cytarabine, and daunorubicin Consolidation: 2 courses of atra plus daunorubicin plus ato | 244 | — | — | 90 | 3-Year: 80 (p<0.0001) | 3-Year: 90 (p<0.0001) | 3-Year: 86 (p=0.059) | — | — | ||||
| Liu et al., 201132 | ||||||||||||||
| Single-centre 1988–2009 | atra with or without ato, plus chemotherapy | atra or ato (or both) with anthracycline-based induction; after 3 courses of consolidation chemotherapy pts (n=279) received 2 years of maintenance | 340 | — | — | cr: 84.7 | — | 5-Year rfs: 83.7±2.6 | 5-Year: 89.0±2.4 | — | Median follow-up: 49 months (range: 6–255 months) During induction, 50 pts died | |||
| Huang et al., 201233 | ||||||||||||||
| — | ato consolidation | ato daily, two 21-day courses | 139 | 42 (range: 18–65) | — | cr: 91.2 | 5-Year: 75 (p<0.001) | — | 5-Year: 83 (p=0.002) | — | who grade 3/4 adverse events (low: 74.82%; high: 25.18%; p ≤0.001): neutropenia, 1.4%; infection, 0.7%; nausea/vomiting, 27.3% | |||
| High-dose cytarabine | High-dose cytarabine (2 courses) plus daunorubicin plus cytarabine (2 courses) | 132 | 37 (range: 17–65) | — | 5-Year: 54 | — | 5-Year: 71 | — | who grade 3/4 adverse events (low: 77.27%; high: 22.73%): neutropenia, 94.7%; infection, 87.1%; nausea/vomiting, 58.3% | |||||
| Iland et al., 20127 | ||||||||||||||
| Phase ii | atra plus idarubicin plus ato | Induction: atra plus idarubicin plus ato, plus prednisone and hemostatic support Consolidation 1 and 2: atra plus ato Maintenance: atra plus methotrexate plus 6-mercaptopurine | 124 | 44 (range: 3–78) | Low: 26 Intermediate: 54 High: 20 | hcr: 95 | 2-Year failure-free survival: 88.1 | 2-Year: 97.5 | 2-Year: 93.2 | 2-Year freedom from relapse: 97.5 | — | |||
| Menon et al., 201234 | ||||||||||||||
| — | ato plus atra plus daunorubicin | Phase 1: ato Phase 2: atra plus daunorubicin Phase 3: atra | 106 | 30 | Sanz risk class: i: 16 ii: 39 iii: 45 | After phase i: cyr: 71; mr: 66.6 After phase ii: mr: 96 | — | rfs: 72 | 86.2 | 8.4 | Median follow-up: 16.5 months Safety: early death, 11.3% differentiation syndrome, 18%; transient QT prolongation, 20%; grade ii peripheral neuropathy, 5% | |||
| Ades et al., 201335 | ||||||||||||||
| Phase iii | A1 (standard) | Induction: atra until cr, plus idarubicin, plus cytarabine First consolidation: same chemotherapy course Second consolidation: idarubicin plus cytarabine Maintenance: intermittent atra and continuous 6-mercaptopurine plus methotrexate | 117 | <70 | wbcs: <10×109/L | cr: 97 | 2-Year: 95.5 (p=ns) | — | 2-Year: 96.6 (p=ns) | 2-Year: 4 pts | Median duration in days (A1 vs. A2 vs. A3) of neutropenia: first consolidation, 24 vs. 24 vs. 17; second consolidation, 23 vs. 19 vs. 13; of thrombocytopenia: first consolidation, 25 vs. 23 vs. 20; second consolidation, 27 vs. 18 vs. 18; of hospitalization: first consolidation, 32 vs. 32 vs. 18; second consolidation, 29 vs. 30 vs. 15 |
|||
| A2 | Same as A1, but cytarabine replaced by ato in both consolidation courses | 118 | 2-Year: 96.5 | — | 96.5 | 2- Year: 0 pts | ||||||||
| A3 | Same as A1, but cytarabine replaced by atra in both consolidation courses | 117 | 2-Year: 95.6 | — | 97.4 | 2-Year: 5 pts | ||||||||
| Lou et al., 201336 | ||||||||||||||
| Retrospective | ato plus atra induction and maintenance, plus chemotherapy consolidation | ato plus atra induction, 3 courses of consolidation chemotherapy, and 2-year sequential maintenance with ato and atra | 137 | — | (A) Low or intermediate: 92 pts (B)High: 45 pts | cr: 93.4 | — | 5-year rfs: A: 98.7 vs. B: 87.9 (p=0.016) | 5-Year: A: 98.9 vs. B: 97.4 (p=0.53) | 4 | Median follow-up: 35 months Early death: 9 pts (6.6%) | |||
| ato plus atra, chemotherapy-free | ||||||||||||||
| Dai et al., 200937 | ||||||||||||||
| — | atra plus ato | atra plus ato for induction and consolidation | 90 | — | — | cr: 93.3 Time to cr: 31 days | — | 3-Year rfs: 92.9±3.2 | — | — | High incidence of hepatotoxicity during remission induction | |||
| atra-based | atra-based induction | 72 | Time to cr: 39 days | — | 3-Year rfs: 72.4±7.6 | — | — | — | ||||||
| Arora et al., 201038 | ||||||||||||||
| — | atra plus ato | Induction: atra plus ato until hcr Consolidation: atra for 6 weeks plus ato 5 days per week (all patients had 3 consolidation therapies, each after a gap of 1 month) Maintenance: atra, 6-mercaptopurine, and methotrexate for 2 years | 25 | 33 (range: 13–48) | — | hcr: 92 Median time to hcr: 35 days (range: 15–49 days) | — | — | — | — | Coagulopathy: 22 (85%) atra syndrome: 9 (36%) QTc prolongation: 2 During therapy, 4 pts died (2 during induction, 1 after successful induction, 1 during maintenance) Median follow-up (remaining 18 pts): 17 months (range: 3–57 months) | |||
| Ravandi et al., 201039 | ||||||||||||||
| Phase ii | atra plus ato with or without go | atra plus ato beginning on day 10 (cohort 1, n=47) or on day 1 (cohort 2, n=57) of atra; if high-risk, pts were given go on day 1 in both cohorts | 104 | 46 (range: 14–81) | Low: 70 High: 30 | cr: 98 | 5-Year: 86 Median follow-up: 115 weeks (range: 4–397 weeks) | — | 5-Year: 88 | 5 | 94 Pts still alive | |||
| Pei et al., 201240 | ||||||||||||||
| — | atra plus ato | atra plus ato induction and consolidation | 73 | — | — | cr: 94.5 Time to cr: 27 days (range: 21–43 days) | — | — | — | — | Median follow-up: 52 months (range: 35–74 months) Early death: 4 pts (5.5%) | |||
| Lo-Coco et al., 20136 | ||||||||||||||
| Phase iii | Arm A: atra plus ato | ato plus atra until cr, then ato 5 days per week, 4 weeks on, 4 weeks off, for a total of 4 courses and atra 2 weeks on, 2 weeks off for a total of 7 courses | 75 | 44.6 (range: 19.1–70.2) | wbcs:≤10×109/L | cr: 100 (p=0.12) | 2-Year: 97 Difference: 11 95% ci: 2 to 22 Noninferior: p<0.001 Superior: p=0.02 | 2-Year: 97 (p=0.11) | 2-Year: 99 (p=0.02) | 2-Year: 1 (p= 0.24) | Median follow-up: 34.4 months Grade 3/4 neutropenia for more than 15 days was significantly more frequent in arm A than in arm B Episodes of fever or infection: 26 pts (p<0.001) Grade 3/4 hepatic toxicity: 63% (p<0.001) differentiation syndrome: 19% (p=0.62) Leukocytosis: 47% (p=0.007) QTc prolongation: 16% (p<0.001) | |||
| Arm B: atra plus idarubicin | Standard atra, plus idarubicin induction followed by 3 cycles of anthracycline-based plus atra consolidation and low-dose chemotherapy and atra for maintenance | 79 | 46.6 (range: 18.7–70.2) | wbcs:≤10×109/L | cr: 95 | 2-Year: 86 | 2-Year: 90 | 2-Year: 91 | 2 Year: 6 | Episodes of fever or infection: 59 Grade 3/4 hepatic toxicity: 6% Differentiation syndrome: 16% Leukocytosis: 24% QTc prolongation: 0% | ||||
Pts = patients; cir= cumulative incidence of relapse; atra= all-trans retinoic acid; cr= complete response; rfs= relapse-free survival; who= World Health Organization; h cr= hematologic complete response; cyr= cytogenetic remission; mr= molecular response; wbcs = white blood cells; ns= nonsignificant;ci= confidence interval; go= gemtuzumab ozogamicin.
ATO Plus ATRA Plus Chemotherapy: Nine studies investigated the role of ato in combination with atra and chemotherapy regimens (idarubicin–cytarabine– daunorubicin) in induction, or consolidation, or both (Table iii). Rates of cr ranged from 84.7% to 97%, and rates of disease-free survival ranged from 72% (median follow-up: 16.5 months) to 97.4% at 5 years7,24,25,31–36. The os rates ranged from 86% at 3 years to 91.7% at 5 years7,24,25,31–36. One study that combined ato, atra, and idarubicin in induction and ato and atra in consolidation demonstrated equally favourable outcomes in 2-year os rates, regardless of Sanz risk category7. The reported early death rates ranged from 5.9% to 11.3%31,34,36. Differentiation syndrome was reported at frequencies ranging from 1.1% to 18%31,34, and one study reported transient QTc prolongation in 20% of patients34.
ATO Plus ATRA: One phase iii randomized clinical trial compared the efficacy and safety of ato plus atra with those of atra plus chemotherapy in newly diagnosed patients with low-to-intermediate–risk apl (Table iii)6. Patients at low or intermediate risk received either ato plus atra (n = 77) for induction and consolidation, or atra plus idarubicin for induction (n = 79), followed by 3 cycles of atra plus chemotherapy for consolidation, and low-dose chemotherapy plus atra for maintenance. Rates of cr in the ato plus atra group and the atra plus chemotherapy group were 100% and 95% respectively (p = 0.12). The 2-year event-free survival (efs) rates were 97.1% in the ato plus atra group and 86% in the atra plus chemotherapy group (p = 0.02, Table iii). Patients in the ato plus atra group experienced more QTc prolongation (16% vs. 0%, p < 0.001), more hepatic toxicity (63% vs. 6%, p < 0.001), and more hyperleukocytosis (47% vs. 24%, p = 0.007).
Four other studies examined the efficacy and safety of ato plus atra as induction and consolidation therapy (Table iii). The cr rate ranged from 92% to 98%37–40, with an early death rate of 5.5% reported in one study40 and an 8% induction failure rate because of death reported in another38. The 5-year os rates ranged from 83% to 98.9%33,36,39,41. One study reported a 3-year disease-free survival rate of 92.9%37, and one study reported a 5-year efs rate of 86%39.
Recommendations:
The panel recommends ato plus atra as induction and consolidation treatment for untreated, low-to-intermediate–risk apl patients. This recommendation is based on the results of the randomized phase iii study by Lo-Coco et al.6, which showed that this combination was noninferior to the aida regimen (atra plus idarubicin). The study also showed that ato plus atra was associated with less hematologic toxicity and infection6. For high-risk patients, the panel recommends induction with combination chemotherapy consisting of idarubicin, atra, and ato, followed by consolidation with ato and atra, as used in the phase ii study reported by Iland et al.7.
4.3. Question 3
What is the role of hsct in the treatment of relapsed apl?
Background:
Although some evidence suggests that treatment intensification with hsct can improve patient outcomes after ato-induced second remission42, the best consolidation treatment in that setting remains unknown8. The selection of the best treatment option (hsct or chemotherapy) in second cr after ato—and also the choice between allogeneic and autologous hsct—depends on several factors, including molecular status at second complete response, duration of first remission, age, and donor availability8. Compared with allogeneic hsct, autologous hsct is associated with a lower risk of transplantation-related morbidity and mortality and might be an appropriate treatment choice for patients with a second remission who do not have minimal residual disease detectable by polymerase chain reaction (pcr) at the time of collection of hematopoietic stem cells. Compared with autologous hsct, allogeneic hsct is associated with a greater risk of transplantation-related death. However, because allogeneic hsct has a strong anti-leukemic effect, it could be considered for patients who have failed to achieve a second molecular remission or for those with a very short first cr duration.
For patients with relapsed apl, the nccn guidelines recommend ato plus atra for induction of remission or for patients who fail to achieve molecular remission after completion of consolidation treatment after relapse17. After a morphologic remission, patients should be evaluated for PML–RARA status by pcr and treated with one of the following options:
Autologous hsct (if pcr-negative)
Further courses of ato (if pcr-negative and not suitable for hsct)
Allogeneic hsct (if pcr-positive)
Enrolment in a clinical trial
For patients who fail to reach a morphologic remission, the nccn recommends allogeneic hsct or enrolment in a suitable clinical trial. Recommendations by the eln are consistent with those of the nccn8.
Evidence:
Nine studies examined the role of hsct in consolidation treatment of apl patients after first relapse (Table iv). Six of the studies examined the role of autologous or allogeneic hsct after remission induction with chemotherapy, cytarabine, or ato, with or without atra. Yanada et al. reported a 5-year os rate of 77% in patients receiving autologous hsct after a median follow-up of 4.9 years43. In their respective studies, Yanada et al., Shepard et al.47, and Ferrara et al.48 reported relapse rates of 8.5%, 25%, and 23% in patients treated with autologous hsct. A study by Thirugnanam et al.49 showed a higher 5-year efs rate in patients treated with autologous hsct than in those receiving only ato with or without atra (83.33% vs. 34.45%, p = 0.001). Ramadan et al.46 examined the role of allogeneic hsct in second remission and beyond, reporting a 4-year os rate of 62% when hsct was performed in the second cr and 31% in patients transplanted beyond the second cr (p = 0.05). A study by Linker et al. showed a 5-year disease-free survival rate of 67% after a median follow-up of 8.2 years in patients receiving two-step autologous hsct50.
Table IV.
Clinical trial data for hematopoietic stem-cell transplantation (hsct) in acute promyelocytic leukemia (apl)
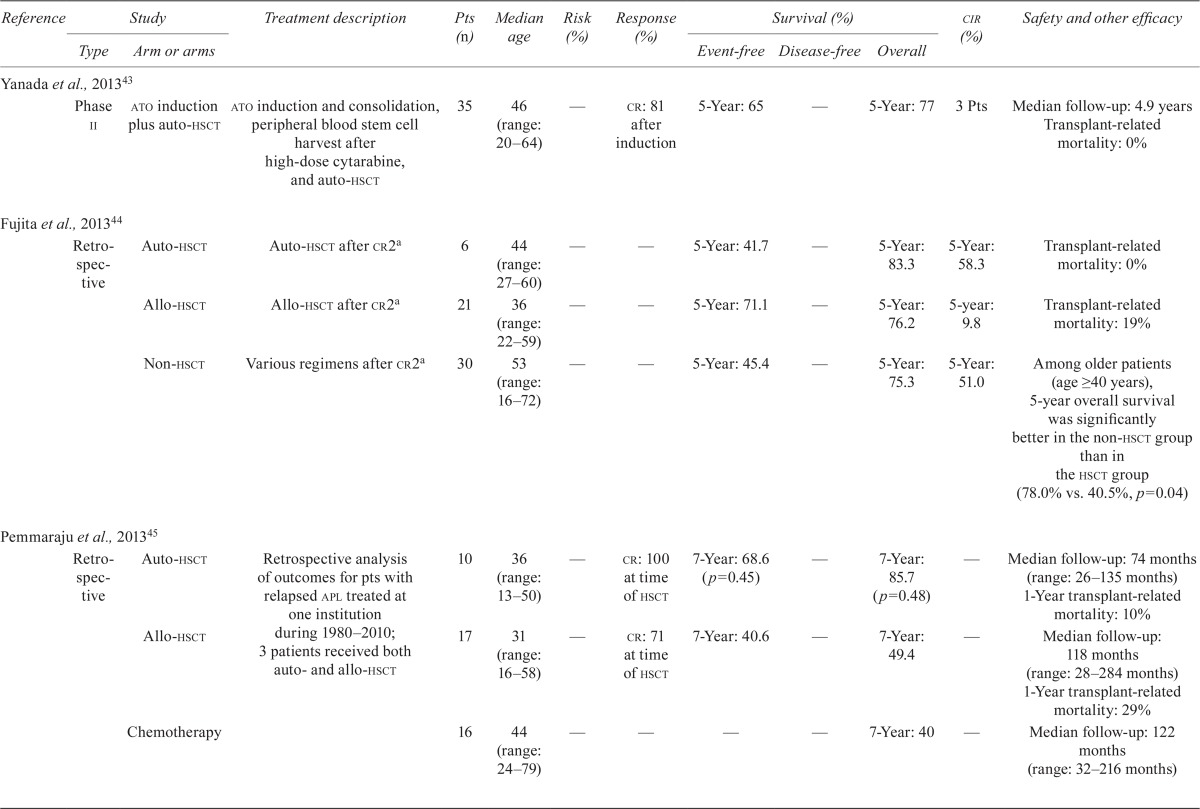

| Reference | Study | Treatment description | Pts (n) | Median age | Risk (%) | Response (%) | Survival (%) | cir (%) | Safety and other efficacy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Type | Arm or arms | Event-free | Disease-free | Overall | ||||||||
| Yanada et al., 2013 43 | ||||||||||||
| Phase ii | ato induction plus auto-hsct | ato induction and consolidation, peripheral blood stem cell harvest after high-dose cytarabine, and auto-hsct | 35 | 46 (range: 20–64) | — | cr: 81 after induction | 5-Year: 65 | — | 5-Year: 77 | 3 Pts | Median follow-up: 4.9 years Transplant-related mortality: 0% | |
| Fujita et al., 201344 | ||||||||||||
| Retro-spective | Auto-hsct | Auto-hsct after cr2a | 6 | 44 (range: 27–60) | — | — | 5-Year: 41.7 | — | 5-Year: 83.3 | 5-Year: 58.3 | Transplant-related mortality: 0% | |
| Allo-hsct | Allo-hsct after cr2a | 21 | 36 (range: 22–59) | — | — | 5-Year: 71.1 | — | 5-Year: 76.2 | 5-year: 9.8 | Transplant-related mortality: 19% | ||
| Non-hsct | Various regimens after cr2a | 30 | 53 (range: 16–72) | — | — | 5-Year: 45.4 | — | 5-Year: 75.3 | 5-Year: 51.0 | Among older patients (age ≥ 40 years), 5-year overall survival was better in the non-hsct group than in the hsct group (78.0% vs. 40.5%, p=0.04) | ||
| Pemmaraju et al., 201345 | ||||||||||||
| Retro-spective | Auto-hsct | Retrospective analysis of outcomes for pts with relapsed apl treated at one institution during 1980–2010; 3 patients received both auto- and allo-hsct | 10 | 36 (range: 13–50) | — | cr: 100 at time of hsct | 7-Year: 68.6 (p=0.45) | — | 7-Year: 85.7 (p=0.48) | — | Median follow-up: 74 months (range: 26–135 months) 1-Year transplant-related mortality: 10% | |
| Allo-hsct | 17 | 31 (range: 16–58) | — | cr: 71 at time of hsct | 7-Year: 40.6 | — | 7-Year: 49.4 | — | Median follow-up: 118 months (range: 28–284 months) 1-Year transplant-related mortality: 29% | |||
| Chemotherapy | 16 | 44 (range: 24–79) | — | — | — | — | 7-Year: 40 | — | Median follow-up: 122 months (range: 32–216 months) | |||
| Ramadan et al., 201246 | ||||||||||||
| — | Allo-hsct | Allo-hsct for pts in cr2 (n=15) or cr3+ (n=16) | 31 | 39 | — | — | — | 4-Year for cr2 vs. cr3+: 62 vs. 31 (p=0.05) | 4-Year for cr2 vs. cr3+: 32 vs. 44 (p=0.37) | Median follow-up: 55 months (range: 4–100 months) 4-Year overall survival (rt-pcr-negative vs. -positive): 64% vs. 27% (p=0.03) 4-Year cir (rt-pcr-negative vs. -positive): 30% vs. 47% (p=0.30) Transplant-related mortality: 19.6% | ||
| Shepard et al., 201147 | ||||||||||||
| — | ato, then auto-hsct | ato re-induction after relapse from cr1 [single-agent ato (n=15), ato plus intrathecal therapy (n=2), ato plus chemotherapy (n=4)] followed by auto-hsct for pts with relapsed apl | 21 | 31 (range: 1–54) | wbcs >10× 109/L (n=4); Platelets <40× 109/L (n=13) | cr2 after ato: 95 | — | Median: 4 years (range: 0.34–10.8) | — | 25 relapsed; median time to relapse: 384 days (range: 126–513 days) | All first-line induction atra, 85% received maintenance after consolidation in cr1. Safety of ato: differentiation syndrome (n=3); prolonged QTc (n=3); grade 3 infection (n=2); grade 2 or 3 transaminitis (n=2) Safety of auto-hsct: grade 3 mucositis (n=4) |
|
| Ferrara et al., 201048 | ||||||||||||
| — | Auto-hsct | Auto-hsct after second mr; 13 no maintenance or consolidation therapy given after auto-hsct | 39 (range: 18–69) | — | — | — | — | — | 11 pts still alive: 10 in mr; 1 in cr3 | Median follow-up: 25 months 2 Pts relapsed after auto-hsct and died in refractory disease; 1 pt relapsed, but achieved cr3 and was awaiting allo-hsct | ||
| Thirugnanam et al., 200949 | ||||||||||||
| Single-centre | Auto-hsct | After mr2 with ato-based therapy, pts opted to undergo auto-hsct | 14 | For all 37 pts: 34 (range: | Median duration of cr1: 20.3 months | mr2: 89 after induction and consolidation | 5-Year: 83.33±15.21 (p=0.001) | — | 5-Year: 100.00 ±0.00 | 7.1 | Median follow-up: 32 months Since January 2000, 37 patients with relapsed apl were treated at the centre | |
| No hsct (ato alone or ato plus atra) | After mr2 with ato-based therapy, pts received monthly cycles of ato as a single agent (n=13) or ato plus atra (n=6) for 6 months | 19 | 6–57) | 5-Year: 34.45±11.24 | — | 5-Year: 38.50 ±11.68 | 63.2 | |||||
| Linker et al., 2009 50 | ||||||||||||
| — | Two-step auto-hsct for pts with aml in cr2 | Step1: Consolidation (cytarabine plus etoposide) Step 2: Auto-hsct with prep regimen of oral busulfan followed by etoposide | 50 total (12 fab M3) | — | — | — | — | 5-Year apl vs. non-apl: 67 vs. 16 (p=0.01) | — | — | Median follow-up: 8.2 years (range: 7.2–9.9 years) | |
| Chakrabarty et al., 201451 | ||||||||||||
| — | Auto-hsct | Patients in cr2 received either auto- or allo-hsct during 1995–2006 | 62 | — | — | — | — | 5-year: 63 (p=0.10) | 5-Year: 75 (p=0.0002) | — | Median follow-up: 115 months in pts who had allo-hsct and 72 months in pts who had auto-hsct 3-Year transplant-related mortality: 2% Multivariate analysis: dfs was worse after allogeneic hsct (hr: 1.88; 95% ci: 1.16 to 3.06; p=0.011) and for those >40 years of age (hr: 2.30; 95% ci: 1.44 to 3.67; p=0.0005); os was worse after allogeneic hsct (hr: 2.66; 95% ci: 1.52 to 4.65; p=0.0006) and for those >40 years of age (hr: 3.29; 95% ci: 1.95 to 5.54; p<0.001) and for those with a cr <12 months (hr: 1.56; 95% ci: 1.07 to 2.26; p=0.021) 3-Year transplant-related mortality: 30% | |
| Allo-hsct | 232 | — | — | — | — | 5-year: 50 | 5-year: 54 | — | ||||
In the Japan Adult Leukemia Study Group APL97 study44.
Pts = patients; cir= cumulative incidence of relapse; ato= arsenic trioxide; auto = autologous; cr[1,2,3] = complete response (1st, 2nd, 3rd); allo = allogeneic; wbcs = white blood cells; atra = all-trans retinoic acid; mr[2] = molecular remission [2nd]; aml= acute myeloid leukemia; fab = French American British classification; dfs= disease-free survival; hr= hazard ratio; ci= confidence interval;os= overall survival.
Three studies compared clinical outcomes in patients treated with autologous or allogeneic hsct and in those who did not receive hsct. The Fujita et al.44 study compared outcomes in patients receiving autologous hsct, allogeneic hsct, and no hsct. The 5-year os and efs rates were 83.3% and 41.7% respectively in the autologous-hsct group, 76.2% and 71.1% in the allogeneic-hsct group, and 77.4% and 50.7% in the non-hsct group. In a retrospective study, Pemmaraju et al.45 demonstrated 7-year os rates of 85.7%, 49.4%, and 40% in patients treated with autologous hsct, allogeneic hsct, and chemotherapy respectively (p = 0.48). In a study by Holter Chakrabarty et al., the 5-year os rate was higher for patients who received autologous hsct than for those who underwent allogeneic hsct (75% vs. 54%, p = 0.002)51. The same study also showed a numerically higher 5-year disease-free survival rate for patients who underwent autologous transplantation (63% vs. 50%, p = 0.10).
Recommendations:
The panel recommends consolidation treatment options consistent with those stated in the nccn guideline: autologous hsct for patients who achieve molecular remission after ato-induced second cr, with allogeneic hsct reserved for patients with persistent disease by molecular monitoring (pcr-positive). Patients who achieve pcr-positive remissions, but who are not suitable for hsct, should be treated with up to 6 cycles of ato for consolidation treatment. The panel recommends consideration of allogeneic hsct or enrolment in clinical trials for patients who do not achieve remission.
5. ACKNOWLEDGMENTS
The authors acknowledge support by Lundbeck Canada for the development of this article, as well as medical writing support provided by Wissam Assaily, Benjamin Fuerth, and Anna Christofides of New Evidence.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114:5126–35. doi: 10.1182/blood-2009-07-216457. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Lo Coco F. Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002;16:1959–73. doi: 10.1038/sj.leu.2402721. [DOI] [PubMed] [Google Scholar]
- 3.Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- 4.Zhou GB, Zhang J, Wang ZY, Chen SJ, Chen Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci. 2007;362:959–71. doi: 10.1098/rstb.2007.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallman M, Lo-Coco F, Kwaan HC, Sanz M, Gore S. Early death in patients with acute promyelocytic leukemia. Proceedings from a live roundtable at the 2010 American Society of Hematology Annual Meeting, December 4–7, 2010, Orlando, Florida. Clin Adv Hematol Oncol. 2011;9:1–16. [PubMed] [Google Scholar]
- 6.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 7.Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (apml4) Blood. 2012;120:1570–80. doi: 10.1182/blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 8.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Tallman MS. Managing acute promyelocytic leukemia without conventional chemotherapy: is it possible? Expert Rev Hematol. 2011;4:427–36. doi: 10.1586/ehm.11.42. [DOI] [PubMed] [Google Scholar]
- 10.Breccia M, Latagliata R, Cannella L, et al. Early hemorrhagic death before starting therapy in acute promyelocytic leukemia: association with high wbc count, late diagnosis and delayed treatment initiation. Haematologica. 2010;95:853–4. doi: 10.3324/haematol.2009.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25:1128–34. doi: 10.1038/leu.2011.78. [DOI] [PubMed] [Google Scholar]
- 13.McClellan JS, Kohrt HE, Coutre S, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97:133–6. doi: 10.3324/haematol.2011.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulson K, Serebrin A, Lambert P Acute promyelocytic leukaemia is characterized by stable incidence and improved survival that is restricted to patients managed in leukaemia referral centres: a pan-Canadian epidemiological study. Br J Haematol. 2014. [Epub ahead of print]. [DOI] [PubMed]
- 15.de la Serna J, Montesinos P, Vellenga E, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 16.Marti–Carvajal AJ, Simancas D, Cardona AF. Treatment for disseminated intravascular coagulation in patients with acute and chronic leukemia. Cochrane Database Syst Rev. 2011:CD008562. doi: 10.1002/14651858.CD008562.pub2. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia. Fort Washington PA: NCCN; 2013. Ver 2.2013. [Current version available online at: http://www.nccn.com/files/cancer-guidelines/breast/index.html (free registration required); cited May 8, 2014] [Google Scholar]
- 18.Kelaidi C, Chevret S, De Botton S, et al. Improved outcome of acute promyelocytic leukemia with high wbc counts over the last 15 years: the European apl group experience. J Clin Oncol. 2009;27:2668–76. doi: 10.1200/JCO.2008.18.4119. [DOI] [PubMed] [Google Scholar]
- 19.Barreto JN, Kuth JC, Peskey CS, Patnaik MM. A comparative study of patients with acute promyelocytic leukemia receiving all-trans retinoic acid with and without voriconazole: effect on differentiation syndrome. Pharmacotherapy. 2012;32:e178. [Google Scholar]
- 20.Chang H, Kuo MC, Shih LY, et al. Clinical bleeding events and laboratory coagulation profiles in acute promyelocytic leukemia. Eur J Haematol. 2012;88:321–8. doi: 10.1111/j.1600-0609.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 21.Ikezoe T, Takeuchi A, Isaka M, et al. Recombinant human soluble thrombomodulin safely and effectively rescues acute promyelocytic leukemia patients from disseminated intravascular coagulation. Leuk Res. 2012;36:1398–402. doi: 10.1016/j.leukres.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Soignet SL, Maslak P, Wang ZG, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–8. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 23.Soignet SL, Frankel SR, Douer D, et al. United States multi-center study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 24.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116:3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore SD, Gojo I, Sekeres MA, et al. Single cycle of arsenic trioxide-based consolidation chemotherapy spares anthracycline exposure in the primary management of acute promyelocytic leukemia. J Clin Oncol. 2010;28:1047–53. doi: 10.1200/JCO.2009.25.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estey E, Garcia–Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemo-therapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–73. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 27.Lazo G, Kantarjian H, Estey E, Thomas D, O’Brien S, Cortes J. Use of arsenic trioxide (As2O3) in the treatment of patients with acute promyelocytic leukemia: the M.D. Anderson experience. Cancer. 2003;97:2218–24. doi: 10.1002/cncr.11314. [DOI] [PubMed] [Google Scholar]
- 28.Au WY, Lie AK, Chim CS, et al. Arsenic trioxide in comparison with chemotherapy and bone marrow transplantation for the treatment of relapsed acute promyelocytic leukaemia. Ann Oncol. 2003;14:752–7. doi: 10.1093/annonc/mdg208. [DOI] [PubMed] [Google Scholar]
- 29.Shigeno K, Naito K, Sahara N, et al. Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: updated outcomes of the phase ii study and postremission therapies. Int J Hematol. 2005;82:224–9. doi: 10.1532/IJH97.05044. [DOI] [PubMed] [Google Scholar]
- 30.Lundbeck Canada . Trisenox (arsenic trioxide) [product monograph] Montreal, QC: Lundbeck Canada; 2013. [Google Scholar]
- 31.Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106:3342–7. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu YJ, Wu DP, Liang JY, et al. Long-term survey of outcome in acute promyelocytic leukemia: a single center experience in 340 patients. Med Oncol. 2011;28(suppl 1):S513–21. doi: 10.1007/s12032-010-9733-7. [DOI] [PubMed] [Google Scholar]
- 33.Huang BT, Zeng QC, Gurung A, et al. The early addition of arsenic trioxide versus high-dose arabinoside is more effective and safe as consolidation chemotherapy for risk-tailored patients with acute promyelocytic leukemia: multicenter experience. Med Oncol. 2012;29:2088–94. doi: 10.1007/s12032-011-0099-2. [DOI] [PubMed] [Google Scholar]
- 34.Menon H, Kumar S, Sengar M, et al. Sequential arsenic tri-oxide, and all trans retinoic acid with daunomycin yield early cytogentic and molecular response in newly diagnosed acute promyelocytic leukemia—data from a tertiary cancer centre [abstract 2628] Blood. 2012;120 [Available online at https://ash.confex.com/ash/2012/webprogram/Paper52514.html; cited August 14, 2014] [Google Scholar]
- 35.Ades L, Chevret S, Raffoux E, et al. Arsenic trioxide (ato) or atra for consolidation treatment of standard risk non elderly newly diagnosed apl—second interim analysis of a randomized trial (apl 2006) by the French Belgian Swiss APL Group [abstract 495] Blood. 2013. p. 121. [Available online at: https://ash.confex.com/ash/2013/webprogram/Paper60074.html; cited August 13, 2014]
- 36.Lou Y, Qian W, Meng H, et al. High efficacy of arsenic trioxide plus all-trans retinoic acid based induction and maintenance therapy in newly diagnosed acute promyelocytic leukemia. Leuk Res. 2013;37:37–42. doi: 10.1016/j.leukres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Dai CW, Zhang GS, Shen JK, et al. Use of all-trans retinoic acid in combination with arsenic trioxide for remission induction in patients with newly diagnosed acute promyelocytic leukemia and for consolidation/maintenance in cr patients. Acta Haematol. 2009;121:1–8. doi: 10.1159/000204472. [DOI] [PubMed] [Google Scholar]
- 38.Arora A, Malhotra P, Das R, Varma S, Suri V, Varma N. Safety and efficacy of all-trans retinoic acid (atra) and arsenic trioxide (ato) combination therapy in acute promyelocytic leukemia [abstract 0648] Haematologica. 2010;95(suppl 2):272. [Available online at: http://online.haematologica.org/eha15/browserecord.php?-action=browse&-recid=6718; cited August 13, 2014] [Google Scholar]
- 39.Ravandi F, Estey EH, Cortes JE, et al. Phase ii study of all-trans retinoic acid (atra), arsenic trioxide (ato), with or without gemtuzumab ozogamicin (go) for the frontline therapy of patients with acute promyelocytic leukemia (apl) [abstract 1080] Blood. 2010:116. [Available online at: https://ash.confex.com/ash/2010/webprogram/Paper29064.html; cited August 13, 2014] [Google Scholar]
- 40.Pei R, Cao J, Ma J, et al. Long term curative effects of sequential therapy with all-trans retinoic acid, arsenious oxide and chemotherapy on patients with acute promyelocytic leukemia. Hematology. 2012;17:311–16. doi: 10.1179/102453312X13451850327262. [DOI] [PubMed] [Google Scholar]
- 41.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–10. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douer D, Hu W, Giralt S, Lill M, DiPersio J. Arsenic trioxide (Trisenox) therapy for acute promyelocytic leukemia in the setting of hematopoietic stem cell transplantation. Oncologist. 2003;8:132–40. doi: 10.1634/theoncologist.8-2-132. [DOI] [PubMed] [Google Scholar]
- 43.Yanada M, Tsuzuki M, Fujita H, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. 2013;121:3095–102. doi: 10.1182/blood-2012-11-466862. [DOI] [PubMed] [Google Scholar]
- 44.Fujita H, Asou N, Iwanaga M, et al. Role of hematopoietic stem cell transplantation for relapsed acute promyelocytic leukemia: a retrospective analysis of jalsg-apl97. Cancer Sci. 2013;104:1339–45. doi: 10.1111/cas.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pemmaraju N, Tanaka MF, Ravandi F, et al. Outcomes in patients with relapsed or refractory acute promyelocytic leukemia treated with or without autologous or allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2013;13:485–92. doi: 10.1016/j.clml.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramadan SM, Di Veroli A, Camboni A, et al. Allogeneic stem cell transplantation for advanced acute promyelocytic leukemia in the atra and ato era. Haematologica. 2012;97:1731–5. doi: 10.3324/haematol.2012.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shepard DD, Kohrt HE, Rosenblat TL, et al. Arsenic trioxide followed by autologous stem cell transplant for patients with relapsed apl [abstract 6619] J Clin Oncol. 2011;29 [Available online at: http://meetinglibrary.asco.org/content/84335-102; cited August 14, 2014] [Google Scholar]
- 48.Ferrara F, Finizio O, Izzo T, et al. Autologous stem cell transplantation for patients with acute promyelocytic leukemia in second molecular remission. Anticancer Res. 2010;30:3845–9. [PubMed] [Google Scholar]
- 49.Thirugnanam R, George B, Chendamarai E, et al. Comparison of clinical outcomes of patients with relapsed acute promyelocytic leukemia induced with arsenic trioxide and consolidated with either an autologous stem cell transplant or an arsenic trioxide-based regimen. Biol Blood Marrow Transplant. 2009;15:1479–84. doi: 10.1016/j.bbmt.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Linker CA, Owzar K, Powell B, et al. Auto-sct for aml in second remission: calgb study 9620. Bone Marrow Transplant. 2009;44:353–9. doi: 10.1038/bmt.2009.36. [DOI] [PubMed] [Google Scholar]
- 51.Holter Chakrabarty JL, Rubinger M, Le–Rademacher J, et al. Autologous is superior to allogeneic hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission. Biol Blood Marrow Transplant. 2014;20:1021–5. doi: 10.1016/j.bbmt.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


