Abstract
Background
Compared with photon therapy, proton-beam therapy (pbt) offers compelling advantages in physical dose distribution. Worldwide, gantry-based proton facilities are increasing in number, but no such facilities exist in Canada. To access pbt, Canadian patients must travel abroad for treatment at high cost. In the face of limited access, this report seeks to provide recommendations for the selection of patients most likely to benefit from pbt and suggests an out-of-country referral process.
Methods
The medline, embase, PubMed, and Cochrane databases were systematically searched for studies published between January 1990 and May 2014 that evaluated clinical outcomes after pbt. A draft report developed through a review of evidence was externally reviewed and then approved by the Alberta Health Services Cancer Care Proton Therapy Guidelines steering committee.
Results
Proton therapy is often used to treat tumours close to radiosensitive tissues and to treat children at risk of developing significant late effects of radiation therapy (rt). In uncontrolled and retrospective studies, local control rates with pbt appear similar to, or in some cases higher than, photon rt. Randomized trials comparing equivalent doses of pbt and photon rt are not available.
Summary
Referral for pbt is recommended for patients who are being treated with curative intent and with an expectation for long-term survival, and who are able and willing to travel abroad to a proton facility. Commonly accepted indications for referral include chordoma and chondrosarcoma, intraocular melanoma, and solid tumours in children and adolescents who have the greatest risk for long-term sequelae. Current data do not provide sufficient evidence to recommend routine referral of patients with most head-and-neck, breast, lung, gastrointestinal tract, and pelvic cancers, including prostate cancer. It is recommended that all referrals be considered by a multidisciplinary team to select appropriate cases.
Keywords: Proton-beam therapy, radiation therapy, review, recommendations
1. INTRODUCTION
In proton-beam therapy (pbt), high-energy protons enter the body, depositing less radiation dose at the body surface and most at the end of their range, deep in tissue (called the Bragg peak). The result is a minimal dose deep to the target, sharply contrasting with the exponential attenuation of dose along the beam path of photons (X-rays). The result is that pbt can spare radiation dose to healthy uninvolved tissues outside the target, potentially reducing the risk of radiation injury1.
By December 2013, 111,088 patients worldwide had been treated with pbt2. Historically, the high capital cost of proton facilities equipped with rotational gantries (exceeding US$160 million)3 has limited the number of facilities in operation; however, that number is now increasing rapidly (see Table i). In the 10 years up to 2014, the number of facilities in the United States alone grew to thirteen from two3. Compact single-room cyclotron centres have drastically reduced the capital cost (estimated at less than US$50 million) and account for 10 of the 22 facilities under construction worldwide2. In Canada, the triumf Proton Treatment Facility in Vancouver, British Columbia, makes a fixed beam suitable for the treatment of intraocular melanomas available episodically through the year4. Gantry-equipped facilities capable of treating a broad range of tumour sites are not currently available in Canada.
TABLE I.
Proton-beam facilities equipped with rotational gantries at March 24, 20142
| Country |
Facilities (n)
|
||
|---|---|---|---|
| In operation | Under constructiona | Planned | |
| United States | 13 | 9 | 1 |
| Japan | 8 | 4 | |
| Germany | 3 | 1 | |
| France | 1 | 1 | |
| South Korea | 1 | 1 | |
| China | 1 | 1 | |
| Switzerland | 1 | 1 | |
| Czech Republic | 1 | ||
| Austria | 1 | ||
| Italy | 1 | ||
| Poland | 1 | ||
| Saudi Arabia | 1 | ||
| Sweden | 1 | ||
| Taiwan | 1 | ||
| Netherlands | 4 | ||
| England | 2 | ||
| TOTAL | 29 | 22 | 9 |
First patient to be treated during or before 2016.
Patients with diagnoses other than intraocular melanoma must travel outside Canada to access pbt. In most cases, decisions to fund referrals are made case-by-case by the provincial ministries of health. The mean cost per referral is estimated at $200,000 for treatment including daily anesthesia, concurrent chemotherapy, and hospitalization when required. Additional costs, including those for travel, accommodation, and meals, can be incurred by patients.
The present report reviews the latest clinical evidence on the efficacy of pbt, provides recommendations for the selection of patients most likely to benefit from referral, and proposes an out-of-country referral process for Canadian patients.
2. METHODS
2.1. Literature Search
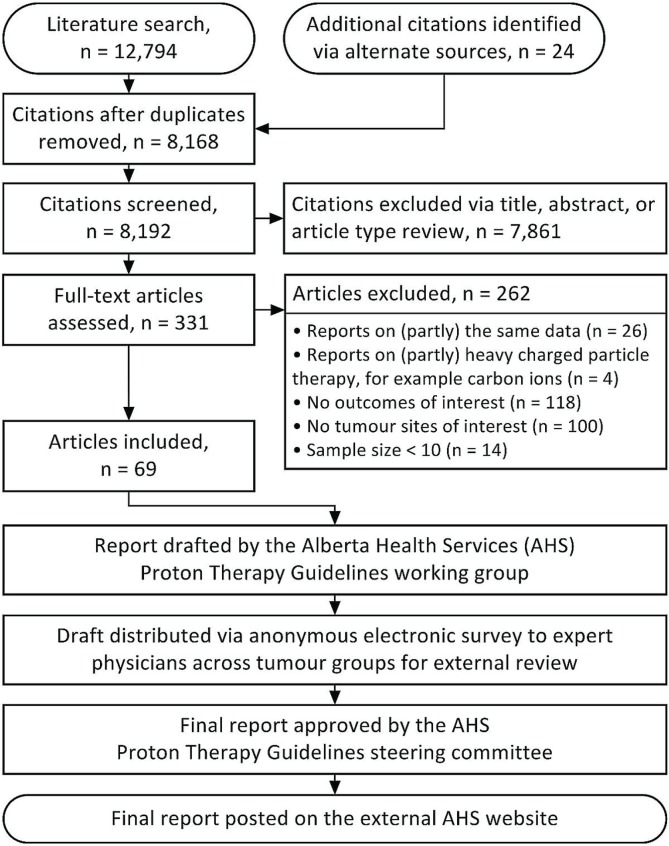
The medline, embase, PubMed, and Cochrane Library databases were systematically searched for studies published in the English language between January 1, 1990, and May 25, 2014. Search terms were “protons” (mesh) and “radiotherapy” (mesh), “proton therapy” (mesh), “proton beam” (key word), “particle beam therapy” (key word), or “charged particle therapy” (key word). Two reviewers (XK, SP) screened citations for studies evaluating clinical outcomes after pbt (Figure 1). Reference lists of publications and personal files were searched for additional citations.
FIGURE 1.
Literature search, draft preparation, and validation process.
A search for ongoing trials at http://clinicaltrials.gov/, performed May 25, 2014, identified 218 trials involving pbt, including 20 randomized controlled trials (rcts), of which only 6 are comparing pbt with photon radiation therapy (rt).
2.2. Recommendation Development
The present report was produced by the Alberta Health Services (ahs) Cancer Care Proton Therapy Guidelines working group through review and interpretation of evidence. The working group was tasked with drafting guidelines and clinical pathways for the care of patients who could potentially be candidates for pbt. The draft report was distributed by electronic survey to 17 Alberta physicians (59% response rate) from across tumour groups for external review. Feedback was incorporated into the draft, which was then approved by the steering committee and posted on the external ahs Web site in March 2013 (updated in June 2014).
The report was developed as part of the mandate of the ahs Proton Therapy Referral Committee to provide advice to the Out-of-Country Health Services Committee that considers funding for patients referred for medical care to facilities outside Canada.
3. RESULTS
3.1. Pediatrics
Childhood cancer survivors who undergo rt are at high risk of deleterious effects on growth and development and of tissue late effects, including secondary malignancies5. Models strongly suggest a lower risk of secondary malignancies in children treated with pbt because of improved dose distribution and a lower volume of normal tissue exposed to radiation6,7. Preliminary data from a review of 86 children with retinoblastoma demonstrated a lower 10-year cumulative incidence of rt-associated or in-field secondary malignancies with pbt than with photon rt (0% vs. 14%, p = 0.015)8. A review of 558 patients treated at the (then) Harvard Cyclotron Laboratory, matched to 558 patients from the Surveillance, Epidemiology, and End Results database who received photon rt, demonstrated fewer subsequent cancer events after pbt (4.2% vs. 7.5%; adjusted hazard ratio: 0.52; p = 0.009)9. However, limitations in epidemiologic data prevent conclusions being drawn10.
The rational for using pbt for central nervous system (cns) lesions is compelling. Compared with photon intensity-modulated rt (imrt), pbt can achieve substantial sparing of normal tissue during craniospinal irradiation in medulloblastoma (including the cochlea and heart11) and during boost therapy (hippocampus12 and subventricular zone13). In Monte Carlo simulations comparing pbt with photon rt for predicted reductions in ototoxicity, endocrine deficiencies, cardiac disease, and secondary malignancies in medulloblastoma survivors, pbt was found to be cost-effective and to be associated with greater quality-adjusted life years14. In 96% of simulations, pbt outperformed photon rt. The significant intellectual and academic decline that follow cranial rt15 were not modelled because of a lack of cost data for productivity loss secondary to cognitive decline. Despite concerns that linear energy transfer variations in proton beams can alter patterns of failure, a review of 109 children with medulloblastoma treated with pbt suggested that the patterns of failure were similar to those with photon rt16.
For craniopharyngiomas, planning studies show that, compared with imrt, pbt delivers a lower integral dose to the hippocampus, dentate gyrus, and subventricular zone17 and a lower dose to the optic chiasm, cochlea, brain, and scanned body18. In 5 children and 10 adults treated with combined proton–photon rt, Fitzek et al.19 reported actuarial 5- and 10-year local control rates of 93% and 85% respectively.
Young age, proximity to critical structures, and expectation for a high tumour control rate make pbt an ideal modality for children with ependymoma20. A review of 70 children with intracranial ependymoma treated with pbt reported high rates of 3-year local control and overall survival (83% and 95% respectively) at a median follow-up of 46 months20. Few children treated with pbt developed growth hormone deficiency (n = 2), central hypothyroidism (n = 1), or hearing loss (n = 2). Amsbaugh et al.21 reported local control and overall survival rates of 100% at a mean follow-up of 26 months in 8 children with spinal ependymoma treated with pbt. No patients experienced grade 3 or greater adverse events.
Studies of tumours outside the cns—including skull base chordoma and chondrosarcoma22,23, uveal melanoma23,24, germ-cell tumours25, high-risk neuroblastoma26,27, parameningeal rhabdomyosarcoma28, bladder and prostate rhabdomyosarcoma29, other soft tissue sarcomas30, Ewing sarcoma31, and mediastinal Hodgkin lymphoma32—uniformly showed that pbt is well tolerated, with local control rates similar to or higher than those achieved with photon rt. Although planning studies and early clinical results are promising, long-term data supporting a clear advantage of pbt over photon rt are not yet available. Increased enrollment into clinical trials, including multicentre and registry trials with appropriate statistical designs and long-term follow-up, is needed33.
3.2. Chordoma and Chondrosarcoma
Chordoma and chondrosarcoma often occur in close proximity to critical neural tissues such as the optic pathway, brainstem, or sacral plexus, posing challenges in the delivery of curative doses of rt. Historical series of photon rt demonstrated 5-year local control rates in the 20% range34,35, which improved in small series of stereotactic36 and image-guided rt37 (published only in abstract form) to 50% and 77% respectively for skull base chordoma and to 100% and 93% respectively for skull base chondrosarcomas. A review of 621 patients treated with combined proton–photon rt at the Harvard Cyclotron Laboratory reported 5-year local relapse-free and overall survival rates of 73% and 80% respectively for skull base chordoma and 98% and 91% respectively for skull base chondrosarcomas38. Similar results from the Paul Scherrer Institute were reported with spot-scanned intensity-modulated proton therapy, including 94% freedom from grade 3 or 4 toxicity at 5 years39. Full-dose re-irradiation for recurrent or progressive chordoma, with or without salvage surgery, demonstrated encouraging 2-year rates of local control (85%) and overall survival (80%), with late grade 3 or 4 toxicity occurring in 3 of 16 cases40.
Delaney et al. reported long-term results of a phase ii study of combined proton–photon rt for spinal chordoma, chondrosarcoma, and other sarcomas. The 8-year actuarial local control was 85% for primary tumours41. Randomized data comparing proton with photon rt are not available. Differences in outcome for those rt modalities are confounded by extent of resection, dose escalation, and patient selection biases.
3.3. Intraocular Melanoma
Management of intraocular melanoma aims to preserve the eye and functional vision while achieving a high rate of tumour control. Protons are useful in treating larger tumours or tumours close to the fovea or optic disc that are not suitable for plaque brachytherapy. Local control and eye preservation rates exceed 90% at 5 years42–46. In 59 patients treated with pbt at triumf in Vancouver, the 5-year actuarial local control rate was 91% (97% in patients treated with 60 GyE), with an overall eye conservation rate of 80%46. The 5-year actuarial rate of neovascular glaucoma was 31% at a median follow-up of 63 months. Radiation retinopathy (74%) and optic neuropathy (64%) were common in-field adverse events. Those results compare favourably with results from series using stereotactic photon rt, which spares the patient from surgical insertion of localization clips47,48. However, in the Canadian series, Krema et al.47 reported higher actuarial rates of neovascular glaucoma (42% after a short median follow-up of 37 months) than were reported with proton rt46.
A single-centre review of 2069 patients treated with pbt demonstrated a 15-year actuarial local control rate of 95%49. The cumulative rate of enucleation was 16%, most frequently because of neovascular glaucoma, blindness with ocular discomfort, or local recurrence. In an effort to reduce toxicity, Gragoudas et al.50 randomized 188 patients to standard or reduced-dose pbt and observed less visual field loss, with maintenance of local control and metastatic death rates, in those receiving the lower dose. In a review of 597 patients, Wilson and Hungerford51 reported similar local control rates with pbt and , 125I plaque brachytherapy. A review of 73 patients with recurrence after pbt suggested that, compared with enucleation, re-irradiation with pbt does not compromise overall survival52.
3.4. Central Nervous System
The dosimetric advantages of pbt compared with photon rt include the former’s ability to deliver high doses with steeper gradients to nearby critical structures. Whether those advantages result in a reduction in late effects such as neurocognitive function, vascular events, and second malignancies requires evaluation. For high-grade glioma, pbt has resulted in survival times typical for highly selected patients53, but pbt is likely more useful in the treatment of benign tumours such as arteriovenous malformations (avms), meningiomas, acoustic neuromas, and pituitary adenomas, where long-term survivors are at risk of significant late effects, including secondary malignancies.
Among 248 patients with 254 cerebral avms (23% in deep locations) treated with single-fraction proton stereotactic radiosurgery (psrs), the median time to total obliteration was 31 months, and the 5- and 10-year cumulative incidences of total obliteration were 70% and 91% respectively, comparing favourably with obliteration rates reported using photon radio-surgery, including Gamma Knife (Elekta, Stockholm, Sweden)54. In 59 patients with high-risk cerebral avms treated with two-fraction psrs because of large size or eloquent brain location, median time to total obliteration was long at 62 months, with a low 5-year actuarial total obliteration rate of 33%55. Median time to obliteration was similar (49 months) in 44 children with high-risk cerebral avms treated with psrs56. Those data suggest that avms can be safely treated with psrs; however, no randomized comparisons between proton and photon rt have been published.
Studies of benign meningiomas treated with pbt show local control rates that are comparable to those achieved with photon rt57–60. In an older study, the use of pbt without modern spot-scanning and image-guidance appeared to be associated with worse morbidity than was seen with contemporaneous photon rt60. For acoustic neuromas, 100% local control at a mean follow-up of 34 months was observed in 30 patients with poor hearing treated with pbt61. Among 88 patients treated with single-fraction psrs, the 5-year actuarial local control was 94%62. The 5-year actuarial rates for preservation of normal facial and trigeminal nerve function were 91% and 89% respectively and appeared to be higher than those seen with low-dose photon radiosurgery63, but the differences could have been a result of patient selection.
Proton craniospinal irradiation has been proposed to spare dose to vertebral bodies and to reduce acute gastrointestinal and hematologic toxicity in adults64. In 40 adults with medulloblastoma, less nausea and vomiting, weight loss, and hematologic toxicity was associated with proton craniospinal irradiation than with photon rt, with 2-year overall survival being similar65.
3.5. Head and Neck
Multiple in silico studies have demonstrated dosimetric advantages for pbt in head-and-neck cancers, but clinical data are limited1. Data for sinonasal tumours are particularly encouraging66–69. Resto et al.66 reviewed 102 patients with locally advanced sinonasal tumours of varying histologies treated with pbt alone or combined with photons. The 5-year rates of local control and overall survival were, respectively, 95% and 90% with complete resection, 82% and 53% with partial resection, and 87% and 49% with biopsy alone. Truong et al.69 reported 2-year local control and overall survival rates of 86% and 53% respectively in 20 patients with primary sphenoid sinus tumours treated with pbt. A meta-analysis of 86 observational and 8 in silico studies demonstrated that, in paranasal and sinonasal cancer, 5-year local control rates were significantly higher for treatment with pbt than for treatment with imrt (88% vs. 66%, p = 0.035)70. In other head-and-neck cancers, limited data indicate that toxicity tends to be lower with pbt and that rates of local control and survival are similar to those achieved with photon rt70.
3.6. Prostate
More than 2000 men with prostate cancer treated with pbt have been reported, and many patients travel considerable distances for pbt71. Three rcts to test the efficacy of dose escalation using pbt have compared combined proton–photon rt with photon rt72,73. Shipley et al.72 treated 202 advanced-stage patients with 4-field photon rt (50.4 Gy), then either proton (25.2 GyE) or photon (16.8 Gy) boost rt. An increase in local control with higher dose was noted for subjects with high-grade disease. In the prog-9509 study, 393 patients with clinically localized prostate cancer received pbt boosts of either 19.6 GyE or 28.8 GyE, and 4-field photon rt (50.4 Gy)73. Higher rt reduced the 10-year biochemical failure rate (7.1% vs. 28.2%, p < 0.0001) without increasing grade 3 or greater late urinary or rectal morbidity. A case-match analysis of the 196 men on the high-dose arm of that trial and 203 similar men treated with brachytherapy revealed similar 8-year biochemical failure rates74. Kim et al.75 randomized 88 T1–3 prostate cancer patients to 5 hypofractionated arms that showed similar biochemical control rates and low rates of grade 3 or greater toxicity (2%); however, a control arm of standard fractionation was not included.
The University of Florida reported 5-year patient-reported outcomes from three prospective trials of image-guided proton-only therapy76. Prospectively collected quality-of-life scores during the first 2 years were similar in 1243 men after pbt and 204 men after imrt77. Gray et al.78 reported that pbt, conformal photon rt, and imrt all result in similar clinically meaningful reductions in bowel (but not urinary) quality-of-life scores at 24 months.
Retrospective data from the Surveillance, Epidemiology, and End Results database comparing pbt with imrt are controversial79,80. A review of 27,647 Medicare beneficiaries demonstrated no reductions in toxicity at 12 months after treatment and substantially higher cost for pbt compared with imrt71. Dosimetric studies suggest that the greatest benefit of using pbt is lower mean integral dose, potentially reducing the risk of secondary malignancies81, but clinical data are lacking. Currently, pbt appears to hold no clear benefit over imrt for the management of patients with prostate cancer.
3.7. Other Sites
Other sites—including breast and lung cancers, gastrointestinal tract malignancies, and lymphoma—are reviewed in the full report available on the external Web site http://www.albertahealthservices.ca/hp/if-hp-cancer-guide-rt002-proton-beam-rt.pdf. For those disease sites, pbt demonstrates no clear advantages over photon rt.
4. DISCUSSION
Adoption of new technologies in situations of uncertain clinical benefit is hotly debated82–84. Many innovations, including cobalt-60 units, linear accelerators, electron beams, imrt, and image-guided rt, have entered into clinical practice without phase iii rct evidence82. The guiding principle for those advances was to deliver doses “as low as reasonably achievable” (alara principle). The current situation with respect to pbt is similar, with numerous dosimetric studies demonstrating potential advantages with pbt85. It has been argued that randomization between proton and photon rt would be unethical because normal tissues would be exposed to extra dose without any anticipated gain in the tumour control achieved with photons82. Fully informed patients aware that the risk of radiation injury increases with dose and volume irradiated might, appropriately, be unwilling to enrol in rcts. Goitein and Cox83 have questioned whether equipoise, a state of genuine uncertainty about the superiority of one arm or the other that ethically justifies a trial between two treatments86, can be met in this situation, but others disagree84. Uncertainties about the range of protons in tissue (arising from patient set-up and motion, beam delivery, and dose calculation), especially in the spot-scanning of moving targets, could limit the physical benefits of pbt87.
Despite the controversies, efforts to generate high-level evidence are under way, including six rcts comparing proton and photon rt. The multicentre partiqol rct in patients with low-risk and low-to-intermediate–risk prostate cancer is comparing bowel quality-of-life as the primary endpoint (http://clinicaltrials.gov/show/NCT01617161). The Radiation Therapy Oncology Group is comparing overall survival in patients with inoperable non-small-cell lung cancer (http://clinicaltrials.gov/show/NCT01993810). These studies include cost effectiveness and quality-of-life as secondary endpoints. Enrollment at the MD Anderson Cancer Centre into four rcts of intensity-modulated proton therapy compared with imrt for glioblastoma (http://clinicaltrials.gov/show/NCT01854554), head-and-neck cancer (http://clinicaltrials.gov/show/NCT01893307), and esophageal cancer (http://clinicaltrials.gov/show/NCT01512589), and stereotactic body proton versus photon rt for non-small-cell lung cancer (http://clinicaltrials.gov/show/NCT01511081) include late effects as a primary endpoint. Large registry studies collecting quality-of-life data in pediatric (http://clinicaltrials.gov/show/NCT01115777) and adult patients (http://clinicaltrials.gov/show/NCT01255748) have proceeded. In silico trials, such as those from the rococo consortium88, could help to guide clinical trial development.
The lack of rcts comparing proton with photon rt required our working group, like other authors1,89–97, to make recommendations based on prospective cohort and retrospective data. Loeffler et al.1 suggested that patients with the highest priority for charged-particle therapy include those with chordomas or chondrosarcomas of the skull base, large uveal and mucosal melanomas, large unresectable sarcomas, renal cell carcinoma, pancreatic and liver cancers, and most pediatric patients. In 2012, the emerging technology committee of the American Society for Radiation Oncology reported on the benefits of pbt for large ocular melanomas and chordomas and the dosimetric benefits for craniospinal irradiation in pediatric patients89. That report suggested the superiority of proton over photon rt for pediatric cns tumours; however, the data were insufficient to support a firm recommendation. The authors suggested that evidence was also insufficient to recommend pbt outside of clinical trials in head-and-neck, lung, gastrointestinal, and non-cns pediatric cancers. Three systematic reviews published in 2007 reported insufficient evidence to recommend pbt for most disease sites90–92; however, one concluded that the evidence was sufficient to support pbt in chordomas and large ocular tumours, while not reviewing pediatric indications92.
In June 2014, the American Society for Radiation Oncology released a model policy to address coverage for pbt93. Their policy considers pbt reasonable when clinical benefit can be expected from the sparing of surrounding normal tissue that cannot be adequately achieved using photon rt—for example, when the target volume is close to one or more critical structures and a steep dose gradient outside the target must be achieved, when a decrease in dose inhomogeneity is needed to avoid an excessive dose hotspot, when a photon technique would increase the risk of clinically meaningful normal-tissue toxicity, or when the same or an immediately adjacent area has previously been irradiated. England, Denmark, and the Netherlands have released national guidelines for pbt referral (Table ii).
TABLE II.
Recommended indications for proton-beam therapy from other nations
| Nation | Recommendations | |
|---|---|---|
| United States (astro)a,93 | Group 1:
|
Group 2: All other indications are suitable when the patient is enrolled in a clinical trial or patient registry. |
| Englandc,94 | All ages:
|
Pediatric (<16years of age):
|
| Denmark a,95 | Adults:
|
Children and young adolescents:
|
| Netherlands a,96 | Standard indications:
|
Potential indications:
|
Indications for proton therapy within a clinical trial or patient registry are not listed.
Where the spinal cord would exceed the tolerance with photon therapy or has previously been irradiated.
Additional requirements: curative intent, good performance status (World Health Organization 0–1), no other coincident diagnoses likely to limit 5-year survival or to make a prolonged period abroad difficult to manage, no metastatic disease, no re-treatment cases, weight 150 kg or less. Rhabdomyosarcoma includes orbital, parameningeal, head and neck, and pelvic sites only.
astro = American Society for Radiation Oncology
Factors other than diagnosis should be taken into account when deciding whether pbt confers a reasonable expectation of benefit over photon rt. The working group recommends that, to ensure that referred patients will have sufficient risk of late effects that could be reduced with pbt, the treatment intent should be curative and the patient’s expected survival should be 5 years or more. In Alberta, criteria do not specifically exclude patients with metastatic disease or cases involving re-irradiation (which are excluded in the English guidelines94). Not all patients will be able and willing to relocate to a foreign city, often for 6–8 weeks, for pbt treatment planning and delivery. The psychosocial, occupational, and financial consequences patients endure while away from home cannot be overstated85. Fully informed patients should be told of the lack of high-level evidence supporting pbt. Patients or parents of pediatric patients who cannot travel for pbt for whatever reason could potentially be left with lifelong guilt that they did not undergo “optimal” treatment85. The working group recommends that every referral be discussed by a multidisciplinary team to provide transparency and accountability in the selection process. In Alberta, that team requests generation of proton and photon rt treatment plans for an assessment of the magnitude of the potential benefit of pbt in cases in which the dosimetric benefit of pbt is unclear.
Proton-beam technology is an evolving field. Passive scattering to produce a proton field is used in most facilities currently in operation98,99. Most published clinical studies are of patients treated with such fields. Faster energy changes between neighboring layers and overall dose delivery are advantages of passive scattering, but the need for patient-specific collimators (apertures) to laterally shape the beam reduces clinical efficiency100. In uniform scanning, a proton pencil beam is magnetically scanned in lateral directions to produce a large field101,102. Because no scattering material is in the beam path, the maximum range of such beams is slightly higher, but as in passive scattering, patient-specific collimators are needed for uniform scanning100. In spot scanning, a proton pencil beam is scanned over the target without the need for an aperture or compensator103. This technology, available in a limited number of facilities, allows for the delivery of intensity-modulated proton therapy. Relative to imrt, neutron contamination from passively-scattered proton beams potentially increases the risk of secondary malignancies5, but spot-scanning drastically reduces neutron production104. An evaluation of whether young children should be referred only to spot-scanning facilities is required.
The economics of referral will become increasingly important as patient volumes increase. In Alberta, the number of referrals more than doubled in the year after recommendations were posted on the external Web site (Table iii). The working group recommends that patients with intraocular melanomas be sent to the triumf facility in Vancouver to minimize out-of-country costs. Cost savings for other diagnoses could potentially be achieved by negotiation of provincial contracts with one or more preferred pbt providers. Referral to a single facility could also potentially promote communication and research collaboration105. Pediatric referrals should be made to a facility with a multidisciplinary pediatric oncology team. A national contract with a preferred provider incorporating provincial cancer agencies and ministries of health could be negotiated, but would depend on the support of all stake-holders. If the 2013 Alberta rates of pbt utilization and health expenditure (11 referrals at a mean cost per referral of approximately $200,000) are extrapolated to Canada (based on 2013 population estimates106), the nationwide cost for an estimated 96 referrals would be $19.2 million annually and growing. Referral patterns and actual costs across Canada have not yet been studied.
TABLE III.
Patients referred out-of-country for proton-beam therapy from Alberta, 2009–2013
| Fiscal yeara |
Referrals
|
||
|---|---|---|---|
| Funding applications | Cases denied | Cases funded | |
| 2009 | 5 | 1 | 4 |
| 2010 | 4 | 0 | 4 |
| 2011 | 7 | 2 | 5 |
| 2012 | 4 | 0 | 4 |
| 2013b | 11 | 0 | 11 |
Each fiscal year starts April 1 and ends March 31 of the following year.
Recommendations for referral were posted on the external Web site in March 2013.
5. SUMMARY
Evidence from a literature review, consensus of expert opinion, feedback obtained through a review process, and final approval given by the steering committee form the basis of these recommendations, which were completed in March 2013 and updated in June 2014.
5.1. Target Population
These recommendations apply to pediatric and adult patients being considered for treatment with rt.
5.2. Recommendations
Factors other than diagnosis should be taken into account in assessing whether pbt confers a reasonable expectation of clinical benefit over photon therapy such as imrt, stereotactic rt, and brachytherapy.
- These general requirements for referral and approval of funding must all be met:
- The treatment is being delivered with curative intent.
- There is a reasonable expectation that overall survival will reach or exceed 5 years.
- The patient’s Eastern Cooperative Oncology Group performance status is 0–2.
- The patient is able and willing to travel.
- Commonly accepted indications for referral include
- chordoma and chondrosarcoma;
- intraocular melanomas that are not suitable for plaque brachytherapy; and
- tumours of children and adolescents, including those requiring craniospinal irradiation, low-grade glioma, ependymoma, craniopharyngioma, germ-cell tumours, pituitary and pineal tumours (not pineoblastomas), rhabdomyosarcoma, Ewing sarcoma, pelvic sarcomas, and mediastinal lymphoma.
- Indications in adults with possible benefit from referral include
- benign tumours of the cns, including avm, benign meningioma, acoustic neuroma, pituitary and pineal tumours (not pineoblastomas), and craniopharyngioma; and
- paranasal sinus and nasal cavity tumours.
Patients with other head-and-neck, breast, lung, gastrointestinal tract, and pelvic cancers, including prostate cancer, are not recommended for routine referral because of an insufficient evidence base. However, individual cases of any diagnosis can be considered in a multidisciplinary setting.
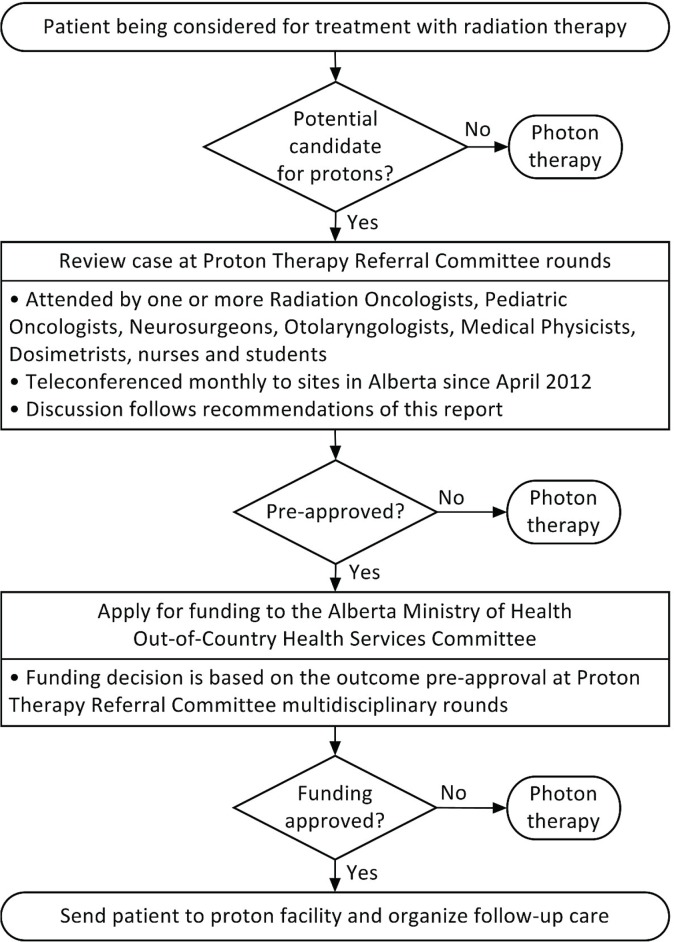
All referred cases should be discussed by a multidisciplinary team that includes a radiation oncologist. After that discussion, approval of funding is required by most provincial ministries of health. Once approved, responsibility for the referral, ongoing communication with the pbt facility, and post-treatment follow-up remains with the referring oncologist (Figure 2).
Patients with intraocular melanomas should be referred to the triumf Proton Treatment Facility in Vancouver. Other patients will require referral to a proton facility outside Canada. A current list of facilities in operation is available on the Particle Therapy Co-Operative Group Web site2.
FIGURE 2.
Clinical care pathway resulting from implementation of the recommendations in Alberta.
5.3. Qualifying Statement
Funding for referral for pbt is generally considered on a case-by-case basis by the Ministry of Health in each province. In Alberta, the Out-of-Country Health Services Committee, a regulated committee operating at arm’s length from the Ministry of Health and Alberta Health Services, reviews applications for hospital and physician services that are not available in Canada. Applications for pbt referral require documentation of case review at the ahs Proton Therapy Referral Committee multidisciplinary rounds, where discussion follows the recommendations reported here.
6. ACKNOWLEDGMENTS
The ahs Proton Therapy Referral Committee thanks the members of the ahs Provincial Tumour Programs and the Out-of-Country Health Services Committee, particularly Stella Hoeksema and Dr. Raymond Howard, for their support and feedback on draft versions of the recommendations.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Loeffler JS, Durante M. Charged particle therapy—optimization, challenges and future directions. Nat Rev Clin Oncol. 2013;10:411–24. doi: 10.1038/nrclinonc.2013.79. [DOI] [PubMed] [Google Scholar]
- 2.Particle Therapy Co-Operative Group (ptcog) Home > Facilities in Operation [Web page] Villigen, Switzerland: PTCOG; 2014. [Current version available at: http://www.ptcog.ch/index.php/facilities-in-operation; cited May 25, 2014] [Google Scholar]
- 3.Kerstiens J, Johnstone PA. Proton therapy expansion under current United States reimbursement models. Int J Radiat Oncol Biol Phys. 2014;89:235–40. doi: 10.1016/j.ijrobp.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 4.triumf . Home > Proton Therapy [Web page] Vancouver, BC: TRIUMF; n.d. [Available at: http://www.triumf.ca/proton-therapy; cited May 25, 2014]. [Google Scholar]
- 5.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Howell RM, Giebeler A, Taddei PJ, Mahajan A, Newhauser WD. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol. 2013;58:807–23. doi: 10.1088/0031-9155/58/4/807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paganetti H. Assessment of the risk for developing a second malignancy from scattered and secondary radiation in radiation therapy. Health Phys. 2012;103:652–61. doi: 10.1097/HP.0b013e318261113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi RV, Shih HA, Yeap BY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120:126–33. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Bekelman JE, Schultheiss T, Berrington De Gonzalez A. Subsequent malignancies after photon versus proton radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87:10–12. doi: 10.1016/j.ijrobp.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 11.St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or imrt in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–34. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 12.Brodin NP, Munck af Rosenschold P, Blomstrand M, et al. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro Oncol. 2014;16:594–602. doi: 10.1093/neuonc/not225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomstrand M, Brodin NP, Munck Af Rosenschold P, et al. Estimated clinical benefit of protecting neurogenesis in the developing brain during radiation therapy for pediatric medulloblastoma. Neuro Oncol. 2012;14:882–9. doi: 10.1093/neuonc/nos120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mailhot Vega RB, Kim J, Bussiere M, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer. 2013;119:4299–307. doi: 10.1002/cncr.28322. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16:1129–36. doi: 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi RV, Giantsoudi D, Raiford M, et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys. 2014;88:655–63. doi: 10.1016/j.ijrobp.2013.11.239. [DOI] [PubMed] [Google Scholar]
- 17.Boehling NS, Grosshans DR, Bluett JB, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2012;82:643–52. doi: 10.1016/j.ijrobp.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Beltran C, Roca M, Merchant TE. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys. 2012;82:e281–7. doi: 10.1016/j.ijrobp.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzek MM, Linggood RM, Adams J, Munzenrider JE. Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys. 2006;64:1348–54. doi: 10.1016/j.ijrobp.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol. 2013;15:1552–9. doi: 10.1093/neuonc/not121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amsbaugh MJ, Grosshans DR, McAleer MF, et al. Proton therapy for spinal ependymomas: planning, acute toxicities, and preliminary outcomes. Int J Radiat Oncol Biol Phys. 2012;83:1419–24. doi: 10.1016/j.ijrobp.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Hoch BL, Nielsen GP, Liebsch NJ, Rosenberg AE. Base of skull chordomas in children and adolescents: a clinicopatho-logic study of 73 cases. Am J Surg Pathol. 2006;30:811–18. doi: 10.1097/01.pas.0000209828.39477.ab. [DOI] [PubMed] [Google Scholar]
- 23.Rombi B, Ares C, Hug EB, et al. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2013;86:578–84. doi: 10.1016/j.ijrobp.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Petrovic A, Bergin C, Schalenbourg A, Goitein G, Zografos L. Proton therapy for uveal melanoma in 43 juvenile patients: long-term results. Ophthalmology. 2014;121:898–904. doi: 10.1016/j.ophtha.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald SM, Trofimov A, Safai S, et al. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79:121–9. doi: 10.1016/j.ijrobp.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 26.Hill–Kayser C, Tochner Z, Both S, et al. Proton versus photon radiation therapy for patients with high-risk neuroblastoma: the need for a customized approach. Pediatr Blood Cancer. 2013;60:1606–11. doi: 10.1002/pbc.24606. [DOI] [PubMed] [Google Scholar]
- 27.Hattangadi JA, Rombi B, Yock TI, et al. Proton radio-therapy for high-risk pediatric neuroblastoma: early outcomes and dose comparison. Int J Radiat Oncol Biol Phys. 2012;83:1015–22. doi: 10.1016/j.ijrobp.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Childs SK, Kozak KR, Friedmann AM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82:635–42. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotter SE, Herrup DA, Friedmann A, et al. Proton radio-therapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:1367–73. doi: 10.1016/j.ijrobp.2010.07.1989. [DOI] [PubMed] [Google Scholar]
- 30.Timmermann B, Schuck A, Niggli F, et al. Spot-scanning proton therapy for malignant soft tissue tumors in childhood: first experiences at the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2007;67:497–504. doi: 10.1016/j.ijrobp.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Rombi B, DeLaney TF, MacDonald SM, et al. Proton radiotherapy for pediatric Ewing’s sarcoma: initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1142–8. doi: 10.1016/j.ijrobp.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Hoppe BS, Flampouri S, Zaiden R, et al. Involved-node proton therapy in combined modality therapy for hodgkin lymphoma: results of a phase 2 study. Int J Radiat Oncol Biol Phys. 2014;89:1053–9. doi: 10.1016/j.ijrobp.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Merchant TE. Clinical controversies: proton therapy for pediatric tumors. Semin Radiat Oncol. 2013;23:97–108. doi: 10.1016/j.semradonc.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catton C, O’Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67–72. doi: 10.1016/S0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 35.Romero J, Cardenes H, la Torre A, et al. Chordoma: results of radiation therapy in eighteen patients. Radiother Oncol. 1993;29:27–32. doi: 10.1016/0167-8140(93)90169-9. [DOI] [PubMed] [Google Scholar]
- 36.Debus J, Schulz–Ertner D, Schad L, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys. 2000;47:591–6. doi: 10.1016/S0360-3016(00)00464-8. [DOI] [PubMed] [Google Scholar]
- 37.Masson–Cote L, Bahl G, Atenafu E, Menard C, Millar B, Catton C, et al. High dose photon radiotherapy for skull base chordomas and chondrosarcomas [abstract 1057] Int J Radiat Oncol Biol Phys. 2011;81:S184. doi: 10.1016/j.ijrobp.2011.06.327. [DOI] [Google Scholar]
- 38.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(suppl 2):57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 39.Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–18. doi: 10.1016/j.ijrobp.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 40.McDonald MW, Linton OR, Shah MV. Proton therapy for reir-radiation of progressive or recurrent chordoma. Int J Radiat Oncol Biol Phys. 2013;87:1107–14. doi: 10.1016/j.ijrobp.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 41.Rieken S, Habermehl D, Nikoghosyan A, et al. Assessment of early toxicity and response in patients treated with proton and carbon ion therapy at the Heidelberg ion therapy center using the raster scanning technique. Int J Radiat Oncol Biol Phys. 2011;81:e793–801. doi: 10.1016/j.ijrobp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Munzenrider JE, Verhey LJ, Gragoudas ES, et al. Conservative treatment of uveal melanoma: local recurrence after proton beam therapy. Int J Radiat Oncol Biol Phys. 1989;17:493–8. doi: 10.1016/0360-3016(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 43.Munzenrider JE, Gragoudas ES, Seddon JM, et al. Conservative treatment of uveal melanoma: probability of eye retention after proton treatment. Int J Radiat Oncol Biol Phys. 1988;15:553–8. doi: 10.1016/0360-3016(88)90294-5. [DOI] [PubMed] [Google Scholar]
- 44.Dendale R, Lumbroso–Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma: results of Curie Institut– Orsay proton therapy center (icpo) Int J Radiat Oncol Biol Phys. 2006;65:780–7. doi: 10.1016/j.ijrobp.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Caujolle JP, Paoli V, Chamorey E, et al. Local recurrence after uveal melanoma proton beam therapy: recurrence types and prognostic consequences. Int J Radiat Oncol Biol Phys. 2013;85:1218–24. doi: 10.1016/j.ijrobp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Tran E, Ma R, Paton K, Blackmore E, Pickles T. Outcomes of proton radiation therapy for peripapillary choroidal melanoma at the BC Cancer Agency. Int J Radiat Oncol Biol Phys. 2012;83:1425–31. doi: 10.1016/j.ijrobp.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Krema H, Somani S, Sahgal A, et al. Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol. 2009;93:1172–6. doi: 10.1136/bjo.2008.153429. [DOI] [PubMed] [Google Scholar]
- 48.Dieckmann K, Georg D, Zehetmayer M, Bogner J, Georgopoulos M, Potter R. linac based stereotactic radiotherapy of uveal melanoma: 4 years clinical experience. Radiother Oncol. 2003;67:199–206. doi: 10.1016/S0167-8140(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 49.Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002;120:1665–71. doi: 10.1001/archopht.120.12.1665. [DOI] [PubMed] [Google Scholar]
- 50.Gragoudas ES, Lane AM, Regan S, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol. 2000;118:773–8. doi: 10.1001/archopht.118.6.773. [DOI] [PubMed] [Google Scholar]
- 51.Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–87. doi: 10.1016/S0161-6420(99)90456-6. [DOI] [PubMed] [Google Scholar]
- 52.Marucci L, Ancukiewicz M, Lane AM, Collier JM, Gragoudas ES, Munzenrider JE. Uveal melanoma recurrence after fractionated proton beam therapy: comparison of survival in patients treated with reirradiation or with enucleation. Int J Radiat Oncol Biol Phys. 2011;79:842–6. doi: 10.1016/j.ijrobp.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Mizumoto M, Tsuboi K, Igaki H, et al. Phase i/ii trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2010;77:98–105. doi: 10.1016/j.ijrobp.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 54.Hattangadi–Gluth JA, Chapman PH, Kim D, et al. Single-fraction proton beam stereotactic radiosurgery for cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2014;89:338–46. doi: 10.1016/j.ijrobp.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 55.Hattangadi JA, Chapman PH, Bussiere MR, et al. Planned two-fraction proton beam stereotactic radiosurgery for high-risk inoperable cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2012;83:533–41. doi: 10.1016/j.ijrobp.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Walcott BP, Hattangadi–Gluth JA, Stapleton CJ, Ogilvy CS, Chapman PH, Loeffler JS. Proton beam stereotactic radio-surgery for pediatric cerebral arteriovenous malformations. Neurosurgery. 2014;74:367–74. doi: 10.1227/NEU.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber DC, Schneider R, Goitein G, et al. Spot scanning–based proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2012;83:865–71. doi: 10.1016/j.ijrobp.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 58.Slater JD, Loredo LN, Chung A, et al. Fractionated proton radiotherapy for benign cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2012;83:e633–7. doi: 10.1016/j.ijrobp.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 59.Halasz LM, Bussiere MR, Dennis ER, et al. Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys. 2011;81:1428–35. doi: 10.1016/j.ijrobp.2010.07.1991. [DOI] [PubMed] [Google Scholar]
- 60.Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363–70. doi: 10.1016/S0360-3016(00)01411-5. [DOI] [PubMed] [Google Scholar]
- 61.Bush DA, McAllister CJ, Loredo LN, Johnson WD, Slater JM, Slater JD. Fractionated proton beam radiotherapy for acoustic neuroma. Neurosurgery. 2002;50:270–5. doi: 10.1097/00006123-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Weber DC, Chan AW, Bussiere MR, et al. Proton beam radio-surgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurgery. 2003;53:577–88. doi: 10.1227/01.NEU.0000079369.59219.C0. [DOI] [PubMed] [Google Scholar]
- 63.Combs SE, Laperriere N, Brada M. Clinical controversies: proton radiation therapy for brain and skull base tumors. Semin Radiat Oncol. 2013;23:120–6. doi: 10.1016/j.semradonc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Barney CL, Brown AP, Grosshans DR, et al. Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro Oncol. 2014;16:303–9. doi: 10.1093/neuonc/not155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86:277–84. doi: 10.1016/j.ijrobp.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Resto VA, Chan AW, Deschler DG, Lin DT. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2008;30:222–9. doi: 10.1002/hed.20681. [DOI] [PubMed] [Google Scholar]
- 67.Fukumitsu N, Okumura T, Mizumoto M, et al. Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys. 2012;83:704–11. doi: 10.1016/j.ijrobp.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 68.Zenda S, Kohno R, Kawashima M, et al. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2011;81:1473–8. doi: 10.1016/j.ijrobp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies: treatment outcome and prognostic factors. Head Neck. 2009;31:1297–308. doi: 10.1002/hed.21092. [DOI] [PubMed] [Google Scholar]
- 70.Ramaekers BL, Pijls–Johannesma M, Joore MA, et al. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: comparing photons, carbon-ions and protons. Cancer Treat Rev. 2011;37:185–201. doi: 10.1016/j.ctrv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shipley WU, Verhey LJ, Munzenrider JE, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 73.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coen JJ, Zietman AL, Rossi CJ, et al. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: a case-matched analysis. Int J Radiat Oncol Biol Phys. 2012;82:e25–31. doi: 10.1016/j.ijrobp.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 75.Kim YJ, Cho KH, Pyo HR, et al. A phase ii study of hypofractionated proton therapy for prostate cancer. Acta Oncol. 2013;52:477–85. doi: 10.3109/0284186X.2013.764011. [DOI] [PubMed] [Google Scholar]
- 76.Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:596–602. doi: 10.1016/j.ijrobp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Hoppe BS, Michalski JM, Mendenhall NP, et al. Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer. 2014;120:1076–82. doi: 10.1002/cncr.28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2013;119:1729–35. doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim S, Shen S, Moore DF, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60:908–16. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray L, Henry A, Hoskin P, Siebert FA, Venselaar J, on behalf of the braphyqs/probate group of the gecestro Second primary cancers after radiation for prostate cancer: a review of data from planning studies. Radiat Oncol. 2013;8:172. doi: 10.1186/1748-717X-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suit H, Kooy H, Trofimov A, et al. Should positive phase iii clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother Oncol. 2008;86:148–53. doi: 10.1016/j.radonc.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 83.Goitein M, Cox JD. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol. 2008;26:175–6. doi: 10.1200/JCO.2007.14.4329. [DOI] [PubMed] [Google Scholar]
- 84.Glatstein E, Glick J, Kaiser L, Hahn SM. Should randomized clinical trials be required for proton radiotherapy? An alternative view. J Clin Oncol. 2008;26:2438–9. doi: 10.1200/JCO.2008.17.1843. [DOI] [PubMed] [Google Scholar]
- 85.Wolden SL. Protons for craniospinal radiation: are clinical data important? Int J Radiat Oncol Biol Phys. 2013;87:231–2. doi: 10.1016/j.ijrobp.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 86.Sheehan M, Timlin C, Peach K, et al. Position statement on ethics, equipoise and research on charged particle radiation therapy. J Med Ethics. 2014;40:572–5. doi: 10.1136/medethics-2012-101290. [DOI] [PubMed] [Google Scholar]
- 87.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99–117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roelofs E, Engelsman M, Rasch C, et al. Results of a multi-centric in silico clinical trial (rococo): comparing radiotherapy with photons and protons for non-small cell lung cancer. J Thorac Oncol. 2012;7:165–76. doi: 10.1097/JTO.0b013e31823529fc. [DOI] [PubMed] [Google Scholar]
- 89.Allen AM, Pawlicki T, Dong L, et al. An evidence based review of proton beam therapy: the report of astro’s emerging technology committee. Radiother Oncol. 2012;103:8–11. doi: 10.1016/j.radonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Brada M, Pijls–Johannesma M, De Ruysscher D. Proton therapy in clinical practice: current clinical evidence. J Clin Oncol. 2007;25:965–70. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 91.Olsen DR, Bruland OS, Frykholm G, Norderhaug IN. Proton therapy—a systematic review of clinical effectiveness. Radiother Oncol. 2007;83:123–32. doi: 10.1016/j.radonc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 92.De Ruysscher D, Mark Lodge M, Jones B, et al. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother Oncol. 2012;103:5–7. doi: 10.1016/j.radonc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 93.American Society for Radiation Oncology (astro) Model Policies: Proton Beam Therapy (PBT) Fairfax, VA: ASTRO; 2014. [Available online at: https://www.astro.org/uploadedFiles/Main_Site/Practice_Management/Reimbursement/ASTRO%20PBT%20Model%20Policy%20FINAL.pdf; cited June 4, 2014]. [Google Scholar]
- 94.U.K. National Health Service (nhs) National Commissioning Group for Highly Specialised Services . Guidance for the Referral of Patients Abroad for NHS Proton Treatment. Leeds, U.K.: NHS; 2011. Ver. 2.3. [Google Scholar]
- 95.Aarhus University . The Danish National Center for Particle Radiotherapy. Aarhus, Denmark: Aarhus University; 2012. [Available online at: http://www.regionmidtjylland.dk/files/Aktuelt/Billeder%20til%20web/Småtterier/Det%20Nationale%20Center%20for%20Partikelterapi%202012.pdf; cited May 25, 2014] [Google Scholar]
- 96.Health Council of the Netherlands . Proton Radiotherapy. The Hague, Netherlands: Health Council of the Netherlands; 2009. [Available online at: http://www.gezondheidsraad.nl/sites/default/files/proton_radiotherapy200917E_0.pdf; cited May 25, 2014] [Google Scholar]
- 97.Institut national d’excellence en santé et an services sociaux (inesss) Note informative : La protonthérapie. Quebec City, QC: INESSS; 2010. [Available online at: https://www.inesss.qc.ca/fileadmin/doc/AETMIS/Rapports/Cancer/NI-2010_03_protontherapie.pdf; cited August 18, 2014.] [Google Scholar]
- 98.Daartz J, Engelsman M, Paganetti H, Bussiere MR. Field size dependence of the output factor in passively scattered proton therapy: influence of range, modulation, air gap, and machine settings. Med Phys. 2009;36:3205–10. doi: 10.1118/1.3152111. [DOI] [PubMed] [Google Scholar]
- 99.Engelsman M, Lu HM, Herrup D, Bussiere M, Kooy HM. Commissioning a passive-scattering proton therapy nozzle for accurate sobp delivery. Med Phys. 2009;36:2172–80. doi: 10.1118/1.3121489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol. 2013;23:88–96. doi: 10.1016/j.semradonc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Farr JB, Mascia AE, Hsi WC, et al. Clinical characterization of a proton beam continuous uniform scanning system with dose layer stacking. Med Phys. 2008;35:4945–54. doi: 10.1118/1.2982248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rana S, Zeidan O, Ramirez E, Rains M, Gao J, Zheng Y. Measurements of lateral penumbra for uniform scanning proton beams under various beam delivery conditions and comparison to the xio treatment planning system. Med Phys. 2013;40:091708. doi: 10.1118/1.4818283. [DOI] [PubMed] [Google Scholar]
- 103.Zhu XR, Poenisch F, Lii M, et al. Commissioning dose computation models for spot scanning proton beams in water for a commercially available treatment planning system. Med Phys. 2013;40:041723. doi: 10.1118/1.4798229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tessa CL, Berger T, Kaderka R, et al. Characterization of the secondary neutron field produced during treatment of an anthropomorphic phantom with X-rays, protons and carbon ions. Phys Med Biol. 2014;59:2111–25. doi: 10.1088/0031-9155/59/8/2111. [DOI] [PubMed] [Google Scholar]
- 105.St. Jude Children’s Research Hospital (stcrh) STCRH and UF Proton Therapy Institute to begin proton therapy clinical trial [Web article] Memphis, TN: STCRH; 2009. [Available at: http://www.stjude.org/stjude/v/index.jsp?vgnextoid=ff768ca830ac4210VgnVCM1000001e0215acRCRD; cited May 25, 2014] [Google Scholar]
- 106.Statistics Canada . Population by year, by province and territory [Web resource] Ottawa, ON: Statistics Canada; 2013. [Available at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm; cited May 25, 2014] [Google Scholar]