Abstract
Purpose
We analyzed patterns and factors associated with receipt of breast and cervical cancer screening in a cohort of colorectal cancer survivors.
Methods
Individuals diagnosed with colorectal cancer in Nova Scotia between January 2001 and December 2005 were eligible for inclusion. Receipt of breast and cervical cancer screening was determined using administrative data. General-population age restrictions were used in the analysis (breast: 40–69 years; cervical: 21–75 years). Kaplan–Meier and Cox proportional hazards models were used to assess time to first screen.
Results
Of 318 and 443 colorectal cancer survivors eligible for the breast and cervical cancer screening analysis respectively, 30.1% [95% confidence interval (ci): 21.2% to 39.0%] never received screening mammography, and 47.9% (95% ci: 37.8% to 58.0%) never received cervical cancer screening during the study period. Receipt of screening before the colorectal cancer diagnosis was strongly associated with receipt of screening after diagnosis (hazard ratio for breast cancer screening: 4.71; 95% ci: 3.42 to 6.51; hazard ratio for cervical cancer screening: 6.83; 95% ci: 4.58 to 10.16).
Conclusions
Many colorectal cancer survivors within general-population screening age recommendations did not receive breast and cervical cancer screening. Future research should focus on survivors who meet age recommendations for population-based cancer screening.
Keywords: Cancer screening, early detection of cancer, neoplasms, second primary, cancer survivorship
1. INTRODUCTION
Since the early 2000s, approximately 840,000 Canadians have been diagnosed with cancer1. Colorectal cancer is the 3rd most common cancer survivorship site, with approximately 105,000 survivors. That high number is the result of both a high incidence (colorectal cancer is the 3rd most frequently diagnosed cancer in both men and women, with 23,900 incident cases per year) and a 65% 5-year relative survival1. With a growing and aging population, the number of new cancer cases in Canada is expected to continue rising1. In addition, people diagnosed with cancer are now living longer than ever2, meaning that a growing number of cancer survivors can be expected to increase the burden on the health care system.
The 2006 U.S. Institute of Medicine report From Cancer Patient to Cancer Survivor: Lost in Transition highlighted the often-overlooked and multiple care needs of cancer survivors, including surveil-lance for local, regional, or distant recurrences of the initial cancer; and screening for new primary cancers at other sites3. Although guidelines for cancer surveillance have been created for many commonly diagnosed cancers (for example, breast4,5, cervical6, endometrial7, colorectal8,9, melanoma10, and prostate11), no guidelines have been developed for the provision of preventive screening for new primary cancers among cancer survivors, despite studies demonstrating that many cancer survivors are at increased risk of developing a second malignancy12–17.
Cancer screening is an important component of preventive health care in defined target populations. Although cancer screening is more frequent for cancer survivors than for the general population, a significant proportion of cancer survivors do not receive screening for new primary cancers18. Little is known about cancer screening practices among Canadian cancer survivors. One Canadian study19 reported that colorectal cancer survivors from Ontario were less likely than a matched non-cancer control population to receive breast cancer screening and no more likely to receive cervical cancer screening.
It is recommended that women 50–69 years of age in Nova Scotia undergo mammography every 2 years for breast cancer screening20. Of N.S. women 50–69 years of age, 48.03% underwent screening mammography in 2006–200721 (the latest two full years also examined during our study). The N.S. breast screening program also targets women 40–49 years of age for annual mammography. A Papanicolaou (Pap) smear is recommended at least every 3 years for women 21–75 years of age for cervical cancer screening22. Of N.S. women 18–69 years of age, 81% had received a Pap smear within the preceding 3 years23.
The objective of the present study was to describe the receipt of breast and cervical cancer screening by colorectal cancer survivors in Nova Scotia and to examine factors associated with such screening practices.
2. METHODS
2.1. Data Sources and Study Cohort
Our retrospective, population-based cohort study used the Canadian Institutes of Health Research and Cancer Care Nova Scotia Team access (Access to Colorectal Cancer Services in Nova Scotia) database24, which includes all individuals diagnosed with colorectal cancer in Nova Scotia between January 1, 2001, and December 31, 2005. The present study included all patients diagnosed with stage i, ii, or iii colorectal cancer. Only those who underwent resection, indicative of curative treatment for colorectal cancer, were included. Because the study was considering receipt of breast and cervical cancer screening, only eligible women were included in the cohort.
We considered the cancer survivorship period to begin immediately after an individual’s colorectal cancer diagnosis. The cancer survivorship period continued until censoring, the end of available data, or the study end date (March 31, 2008, for 62.8% of the cohort). Censoring dates were calculated to be 90 days before
diagnosis of a new primary cancer,
evidence of colorectal cancer recurrence,
initiation of chemotherapy or radiotherapy more than 365 days after diagnosis,
surgical resection of common distant recurrence sites (liver and lung),
enrollment in a palliative care program, and
date of death.
We excluded individuals with evidence of recurrence or advanced disease within the first year after diagnosis, and those with a previous cancer diagnosis. Individuals residing in the Cumberland District Health Authority were excluded, because some of their health care might have been delivered in a nearby province and would not have been captured in the database we used. The cervical cancer screening analysis excluded individuals with a prior hysterectomy, and the hysterectomy date was used as a censoring criterion. Table i summarizes the inclusion, exclusion, and censoring criteria.
TABLE I.
Inclusion, exclusion and censorship criteria
| Factor | Criteria |
|---|---|
| Inclusion | Women diagnosed with stage i, ii, or iii colorectal cancer in Nova Scotia between January 1, 2001, and December 31, 2005 |
| Underwent resection | |
| Exclusion | Evidence of recurrence within the first year after diagnosis |
| Previous cancer diagnosis | |
| Residence in the Cumberland District Health Authority | |
| Censorship | 90 Days before diagnosis of a new primary cancer, upon evidence of colorectal cancer recurrence, at initiation of chemotherapy or radiotherapy more than 365 days after diagnosis, at resection of common distant recurrence sites (liver and lung), upon enrollment in a palliative care program, or at date of death |
| End of study data (March 31, 2008) or date of hysterectomy with removal of cervix (cervical cancer screening cohort only) |
Breast cancer screening was defined as receipt of screening mammography during the survivor-ship period. This information was obtained through the Nova Scotia Breast Screening Program. Only mammography booked through the central mammography booking system is included in the Nova Scotia Breast Screening Program database. Because some locations within Nova Scotia potentially provided screening mammography outside the booking system for part of the study period, we examined potential ascertainment bias by conducting a sensitivity analysis of screening in individuals living in the Capital District Health Authority, which used central mammography booking throughout the study period. Screening tests captured by the Nova Scotia Breast Screening Program differentiate between mammography conducted with screening and with diagnostic intent. We included only screening mammography in the study.
Cervical cancer screening was defined as receipt of a Pap test during survivorship. This information was collected from the Cervical Cancer Prevention Program database, which records all Pap tests conducted in Nova Scotia.
2.2. Statistical Analysis
We used Kaplan–Meier and Cox proportional hazards models to assess time to first breast and cervical cancer screening test. This analysis technique allowed us to account for the variable lengths of follow-up in the data (a result of the staggered entry and censoring of the cancer survivors throughout the study period).
We stratified the cohort into age groups representative of the guidelines for general-population breast and cervical cancer screening, focusing our analyses on survivors within the recommended age groups set out by the N.S. screening guidelines25,26 (breast cancer screening: ages 40–69; cervical cancer screening: ages 21–75). For individuals whose age exceeded the upper age limit in the cancer screening guidelines, we report results separately for those who were less than and greater than 10 years above the recommended upper age limit. Age groups were defined based on the age of each survivor at time of the colorectal cancer diagnosis; survivors did not switch age groups as they aged.
To identify factors that affected time to first breast or cervical cancer screen during the survivor-ship period, we conducted univariate analyses using Cox proportional hazards models. Those univariate comparisons were planned a priori. The following variables were considered: 5-year age group at diagnosis, cancer site (colon vs. rectal), stage (i, ii, or iii), comorbidity (modified Elixhauser scale27: coded as 0, 1, ≥2 comorbid conditions, excluding cancer-related comorbid categories), urban or rural residency, receipt of breast or cervical cancer screening before the colorectal cancer diagnosis (within the preceding 3 years for breast cancer screening and within the preceding 4 years for cervical cancer screening), utilization of a primary care physician (annualized rate, excluding visits within the first year after diagnosis, grouped into quartiles), and oncology specialist utilization (annualized rate, excluding visits within the first year after diagnosis, and rounded into one these categories: 0, 1, ≥2 visits per year). Physician utilization within the first year after diagnosis was likely to have been focused on primary colorectal cancer treatment and was therefore not examined. All proportional hazards models satisfied the proportionality assumption (p > 0.05), with one exception: urban or rural residency in the breast cancer screening cohort (p = 0.02). That exception was analyzed using a stratified Kaplan–Meier curve and log-rank test.
An exploratory analysis examined time to a second screening; it included those who had a first breast or cervical cancer screening event during the survivorship period. We then observed the time to the survivor’s second screening event, with “time zero” being the date of the first screening event.
All analyses were conducted using the Statistical Analysis System (version 8: SAS Institute, Cary, NC, U.S.A.). Statistical significance was considered at α = 0.05. The Cox proportional hazards models had 80% power to detect significant hazard ratios of 1.53 and 1.43 for the breast and cervical cancer age-restricted cohorts respectively. Data access was approved by the Capital District Health Authority and Dalhousie University research ethics boards.
3. RESULTS
Table ii presents the characteristics of the 705 women who were diagnosed with colorectal cancer in Nova Scotia during the study period and who were eligible for inclusion in the analysis. Our age-restricted study cohorts contained 318 and 443 survivors who were diagnosed with colorectal cancer between the ages of 40–69 and 21–75 respectively.
TABLE II.
Characteristics of the study cohort, by screening type
| Characteristic |
Screening group
|
|
|---|---|---|
| Breast cancer | Cervical cancer | |
| Participants (n) | 318 | 443 |
| Time on study (days) | ||
| Median | 990 | 1013 |
| Range | 366–2256 | 366–2270 |
| Age range (years) | 40–69 | 21–75 |
| Age at diagnosis | ||
| Median (years) | 60 | 64 |
| Group [n (%)] | ||
| <50 Years | 40 (12.6) | 46 (10.4) |
| 50–69 Years | 278 (87.4) | 278 (62.7) |
| 70–79 Years | 0 | 119 (26.9) |
| ≥80 Years | 0 | 0 |
| Cancer site [n (%)] | ||
| Colon | 212 (66.7) | 304 (68.6) |
| Rectum | 106 (33.3) | 139 (31.4) |
| Comorbidity [n (%)] | ||
| 0 | 226 (71.0) | 305 (68.9) |
| 1 | 59 (18.6) | 82 (18.5) |
| ≥2 | 33 (10.4) | 56 (12.6) |
| Location [n (%)] | ||
| Urban | 233 (73.3) | 309 (69.8) |
| Rural | 82 (25.8) | 130 (29.3) |
| Missing | 3 (0.9) | 4 (0.9) |
| Stage at diagnosis [n (%)] | ||
| i | 77 (24.2) | 110 (24.8) |
| ii | 124 (39.0) | 172 (38.8) |
| iii | 117 (36.8) | 161 (36.3) |
| Median annualized rate of visits with | ||
| Primary care physician | 7 | 7 |
| Oncology specialist | 1 | 1 |
| Other physicians | 1 | 1 |
| All physicians | 10 | 10 |
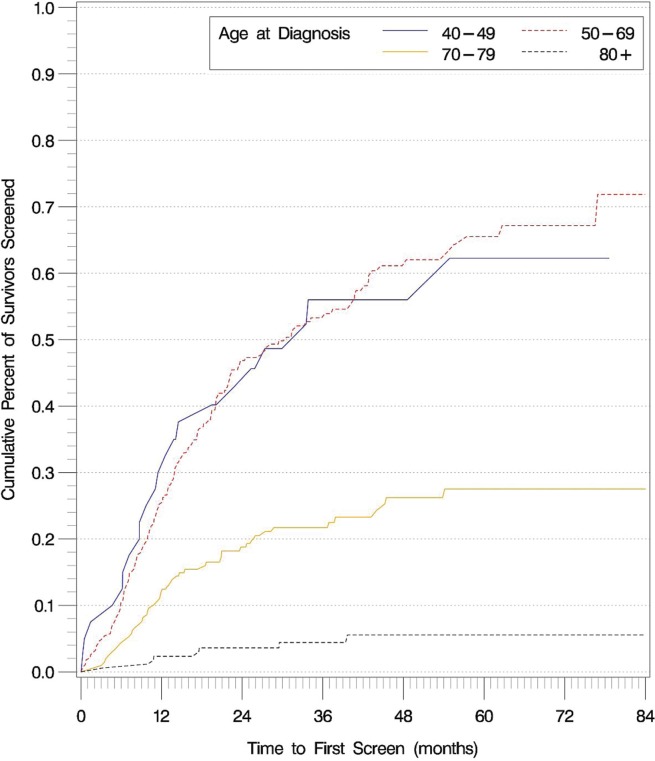
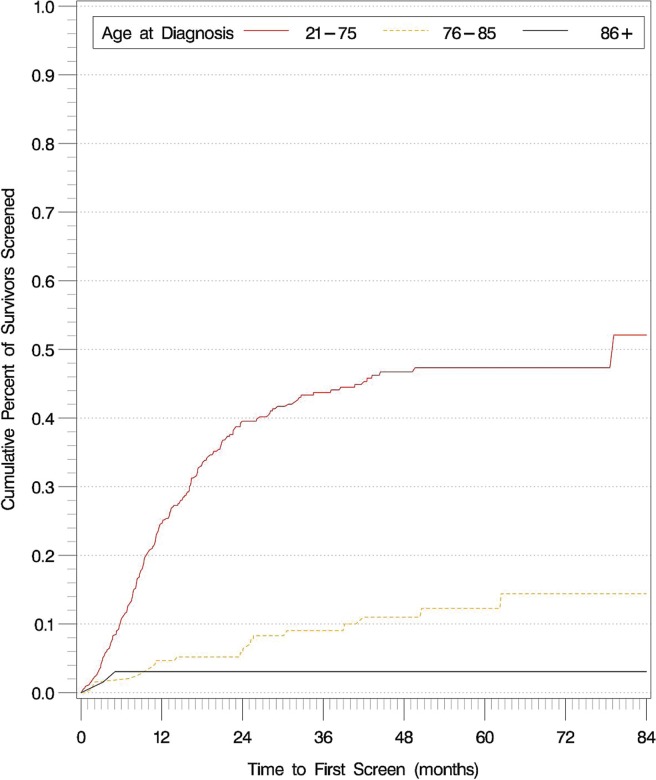
Figures 1 and 2 show the time to first breast and cervical screens for the entire colorectal cancer survivor cohort, stratified by age. No significant difference in time to first breast screen was observed between the 40–49 and 50–69 age groups (p = 0.99). Compared with colorectal cancer survivors who were within the guideline-recommended age groups, those older than the guideline-recommended age were less likely to receive both breast and cervical cancer screening (p < 0.001 for all comparisons).
FIGURE 1.
Time to first breast cancer screen in the entire colorectal cancer survivor cohort, stratified by age at diagnosis.
FIGURE 2.
Time to first cervical cancer screen in the entire colorectal cancer survivor cohort, stratified by age at diagnosis.
A significant proportion of colorectal cancer survivors never received breast or cervical cancer screening during the study period and after their colorectal cancer diagnosis: 30.1% of survivors 40–69 years of age [95% confidence interval (ci): 21.2% to 39.0%] never received a screening mammogram, and 47.9% of survivors 21–75 years of age (95% ci: 37.8% to 58.0%) never received cervical cancer screening.
Table iii presents factors associated with receiving or not receiving breast or cervical cancer screening in the age-restricted cohorts. An older age at diagnosis (per 5-year increase in age at diagnosis) was associated with a lesser likelihood of receiving cervical cancer screening, but was not significantly associated with receiving breast cancer screening [hazard ratio (hr) for cervical cancer screening: 0.76; 95% ci: 0.71 to 0.86; hr for breast cancer screening: 0.92; 95% ci: 0.83 to 1.01].
TABLE III.
Factors associated with use of screeninga, by age-restricted groups
| Variable |
Screening group
|
||||
|---|---|---|---|---|---|
| Breast cancer (ages 40–69) | Cervical cancer (ages 21–75) | ||||
| hr | 95% ci | hr | 95% ci | ||
| Age at diagnosis | Per 5-year increase | 0.98 | 0.96 to 1.00 | 0.95 | 0.94 to 0.96 |
| Cancer site | |||||
| Colon | Reference | Reference | |||
| Rectum | 1.20 | 0.88 to 1.63 | 1.03 | 0.76 to 1.41 | |
| Stage at diagnosis | |||||
| i | Reference | Reference | |||
| ii | 0.71 | 0.49 to 1.03 | 0.96 | 0.68 to 1.36 | |
| iii | 0.86 | 0.60 to 1.25 | 0.73 | 0.50 to 1.06 | |
| Comorbidity | |||||
| 0 | Reference | Reference | |||
| 1 | 0.86 | 0.57 to 1.27 | 0.83 | 0.57 to 1.21 | |
| ≥2 | 0.96 | 0.60 to 1.56 | 0.65 | 0.40 to 1.07 | |
| Location | |||||
| Urban | Reference | Reference | |||
| Rural | p=0.10b | 0.75 | 0.54 to 1.04 | ||
| Pre-diagnosis screeningc | |||||
| No | Reference | Reference | |||
| Yes | 4.71 | 3.42 to 6.51 | 6.83 | 4.58 to 10.16 | |
| Median annualized rate of visits with | |||||
| Primary care physician | Per quartile | ||||
| 1 (low) | Reference | Reference | |||
| 2 | 1.04 | 0.70 to 1.55 | 1.14 | 0.78 to 1.67 | |
| 3 | 1.46 | 0.96 to 2.28 | 0.86 | 0.56 to 1.33 | |
| 4 (high) | 1.10 | 0.72 to 1.68 | 1.08 | 0.72 to 1.64 | |
| Oncology specialist | |||||
| 0 | Reference | Reference | |||
| 1 | 1.57 | 1.05 to 2.36 | 1.46 | 1.00 to 2.12 | |
| ≥2 | 1.37 | 0.92 to 2.03 | 1.25 | 0.86 to 1.82 | |
Hazard ratios shown in boldface type are statistically significant.
Proportional assumption not met; log-rank test on Kaplan–Meier curves used instead.
For breast cancer, within the 3 years before diagnosis; for cervical cancer, within the preceding 4 years.
Receipt of a pre-diagnosis breast or cervical cancer screening test was highly predictive of receiving the same screening test during the survivorship period (hr for breast cancer screening: 4.71; 95% ci: 3.42 to 6.51; hr for cervical cancer screening: 6.83; 95% ci: 4.58 to 10.16).
Survivors who had an average of 1 oncology specialist visit per year in the survivorship period (excluding the first year after diagnosis) were more likely than those who received fewer follow-up visits from an oncology specialist to receive screening mammography (hr: 1.57; 95% ci: 1.05 to 2.36; p = 0.028) and borderline significantly more likely to receive a Pap test (hr: 1.46; 95% ci: 1.00 to 2.12; p = 0.051). However, no dose–response effect was observed; individuals who visited their oncology specialists more frequently (≥2 times annually) did not receive more frequent screening. No other factors in the analysis were statistically significant.
Cancer survivors who received screening mammography or a Pap test during the study period were very likely to receive a second screen. Of those who received screening mammography during the survivorship period, 89.6% (95% ci: 82.6% to 96.7%) had a second mammogram during the survivorship period, and of those who received a Pap test during the survivor-ship period, 82.2% (95% ci: 74.3% to 90.2%) received a second Pap test during the survivorship period.
We found that, of colorectal cancer survivors within the Capital District Health Authority, 52.9% (95% ci: 44.1% to 61.6%) received screening mammography within the first 2 years after diagnosis; in the entire cohort, 46.4% (95% ci: 40.3% to 52.5%) received screening mammography within the first 2 years after diagnosis, which indicates a small but detectable difference in the screening rate.
4. DISCUSSION
In our population-based study of N.S. colorectal cancer survivors, we found that, of colorectal cancer survivors who were within general-population age recommendations for screening, 30.1% never received screening mammography and 47.9% never received a Pap test during the survivorship period.
The strongest predictive factor for receiving breast or cervical cancer screening was receipt of the same screening test before the colorectal cancer diagnosis. Prior screening has been shown to be a predictive utilization measure of repeat mammography in the general population28, and rates of repeat mammography screening are much higher in the N.S. general population (80.8% vs. 57.6%, 2008–2009 data)29. We observed similar results in our time-to-second-screen analysis, in which 89.6% and 82.2% of survivors who received one screening event during the survivorship period received a second screen during the study period (breast and cervical cancer respectively).
The Nova Scotia Breast Screening Program sends reminder cards to women 40–69 years of age who are enrolled in the screening program, which might be responsible for the high proportion of survivors who received a second breast screen. After age 70, women are no longer sent reminder cards, but can still receive screening mammography.
The organization of screening programs can be a key determinant of screening patterns in cancer survivors. Previous literature has shown that reminders can be an effective method of increasing adherence to cancer screening in the general population30, which is consistent with our findings in a colorectal cancer survivor cohort. Despite the Cervical Cancer Prevention Program not using a reminder card system, we observed results for pre-diagnostic cervical cancer screening and time-to-second-screen that were similar to those for breast cancer screening. One of the top self-reported reasons that women undergo cervical cancer screening is receipt of the reminder card sent by the Nova Scotia Breast Screening Program29.
In the age-restricted analysis, a younger age at diagnosis was predictive of receiving a cervical cancer screen, but not a breast cancer screen. In previously published literature examining cancer survivors, older age was found to be predictive of not receiving cancer screening31–33. However, those studies included cancer survivors who were older than those included in our age-restricted analyses. The most probable reason that we did not see decreased screening in our breast cancer screening analysis is our use of an upper age limit of 69 in the analysis. Given that we found significantly less receipt of breast cancer screening in individuals 70 years of age and older, we would likely have observed a trend similar to that in the published literature had we removed the upper age limit.
In previous studies, increasing numbers of total physician visits33 and primary care visits34 have been shown to correlate with increased cancer screening uptake among cancer survivors. However, primary care and oncology specialist visit rates did not appear to have much influence in our cohort, except for one oncology specialist visit, which had borderline statistical significance in breast and cervical cancer screening. The discrepancy could be a result of our analysis technique in a cohort with variable lengths of follow-up. Previous research in a population of breast cancer survivors showed that physician utilization changes over the course of the survivorship period, with an increasing number of visits with the primary care physician and a decreasing number of visits with the oncology specialist over time35. We measured physician utilization as an annualized rate that would not have captured such changes.
Previous studies in colorectal36 and breast cancer survivors37 demonstrated an association of stage at diagnosis and comorbidity with not receiving cancer screening. In our cohort, those predictors were not significantly associated with receipt of cancer screening. It is possible that we did not observe an effect of comorbid conditions because our cohort was restricted to much younger ages than are seen in other studies (40–69 years vs. 67–79 years), with the result that the influence of comorbidity might have been lessened. It is less clear why stage at diagnosis did not have an effect on screening receipt in our cohort.
We focused our analyses on survivors within recommended general-population age limits for breast and cervical cancer screening. When we examined receipt of screening in individuals just above the age recommendations, the screening rate was small but not nil. That finding could potentially present a bias in studies comparing cancer survivors with a general population, because cancer survivors might preferentially be encouraged to receive screening above general-population age recommendations because of their higher risk for developing secondary cancers. For that reason, we recommend that future research use age-restricted cohorts until screening guidelines specifically for cancer survivors are developed.
We are aware of no studies that have examined whether the benefits of cancer screening differ for cancer survivors and a general population, but the existing literature shows that colorectal cancer survivors are, at best, as likely as a general population to develop secondary breast and cervical cancers and are often at a greater risk than the general population of developing second primary cancers15–17. Future research should seek to compare the survival benefits of screening for new primary cancers in cancer survivors and in general populations.
Our study has many strengths. We used data from numerous administrative databases to create explicit inclusion, exclusion, and censoring criteria. The censoring criteria allowed us to identify colorectal cancer survivors with variable lengths of follow-up and to use survival analysis techniques on the resulting data. Our source population included all incident cases of colorectal cancer in Nova Scotia diagnosed during 2001–2005, eliminating the potential response bias inherent in many self-reported surveys. We were also able to differentiate screening and diagnostic mammography, and we included only screening mammography in our analyses.
The limitations of our study largely ref lect limitations in the data set. One limitation was our inability to include all mammograms conducted within Nova Scotia into our study cohort, because mammography conducted outside the screening program was not captured. That limitation was confirmed by our observation that a slightly higher proportion of cancer survivors were screened within 2 years after diagnosis in the Capital District Health Authority than in the cohort overall. No data are available to indicate whether that situation represents a data limitation or an inherent difference in population screening behaviour. Another limitation in our study was data availability. On the study end date (March 31, 2008), 62.8% of the cohort was censored because of a lack of data. Given that we included all incident colorectal cancer cases to December 31, 2005, some survivors analyzed in our cohort had less than 2.5 years of follow-up on study. Furthermore, because we lacked access to test results and family history, we could not examine possible overutilization of cancer screening in the cohort. We could not measure clinical or prognostic factors such as frailty, postoperative complications, or treatment side effects that might have influenced receipt of cancer screening. Another limitation of our study is power. Although our study population included all women diagnosed with colorectal cancer in Nova Scotia during a 5-year period, we were able to reach only 80% power to detect hazard ratios of 1.53 and 1.43 in our Cox proportional hazards models for breast and cervical cancer screening respectively.
5. CONCLUSIONS
In our population-based study of colorectal cancer survivors in Nova Scotia, we found that a significant proportion of cancer survivors within general-population screening age recommendations did not receive breast and cervical cancer screening. Receipt of breast and cervical cancer screening varied substantially for individuals within and outside the age recommendations for population-based screening. Future research in this area should focus on individuals who meet the age recommendations for population-based cancer screening.
6. ACKNOWLEDGMENTS
We thank Dr. Judy Caines and Dr. Jennifer Payne of the Nova Scotia Breast Screening Program for their assistance in interpreting the data obtained from the Nova Scotia Breast Screening Program in the context of this study.
7. CONFLICT OF INTEREST DISCLOSURES
The authors of this manuscript have no financial conflicts of interest to disclose.
8. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee. Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 2.Ellison LF, Wilkins K. An update on cancer survival. Health Rep. 2010;21:55–60. [PubMed] [Google Scholar]
- 3.Hewitt ME, Gantz PA, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 4.Grunfeld E, Dhesy–Thind S, Levine M, on behalf of the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update) CMAJ. 2005;172:1319–20. doi: 10.1503/cmaj.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–7. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 6.Elit L, Fyles AW, Oliver TK, Devries–Aboud MC, Fung-Kee-Fung M, on behalf of the members of the Gynecology Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care Follow-up for women after treatment for cervical cancer. Curr Oncol. 2010;17:65–9. doi: 10.3747/co.v17i3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T, on behalf of the Gynecology Cancer Disease Site Group . Follow-Up After Primary Therapy for Endometrial Cancer. Toronto, ON: Cancer Care Ontario; 2006. [Available online at: http://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=14110; cited January 6, 2011] [DOI] [PubMed] [Google Scholar]
- 8.Desch CE, Benson AB, 3rd, Somerfield MR, et al. on behalf of the American Society of Clinical Oncology Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–19. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 9.Figueredo A, Rumble RB, Maroun J, et al. Follow-Up of Patients with Curatively Resected Colorectal Cancer. Toronto, ON: Cancer Care Ontario; 2004. [archived in 2012]. [Available online at: https://www.cancercare.on.ca/common/pages/UserFile.aspx?serverId=6&path=/File%20Database/CCO%20Files/PEBC/pebc2-9f.pdf; cited January 6, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dummer R, Hauschild A, Pentheroudakis G. Cutaneous malignant melanoma: esmo clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl 4):129–31. doi: 10.1093/annonc/mdp152. [DOI] [PubMed] [Google Scholar]
- 11.BC Cancer Agency (bcca) Home > Health Professionals Info > Cancer Management Guidelines > Genitourinary > Prostate > 5. Management > Follow-up. Vancouver, BC: BCCA; n.d. [Available online at: http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Genitourinary/Prostate/Management/Followup/default.htm; cited January 6, 2011] [Google Scholar]
- 12.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–55. W144–54. doi: 10.7326/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KD, Lu CH, Chen PT, et al. The incidence and risk of developing a second primary esophageal cancer in patients with oral and pharyngeal carcinoma: a population-based study in Taiwan over a 25 year period. BMC Cancer. 2009;9:373. doi: 10.1186/1471-2407-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89:1429–39. doi: 10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]
- 15.Youlden DR, Baade PD. The relative risk of second primary cancers in Queensland, Australia: a retrospective cohort study. BMC Cancer. 2011;11:83. doi: 10.1186/1471-2407-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemminki K, Li X, Dong C. Second primary cancers after sporadic and familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:793–8. [PubMed] [Google Scholar]
- 17.Evans HS, Moller H, Robinson D, Lewis CM, Bell CM, Hodgson SV. The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut. 2002;50:647–52. doi: 10.1136/gut.50.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corkum M, Hayden JA, Kephart G, Urquhart R, Schlievert C, Porter G. Screening for new primary cancers in cancer survivors compared to non-cancer controls: a systematic review and meta-analysis. J Cancer Surviv. 2013;7:455–63. doi: 10.1007/s11764-013-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunfeld E, Moineddin R, Gunraj N, et al. Cancer screening practices of cancer survivors: population-based, longitudinal study. Can Fam Physician. 2012;58:980–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Toward Optimized Practice. Breast Cancer Screening: Clinical Practice Guideline. Edmonton, AB: TopAlbertaDoctors.org; 2013. [Available online at: http://topalbertadoctors.org/download/243/breast_cancer_guideline.pdf; cited August 8, 2014] [Google Scholar]
- 21.Nova Scotia Breast Screening Program (nsbsp) Annual Report 2008 (2007 Data) [Web page] Halifax, NS: NSBSP; 2008. [Available at: http://www.breastscreening.ns.ca/webReport08/index.html; cited August 8, 2014] [Google Scholar]
- 22.Toward Optimized Practice. Guideline and Screening for Cervical Cancer. Edmonton, AB: TopAlbertaDoctors.org; 2011. [Available online at: http://topalbertadoctors.org/download/587/cervical%20cancer%20guideline.pdf; cited August 8, 2014] [Google Scholar]
- 23.Statistics Canada. Home > CANSIM > Table 105-4042: Pap smear, females aged 18 to 69 years, Canada, provinces and territories [Web resource] Ottawa, ON: Statistics Canada; 2006. [Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=1054042&paSer=&pattern=&stByVal=1&p1=1&p2=-1&tabMode=dataTable; cited August 8, 2014] [Google Scholar]
- 24.Urquhart R, Grunfeld E. Building tools to measure and improve access to and quality of colorectal cancer care in Nova Scotia [abstract A71] Can J Gastroenterol. 2010;24(suppl SA):91A. [Google Scholar]
- 25.Nova Scotia Breast Screening Program (nsbsg) Home > Breast Imaging Guidelines [Web page] Halifax, NS: NSBSP; 2014. [Available online at: http://breastscreening.nshealth.ca/breast-imaging-guidelines; cited August 8, 2014] [Google Scholar]
- 26.Cancer Care Nova Scotia. Home > Prevention & Screening > Cervical Cancer Prevention Program > Pap Test Q&A [Web resource] Halifax, NS: Cancer Care Nova Scotia; n.d. [Available at: http://www.cancercare.ns.ca/en/home/preventionscreening/cervicalcancerprevention/paptestinformation.aspx; cited January 7, 2011] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Fulton JP, Buechner JS, Scott HD, et al. A study guided by the health belief model of the predictors of breast cancer screening of women ages 40 and older. Public Health Rep. 1991;106:410–20. [PMC free article] [PubMed] [Google Scholar]
- 29.Nova Scotia Breast Screening Program (nsbsp) Annual Report 2010 (2009 Data) Halifax, NS: NSBSP; 2010. [Available online at: http://breastscreening.nshealth.ca/ann_rpt_2010.pdf; cited June 24, 2011] [Google Scholar]
- 30.Brouwers M, De Vito C, Carol A, et al. Interventions to Increase the Uptake of Cancer Screening. Toronto, ON: Cancer Care Ontario; 2009 [archived 2014]. [Available online at: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=80349; cited June 24, 2011] [Google Scholar]
- 31.Breslau ES, Jeffery DD, Davis WW, Moser RP, McNeel TS, Hawley S. Cancer screening practices among racially and ethnically diverse breast cancer survivors: results from the 2001 and 2003 California health interview survey. J Cancer Surviv. 2010;4:1–14. doi: 10.1007/s11764-009-0102-5. [DOI] [PubMed] [Google Scholar]
- 32.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 33.McBean AM, Yu X, Virnig BA. The use of preventive health services among elderly uterine cancer survivors. Am J Obstet Gynecol. 2008;198:86, e1–8. doi: 10.1016/j.ajog.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 34.McBean AM, Yu X, Virnig BA. Screening mammography rate and predictors following treatment for colorectal cancer. J Cancer Surviv. 2009;3:12–20. doi: 10.1007/s11764-009-0080-7. [DOI] [PubMed] [Google Scholar]
- 35.Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med. 2009;24:469–74. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer DK, Terrin NC, Menon U, et al. Screening practices in cancer survivors. J Cancer Surviv. 2007;1:17–26. doi: 10.1007/s11764-007-0007-0. [DOI] [PubMed] [Google Scholar]
- 37.Snyder CF, Frick KD, Kantsiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27:1054–61. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]