Abstract
Objective
High-dose methotrexate (hdmtx) is a common therapeutic agent in the treatment of osteosarcoma. However, hdmtx is highly toxic and requires complex pharmacokinetic monitoring and leucovorin rescue. Thus, alternative therapeutic strategies are necessary. Here, we analyzed the clinical efficacy of a dia regimen (cisplatin–ifosfamide–doxorubicin) to evaluate its potential as an alternative to hdmtx–based therapy.
Methods
Patients received 12 cycles of chemotherapy administered over 2 years (2 preoperative cycles and 10 postoperative cycles). Cumulative dose was the same in all cycles: cisplatin 120 mg/m2 on day 1 of week 1, followed by ifosfamide 2.0 g/m2 days 1–5 of week 2, and doxorubicin 20 mg/m2 days 1–3 of week 2.
Results
Between January 2004 and October 2008, 39 eligible patients (median age: 16 years) were enrolled, with 36 being evaluable for the study. Of those 36 patients, 20 (55.6%) had a good histologic response to preoperative chemotherapy (>90% tumour necrosis). The estimated 5-year rates of event-free survival (efs) and overall survival were 54.8% and 61.5% respectively.
Conclusions
The results of our study suggest that, in osteosarcoma patients, the dia regimen produces an efs rate and survival outcomes comparable to those attained with hdmtx–containing regimens, with fewer adverse reactions. The dia regimen is well tolerated, and we observed a high level of patient compliance. Our results demonstrate that hdmtx-free osteosarcoma treatment regimens can be effective, warranting further investigation.
Keywords: Osteosarcoma, chemotherapy, methotrexate-free regimens, survival
1. INTRODUCTION
Since the 1980s, osteosarcoma has commonly been treated with neoadjuvant chemotherapy, and over time, the 5-year patient survival rate has improved to between 60% and 80%. The most common first-line therapeutic strategy for osteosarcoma is high-dose methotrexate (hdmtx) in combination with cisplatin, ifosfamide, and doxorubicin1. Several hdmtx-based regimens using various combinations of cycles and doses have been developed, all with similar survival rates2.
Despite its efficacy, hdmtx is associated with serious side effects, including nephrotoxicity, mucositis, hepatotoxicity, pulmonary toxicity, and neurotoxicity3,4. The standard chemotherapy dose of 8–12 g/m2 hdmtx is much higher than the absolutely lethal dose of 2–4 mg/kg5,6. Although leucovorin is widely co-administered to reduce the toxic effects of hdmtx, therapy is still harmful to the patients. Some hdmtx-associated side effects such as mucositis and nephrotoxicity are associated with delayed clearance of methotrexate7. About 1.8% of patients develop acute renal failure5, the leading cause of death related to hdmtx chemotherapy. Because of those complications, therapy with hdmtx properly requires rigorous pharmacokinetic monitoring and rescue with leucovorin7–9. Moreover, the expertise and technology required for proper hdmtx administration is unavailable in many hospitals, particularly in China. In addition, hdmtx toxicity can interfere with the delivery of other active agents in combination therapies. As a result, the necessity for hdmtx in osteosarcoma chemotherapy strategies has been questioned7,10,11.
In the present study, we assessed the efficacy of a hdmtx-free chemotherapy regimen of cisplatin–ifosfamide–doxorubicin (dia) in osteosarcoma patients treated between 2004 and 2008 to determine whether hdmtx is required for effective osteosarcoma chemotherapy.
2. METHODS
2.1. Patients
The maximum age for inclusion in this study was 40 years, and only patients who had not received prior treatment were included. All patients had been diagnosed with nonmetastatic, histologically proven, Enneking stage iib osteosarcoma (cases of parosteal or periosteal osteosarcoma were excluded) that was resectable by either limb-sparing surgery or amputation. Other inclusion criteria were a total serum bilirubin level less than 1.5 times the upper limit of normal, serum creatinine less than twice the upper limit of normal, and no evidence of cardiac rhythm disturbance or cardiomyopathy. All subjects provided informed consent consistent with hospital procedures.
Given those selection criteria, our retrospective study included 39 osteosarcoma patients [24 boys and men, 15 girls and women; age range: 6–39 years (mean: 17 years; median: 16 years)] during the period of interest (January 2004 to October 2008). Tumours were found in the distal femur in 21 cases, in the proximal tibia in 13 cases, in the proximal humerus in 4 cases, and in the proximal fibula in 1 case.
2.2. Treatment Plan
The dia chemotherapy regimen (Figure 1) was administered preoperatively and postoperatively. Preoperatively, patients received 2 cycles of chemotherapy (cisplatin 120 mg/m2 on day 1 of week 1, followed by ifosfamide 2.0 g/m2 days 1–5 of week 2, and doxorubicin 20 mg/m2 days 1–3 of week 2), with a 2-week interval between the preoperative cycles. Surgery was performed 2 weeks after the 2nd cycle, and chemotherapy was resumed 2 weeks after surgery. Patients received 6 cycles of chemotherapy with 3-week intervals between the cycles, and then 2 cycles separated by 3-month intervals, and 2 cycles separated by 6-month intervals. The planned overall duration of treatment after surgery was 10 cycles over 2 years, and drug doses were identical across all treatment cycles.
FIGURE 1.

Treatment schema for the cisplatin (D), ifosfamide (I), doxorubicin (A) protocol. S = surgery.
2.3. Evaluation of Chemotherapy
Tumours were evaluated using any or all of radiography, computed tomography, and magnetic resonance imaging before surgery, before chemotherapy start, and after chemotherapy completion. Each patient’s response to chemotherapy was evaluated using the Response Evaluation Criteria in Solid Tumors (recist), version 1.112. Patient responses were categorized as complete remission (cr), partial remission (pr), stable disease (sd), and progressive disease (pd). Complete remission was defined as complete disappearance of all lesions; pr was defined as a 30% or greater decrease from pre-therapy measurements of the longest diameter of target lesions. Progressive disease was defined as any one or more of a greater than 20% increase in the longest diameter of target lesions compared with pre-therapy measurements, the appearance of new lesions, or an absolute increase of 5 mm or more in lesion size. Stable disease indicated that lesions had neither shrunk sufficiently to qualify for pr nor grown sufficiently to qualify for pd, taking as reference the sum of the smallest diameters recorded during the study. The objective response rate (orr) was defined as the sum of the cr and pr rates, and the disease control rate was defined as the sum of the cr, pr, and sd rates.
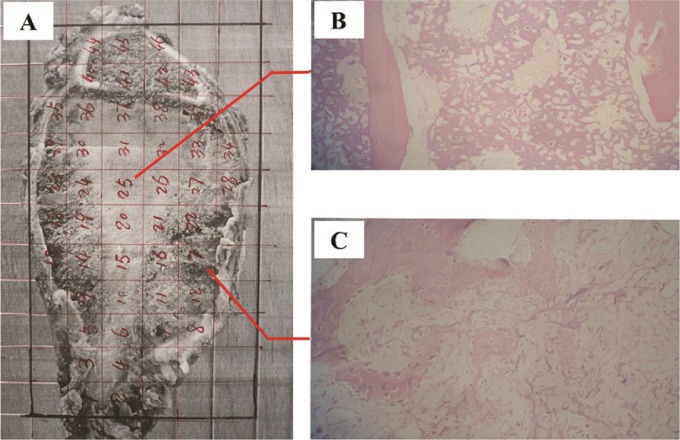
The tumour-cell necrosis rate (tcnr) was calculated after chemotherapy to assess therapeutic efficiency. Briefly, after excision, the largest section of the tumour, as determined by preoperative magnetic resonance imaging, was divided into blocks (10×10×4 mm). Specimens were prepared using standard histologic methods and were stained with hematoxylin and eosin. Tumour necrosis was calculated for each specimen based on histologic analysis, and the tcnr was determined by the sum of the cross-section scores for the entire tumour section (Figure 2).
FIGURE 2.
Representative osteosarcoma surgical specimen. (A) Pathology specimen after resection. The specimen is marked in 1×1-cm sections. (B,C) Sections showing tumour, categorized as Huvos class iv. Hematoxylin and eosin, 200× original magnification.
The grade of tcnr was classified using the Huvos necrosis grading system13: grade i, rare necrosis; grade ii, less-than-50% to 90% necrosis; grade iii, 90% or more necrosis; and grade iv, 100% necrosis. Grades i and ii were considered a poor response to therapy; grades iii and iv were considered a good therapeutic response.
2.4. Evaluation of Toxicity
Chemotherapy-associated toxicity was assessed according to the Common Terminology Criteria for Adverse Events, version 4.0, issued by the U.S. National Cancer Institute14. Adverse reactions are reported by grade (level of severity) on a scale of 1–5, with 1 being mild (no or mild symptoms, and no intervention required); 2 being moderate (some limitation of activities, with minimal local or noninvasive treatment required); 3 being severe (medically significant, but not immediately life-threatening; potentially requiring hospitalization or prolongation of existing hospitalization and posing limitations on personal activities of daily living); 4 being life-threatening (emergency treatment necessary); and 5 being patient death related to the adverse event.
2.5. Surgical Procedures and Postoperative Follow-Up Plan
Eligible patients underwent surgery 2 weeks after the second cycle of preoperative chemotherapy (that is, at week 9 of treatment). Of the 39 study patients, 38 received routine limb salvage surgery; 1 patient underwent amputation. After 10 cycles of postoperative chemotherapy had been completed, patients were examined by radiography of the operated limb and computed tomography of the chest every 3 months for 2 years, and at 6-month intervals during years 3–5 of follow-up. Recurrence, metastasis, and death were recorded.
2.6. Statistical Methods
All statistical analyses were performed using IBM SPSS Statistics (version 20.0: IBM, Armonk, NY, USA). The 5-year overall survival (os) and event-free survival (efs) rates were analyzed using the Kaplan–Meier method. Correlations between patient survival and the various chemotherapy regimens were assessed using the chi-square test and Huvos analysis.
3. RESULTS
3.1. Chemotherapy Cycles
Of the 39 study patients, 19 were unable to complete all planned dia chemotherapy. Early progression was documented in 16 of the 19 during chemotherapy. An intracranial hemorrhage led to the death of 1 patient after 3 cycles of chemotherapy. For financial reasons, 2 patients received only a single preoperative therapy cycle. Of those 2 patients, 1 completed all 10 cycles of postoperative chemotherapy, and 1 developed local recurrence and lung metastasis after 3 cycles. Of the remaining 20 patients, 18 received all 12 cycles of chemotherapy, 1 received 11 cycles, and 1 received 10 cycles. Over the course of the study, the 39 patients received a total of 399 cycles of chemotherapy.
3.2. Evaluation of Chemotherapy Outcomes
By recist 1.1 criteria, none of the 39 patients experienced a cr, 18 experienced a pr, 19 experienced sd, and 2 experienced pd (Figure 3). The orr was 46.2%, and the disease control rate was 94.9%.
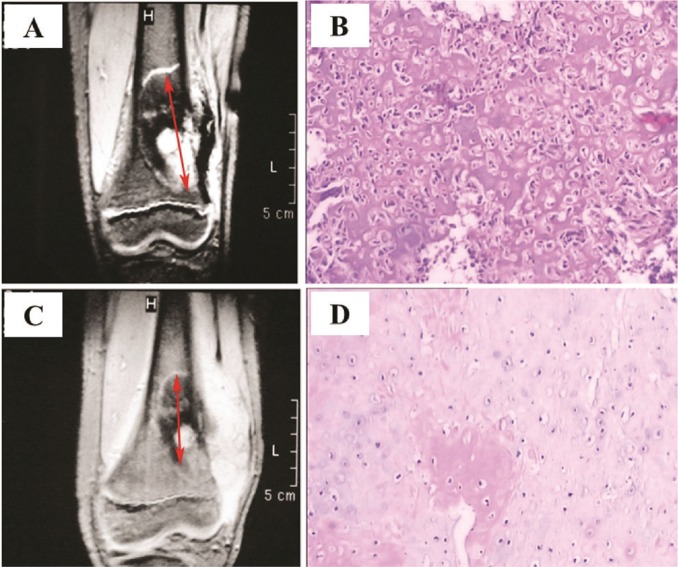
FIGURE 3.
Osteosarcoma arising from the left distal femur in a 15-year-old boy. (A) Magnetic resonance image obtained before neoadjuvant chemotherapy. Arrows show longest diameter of lesion. (B) Tumour cells from the lesion. Hematoxylin and eosin, 40× original magnification. (C) Magnetic resonance image of the same tumour after chemotherapy. Calcification is extensive, and the lesion (arrow) is significantly smaller (≥30% reduction), indicating a good response to therapy. (D) Tumour cells show significant degeneration and necrosis. Hematoxylin and eosin, 40× original magnification. The patient was evaluated as having a partial response by the Response Evaluation Criteria in Solid Tumors (version 1.1).
3.2.1. Tumour-Cell Necrosis after DIA Chemotherapy
Necrosis was not assessed in 3 of the 39 patients, and 2 patients were excluded from analysis because they did not complete the entire chemotherapy regimen (1 received a composite artificial joint replacement at an outside institution). In the 36 evaluable patients, 20 (55.6%) responded well to dia chemotherapy (tcnr > 90%, with 12 patients being classified grade iii, and 8 being classified grade iv). Of the 16 patients (44%) who did not respond well to the dia regimen (tcnr < 90%), 12 were classified grade ii, and 4 were classified grade i. When tcnr status was compared with the recist orr, a strong correlation between the two evaluation methods was identified (Table i). In particular, we found that patients who responded well to dia therapy tended to have experienced a pr by recist (χ2 = 22.05, contingency coefficient = 0.616, p = 0.000). Those results indicate that the dia regimen can promote tumour necrosis, suggesting this treatment strategy can be clinically effective.
TABLE I.
Correlation between the Huvos grading system and the Response Evaluation Criteria in Solid Tumors (recist)
| recist outcome |
Huvos classification based on histologic response
|
|
|---|---|---|
| Good (iii and iv) | Poor (i and ii) | |
| cr+pr (n) | 17 | 1 |
| sd+pd (n) | 3 | 15 |
|
| ||
| χ2=22.05, p=0.000 Contingency coefficient=0.616, p=0.000 | ||
cr = complete response; pr = partial response; sd = stable disease; pd = progressive disease.
3.2.2. Patient Response to DIA Therapy
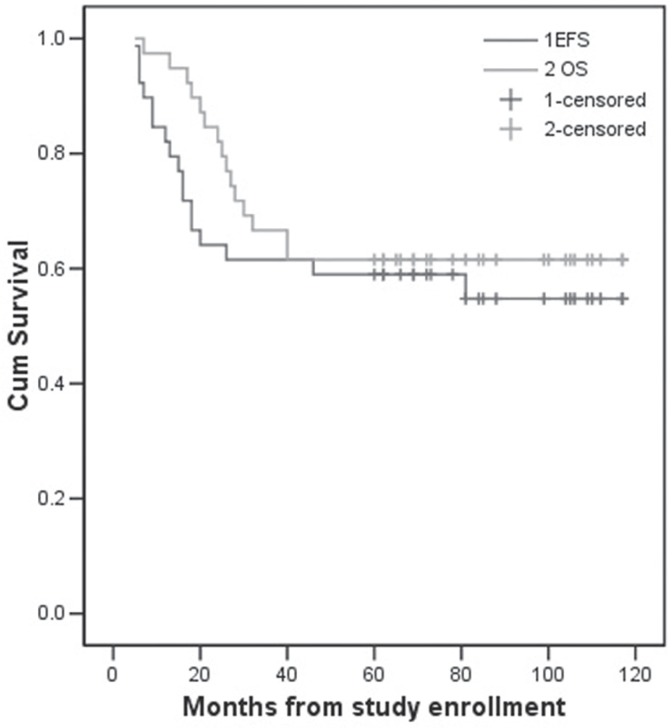
At the time of writing (October 2013), patients had been followed for between 7 and 117 months, with a median follow-up of 66 months. Of the 39 enrolled patients, 23 were experiencing tumour-free survival (that is, survival for 5 years or more), 1 was surviving with tumours, and 15 had died. Of the 15 deceased patients, 10 had experienced tumour relapse (with 9 developing lung metastasis) ultimately leading to death. One patient experienced local recurrence after 81 months of disease-free survival. Another 6 patients developed lung metastases alone, and 5 of them died. One patient developed brain metastasis, but was still alive at the time of writing. One patient developed thrombocytopenia 7 months after therapy and subsequently died of an intracranial hemorrhage. The 5-year os rate in the cohort was 61.5%, and the 5-year efs rate was 54.8% (Figure 4).
FIGURE 4.
Five-year event-free survival [efs (54.8%)] and overall survival [os (61.5%)] for patients with osteosarcoma, by treatment protocol.
3.3. Chemotherapy Toxicity Evaluation
During the 399 cycles of dia chemotherapy, our patient cohort tolerated treatment well. Few side serious side effects were reported, and the most common events—including myelosuppression, nausea, vomiting, abnormal liver function, and alopecia—were transient (Table ii). Those results suggest that toxicity for the dia regimen is very low, with few serious or long-term side effects.
TABLE II.
Adverse reactions observed in 399 chemotherapy cycles, by grade
| Adverse reactions |
Grade
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Febrile neutropenia | 0 | 0 | 5 | 12.80 | 0 | 0 | ||||
| Vomiting | 11 | 28.20 | 16 | 41.00 | 10 | 25.60 | 0 | 0 | ||
| High alt | 6 | 15.40 | 3 | 7.700 | 0 | 0 | — | |||
| Neutropenia | 16 | 41.00 | 9 | 23.10 | 8 | 20.50 | 6 | 15.40 | — | |
| Thrombocytopenia | 12 | 30.80 | 6 | 15.40 | 2 | 5.10 | 2 | 5.10 | — | |
| Alopecia | 14 | 35.80 | 25 | 64.10 | — | — | — | |||
alt = alanine aminotransferase.
4. DISCUSSION
Although hdmtx-based therapy has provided substantial benefits for osteosarcoma patients, its utility is limited by high toxicity and complex monitoring requirements. The monitoring requirements are a particular problem in developing nations such as China, where many hospitals lack the technology and expertise necessary to properly monitor hdmtx-associated toxicity. Investigation of alternative therapies is therefore necessary. In the present study, a hdmtx-free regimen comprising cisplatin, ifosfamide, and doxorubicin was administrated to 39 osteosarcoma patients between 2004 and 2008. Our results demonstrate that administration of this dia regimen in osteosarcoma patients produces efs and survival outcomes comparable to those obtained with hdmtx-containing regimens, without serious long-term side effects.
To compare our results with other studies of chemotherapy in osteosarcoma patients, we reviewed the patient outcomes reported in studies of hdmtx-containing and hdmtx–free chemotherapy regimens (all using 3 or more drugs) published between 1998 and 2011. In 2147 patients participating in 6 studies with hdmtx-containing regimens, 5-year os rates were in the range 57%–87%, and efs rates were in the range 64%–73% (Table iii). In 212 patients participating in 5 studies with hdmtx-free regimens, similar outcomes were observed: 5-year os rates were in the range 69%–78.9%, and efs rates were in the range 60.4%–66.7% (Table iii). This comparative analysis indicates that hdmtx-free regimens have chemotherapeutic efficacies comparable to those with hdmtx-containing regimens.
TABLE III.
Chemotherapy outcomes for high-dose methotrexate-containing and high-dose methotrexate-free regimens (3 drugs or more) in osteosarcoma, 1998–2011
| Reference | Pts (n) | Regimens | Median follow-up |
Survival (%)
|
|
|---|---|---|---|---|---|
| 5-Year overall | Tumour-free | ||||
| Fuchs et al., 199815 | 128 | Methotrexate–cisplatin–doxorubicin | 8.35 Years | 72a | 66a |
| Bacci et al., 200216 | 70 | Methotrexate–cisplatin–doxorubicin–iphosphamide | 60 Months | 87 | 73 |
| Patel et al., 200217 | 20 | Cisplatin–doxorubicin–iphosphamide | 5.5 Years | 74 | 68 |
| Crews et al., 20043 | 48 | Methotrexate–cisplatin–doxorubicin–iphosphamide | 11.1 Years | 70.6 | 67.6 |
| Ferrari et al., 20052 | 182 | Methotrexate–cisplatin–doxorubicin–iphosphamide | 55 Months | 77 | 64 |
| Piperno–Neumann et al., 200618 | 32 | Cisplatin–doxorubicin–iphosphamide | 36 Months | 86b | 74b |
| Tunn and Reichardt 200719 | 53 | Cisplatin–cyclophosphamide–vincristine–doxorubicin | 151 Months | 71 | 60.4 |
| Smeland et al., 200920 | 63 | Methotrexate–cisplatin–doxorubicin | 64 Months | 77 | 69 |
| Methotrexate–cisplatin–doxorubicin–iphosphamide | |||||
| Assi et al., 201021 | 32 | Cisplatin–doxorubicin–iphosphamide | 64 Months | 69 | 65 |
| Picci et al., 201022 | 1656 | Methotrexate–cisplatin–doxorubicin | |||
| Methotrexate–cisplatin–doxorubicin–iphosphamide | 12 Years | 57 | — | ||
| Daw et al., 201123 | 75 | Carboplatin–doxorubicin–iphosphamide | 5.1 Years | 78.9 | 66.7 |
10-Year.
2-Year.
In two studies using different protocols, hdmtx-free dia regimens resulted in 5-year os and efs rates of 69% and 74% and of 65% and 68% respectively17,21, which are comparable to rates achieved with hdmtx-containing regimens. In a recent phase ii clinical trial (OS99), a hdmtx-free regimen comprising carboplatin, ifosfamide, and doxorubicin resulted in 5-year os and efs rates of 78.9% and 61% respectively23. Those results are consistent with the results of our own study of 39 osteosarcoma patients receiving hdmtx-free dia therapy. After follow-up for an average of 66 months, the 5-year os was 61.5% and the disease-free survival was 54.8% in our cohort. Taken together, the data indicate that hdmtx-free chemotherapy regimens produce survival outcomes comparable to those with hdmtx-containing regimens in osteosarcoma patients3,15,16,20,21. The hdmtx-free regimens are well tolerated, do not require pharmacokinetic monitoring, and avoid the toxicities and side effects associated with hdmtx and cisplatin.
Many recent studies have aimed to identify the most effective chemotherapy regimens to treat osteosarcoma24,25. In a European study26, a two-drug regimen (6 cycles of doxorubicin 25 mg/m2 on days 1–3 and cisplatin 100 mg/m2 on day 1 for 18 weeks) and a multidrug regimen (preoperative vincristine, hdmtx, and doxorubicin; postoperative bleomycin, cyclophosphamide, dactinomycin, vincristine, and methotrexate for 44 weeks) were compared in 407 patients with operable nonmetastatic osteosarcoma. The two-drug regimen was shorter in duration and better tolerated, but the 5-year survival rate was unsatisfactory. As that study indicates, long-term multidrug protocols typically provide greater clinical benefit than short-term, less-intensive two-drug regimens. Our study evaluated a multidrug dia protocol and observed favourable results. Compared with the previously reported two-drug regimen, our dia regimen resulted in os and efs rates that were improved to 61.5% and 54.8% from 55% and 44%25.
The methodology for evaluating tumour response to chemotherapy has evolved substantially in recent years. Histopathologic evaluation of the tumour necrosis rate has proved to be a reliable prognostic tool. The Huvos necrosis grading system in particular is widely used for evaluation of chemotherapy in osteosarcoma. Using the percentage of tumour necrosis after chemotherapy as an indicator, patients can be classified as poor responders (that is, low necrosis) or good responders (that is, high levels of necrosis), which in turn can predict long-term prognosis. However, despite its predictive strengths, the Huvos grading system is not widely used in the clinical setting because of the time-consuming, and sometimes subjective, analysis required to assess necrosis27. In contrast, the imaging-based recist approach is widely used clinically, including in phase ii/iii trials of osteosarcoma chemotherapy regimens. Established in 2009, recist 1.1 standardizes the assessment of therapeutic response, allowing for meaningful comparisons of drug efficacy between individuals in the same study and across different studies12. Using recist 1.1, chemotherapy efficacy is determined primarily by the size of bone and cystic lesions measured by computed tomography or magnetic resonance imaging. Specifically, the sum of the measurements of the greatest longitudinal dimension of each lesion is used to determine response. To date, the application of recist 1.1 to osteosarcoma chemo-therapy has not been carefully studied28. Here, we assessed chemotherapy efficacy using both the Huvos grading system and recist. The correlation between therapeutic response as assessed by the Huvos system and orr as derived from recist was good, suggesting that both approaches can effectively measure therapeutic response in osteosarcoma patients. Given the relative ease of the recist approach compared with the Huvos method, we suggest that recist can be used to provide timely, accurate evaluation of response to chemotherapy for osteosarcoma.
Given the significant toxicity and treatment-related complications associated with hdmtx-based osteosarcoma therapy, it is important to develop alternative therapies that avoid hdmtx. In our study of a hdmtx-free dia regimen, 39 patients underwent a total of 399 cycles of chemotherapy. The chemotherapy was well-tolerated in this patient cohort, and the primary adverse events—including myelosuppression, nausea, vomiting, abnormal liver function, and alopecia—were transient. Severe intracranial hemorrhage resulting from thrombocytopenia was observed in a single patient. Thus, chemotherapy-associated complications were fewer with our hdmtx-free dia regimen than with the hdmtx-containing regimens reported in the literature3,4.
5. CONCLUSIONS
We demonstrated that, in osteosarcoma patients, a hdmtx-free dia regimen produces efs and os outcomes comparable to those reported for hdmtx-containing regimens, but with fewer adverse reactions. The dia regimen is a well-tolerated treatment, with good patient compliance, and is worthy of further research and clinical application.
6. ACKNOWLEDGMENT
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
7. CONFLICT OF INTEREST DISCLOSURES
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence their work and that there are no professional or other personal interests of any nature or kind in any product, service, or company that could be construed as influencing the position presented in, or the review of, the present manuscript.
8. REFERENCES
- 1.Hogendoorn PC, Athanasou N, Bielack S, et al. on behalf of the esmo/eurobonet Working Group Bone sarcomas: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v204–13. doi: 10.1093/annonc/mdq223. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Smeland S, Mercuri M, et al. on behalf of the Italian and Scandinavian Sarcoma Groups Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 3.Crews KR, Liu T, Rodriguez–Galindo C, et al. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer. 2004;100:1724–33. doi: 10.1002/cncr.20152. [DOI] [PubMed] [Google Scholar]
- 4.Janeway KA, Grier HE. Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol. 2010;11:670–8. doi: 10.1016/S1470-2045(10)70062-0. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe N, Gorlick R. High-dose methotrexate in osteosarcoma: let the questions surcease—time for final acceptance. J Clin Oncol. 2008;26:4365–6. doi: 10.1200/JCO.2007.14.7793. [DOI] [PubMed] [Google Scholar]
- 6.Graf N, Winkler K, Betlemovic M, Fuchs N, Bode U. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol. 1994;12:1443–51. doi: 10.1200/JCO.1994.12.7.1443. [DOI] [PubMed] [Google Scholar]
- 7.Lewis IJ, Weeden S, Machin D, Stark D, Craft AW. Received dose and dose-intensity of chemotherapy and outcome in nonmetastatic extremity osteosarcoma. European Osteosarcoma Intergroup. J Clin Oncol. 2000;18:4028–37. doi: 10.1200/JCO.2000.18.24.4028. [DOI] [PubMed] [Google Scholar]
- 8.Bielack SS, Kempf–Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 9.Holmboe L, Andersen AM, Morkrid L, Slordal L, Hall KS. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73:106–14. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study ssg viii: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39:488–94. doi: 10.1016/S0959-8049(02)00747-5. [DOI] [PubMed] [Google Scholar]
- 11.Smeland S, Bruland OS, Hjorth L, et al. Results of the Scandinavian Sarcoma Group xiv protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop. 2011;82:211–16. doi: 10.3109/17453674.2011.566141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Huvos AG. Bone Tumors: Diagnosis, Treatment, and Prognosis. 2nd ed. London, U.K: W.B. Saunders; 1991. [Google Scholar]
- 14.Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events version 4.0. J Am Acad Dermatol. 2012;67:1025–39. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs N, Bielack SS, Epler D, et al. Long-term results of the co-operative German–Austrian–Swiss osteosarcoma study group’s protocol coss-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–9. doi: 10.1023/A:1008391103132. [DOI] [PubMed] [Google Scholar]
- 16.Bacci G, Ferrari S, Longhi A, et al. High dose ifosfamide in combination with high dose methotrexate, Adriamycin and cisplatin in the neoadjuvant treatment of extremity osteosarcoma: preliminary results of an Italian Sarcoma Group/Scandinavian Sarcoma Group pilot study. J Chemother. 2002;14:198–206. doi: 10.1179/joc.2002.14.2.198. [DOI] [PubMed] [Google Scholar]
- 17.Patel SJ, Lynch JW, Jr, Johnson T, et al. Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for osteosarcoma in adults. Am J Clin Oncol. 2002;25:489–95. doi: 10.1097/00000421-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Piperno–Neumann S, Bui B, Blay J, et al. A multicentric prospective study of intensive induction chemotherapy (api-ai) in localized osteosarcoma patients: results of a phase ii trial coordinated by the French Sarcoma Group (fsg) and the fnclcc bect. J Clin Oncol. 2006;24:9521. [Google Scholar]
- 19.Tunn PU, Reichardt P. Chemotherapy for osteosarcoma without high-dose methotrexate: a 12-year follow-up on 53 patients. Onkologie. 2007;30:228–32. doi: 10.1159/000100776. [DOI] [PubMed] [Google Scholar]
- 20.Smeland S, Bruland OS, Hjorth L, et al. Scandinavian experience in classical osteosarcoma. Results of the ssg xiv protocol. Acta Orthop. 2009;80:60–6. doi: 10.3109/17453674.2011.566141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assi H, Missenard G, Terrier P, et al. Intensive induction chemotherapy without methotrexate in adult patients with localized osteosarcoma: results of the Institut Gustave-Roussy phase ii trial. Curr Oncol. 2010;17:23–31. doi: 10.3747/co.v17i6.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picci P, Mercuri M, Ferrari S, et al. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol. 2010;21:1366–73. doi: 10.1093/annonc/mdp502. [DOI] [PubMed] [Google Scholar]
- 23.Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children’s Research Hospital OS99 trial. Cancer. 2011;117:2770–8. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 25.Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10:1579–91. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 26.Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–17. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 27.Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23:176–81. doi: 10.1177/1043454206289786. [DOI] [PubMed] [Google Scholar]
- 28.Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: recist 1.1, mda and percist. J Cancer. 2010;1:80–92. doi: 10.7150/jca.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]