Abstract
The characterization of cell viability is a challenging task in applied biotechnology, as no clear definition of cell death exists. Cell death is accompanied with a change in the electrical properties of the membrane as well as the cell interior. Therefore, changes in the physiology of cells can be characterized by monitoring of their dielectric properties. We correlated the dielectric properties of industrially used mammalian cells, sedimented over interdigitated microelectrodes, to the AC signal response across the chip. The voltage waveforms across the electrodes were processed to obtain the circuit impedance, which was used to quantify the changes in cell viability. We observed an initial decrease in impedance, after which it remained nearly constant. The results were compared with data from the dye exclusion viability test, the cell specific oxygen uptake rate, and the online viable cell density data from capacitance probes. The microelectrode technique was found to be sensitive to physiological changes taking place inside the cells before their membrane integrity is compromised. Such accurate determination of the metabolic status during this initial period, which turned out to be less well captured in the dye exclusion tests, may be essential for several biotechnology operations.
I. INTRODUCTION
Technologies based on mammalian cell cultures have been widely used by the biopharmaceutical industry to manufacture therapeutic proteins. It is desirable to maintain cells at active physiological state to maintain high production rate. Cell physiological changes such as apoptosis and decrease in viability should be monitored closely over the course of bioreactor operation. Timely detection and accurate characterization of these cell changes have been a challenging task faced by both industry and academia. For example, there is no clear definition of cell death; instead, there are several criteria for cell demise, which are defined by secondary characteristics associated with their status, making it difficult to precisely determine when a cell dies.1–3 The current test used by biotechnology companies to monitor cell death is based on dye exclusion, which involves staining the cells with a dye such as Trypan Blue. The cells with non-intact membranes get stained due to compromised membranes, whereas cells with fully intact membranes do not.4–10 An image analyzer is used to count the number of stained versus non-stained cells to estimate viability, which is defined as the percentage of the total non-stained cells out of all the cells counted. This viability profile is considered as one of the most important parameters to be monitored during cell culture processes.
An alternate method of characterizing cell physiology is based on the response of cells towards electric fields. The lipid bilayer membrane surrounding (and insulating) the cell cytoplasm has distinct electrical properties, which are considerably different than the cell's intracellular contents. Cell death is accompanied with changes in cell physiology, which changes the surface conductivity and capacitance of the cell membrane, as well as the conductivity of the cell interior. Therefore, cell death can be characterized by monitoring the changes in the cell dielectric properties.11,12 The characterization of cell death and dielectric properties using AC electric fields is a rapidly growing research area.13–39
We report results from a new type of miniaturized viability sensor, where an array of interdigitated microelectrodes in a microchamber is used for monitoring of the change in the AC impedance of the layer of mammalian cells sedimented over the electrode area. The impedance data obtained through this AC electrokinetics technique were compared to the viability data obtained through the dye exclusion test, the cell specific oxygen uptake rate, and the AC online capacitance probes installed in commercial bioreactors. The similarities and differences between the data on the viability status of the cells, determined by these techniques, are outlined and analyzed from the perspective of sensing for real-world bioreactor operation.
II. METHODOLOGY
A. Sample preparation
This study was based in NS0 murine myeloma cell lines,40 one of the two primary hosts extensively used in biomanufacturing of therapeutic antibodies, collected from bioreactor samples. A frozen vial was thawed and subsequently passaged in intervals of two days in shake flasks with basal media. The shake flasks were placed on a 19 mm orbital diameter shaker platform at 125 rpm in an incubator maintained at 37°C and 7.5% CO2. In order to induce cell death, the shake flasks were left in the incubator for an extended period of time. Cell samples were taken periodically for measurements of viable cell density (vcd), viability, and cell diameter by dye exclusion test based semi-automated image analyzer Cedex (Roche Innovatis, Germany). In some cases, the culture was inoculated at high seed densities to expedite the cell death process.
Cell samples at different Cedex viabilities were taken at different time points from the shake flask for impedance measurements. The samples were first centrifuged at 1500 rpm for 5 min in IEC Centra GP8 centrifuge (Thermo Fisher) and the resulting cell culture supernatant was removed. Based on the total cell density (tcd) data obtained with Cedex, the volume of the solution was adjusted such that the number of centrifuged cells was ∼20 × 105 cells. The cells were then re-suspended in 100 μl of an isotonic sugar buffer comprising of 8.5% w/v sucrose, 0.3% w/v glucose, and 0.2% w/v Pluronic F68 in deionized (DI) water. The thus obtained cell density in the sugar buffer was ∼200 × 105 cells/ml. The conductivity of the resulting suspension of cells in sugar buffer was measured to be ∼9.28 mS/cm across a range of viabilities using CDM230 conductivity meter (Radiometer Analytical, Lyon, France) at 25°C. The volume of the centrifuged solution could be adjusted to obtain the desired cell density in the re-suspension media (sugar buffer).
In a separate set of experiments, centrifuged cells were re-suspended in culture supernatant (conductivity ∼10.90 mS/cm) in place of sugar buffer. The same supernatant was used for re-suspending cells at different viabilities to eliminate the variability from the supernatant composition.
B. Interdigitated electrode chip fabrication
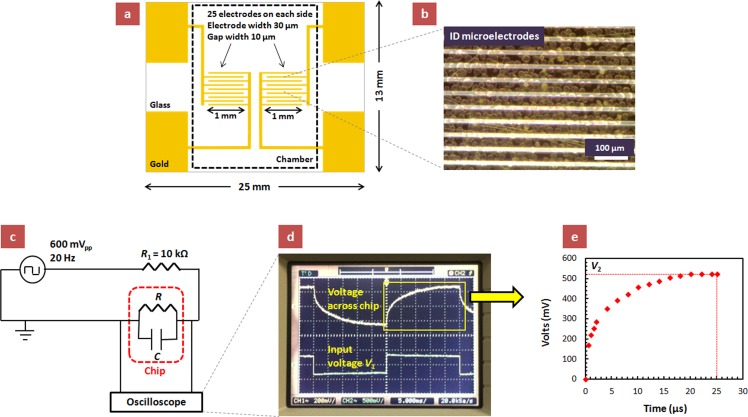
Interdigitated electrode patterns with gap sizes of 10 μm were microfabricated on a glass surface via photolithography. The electrodes were formed by depositing a 100 nm thick layer of gold onto a 10 nm thick layer of chromium. A photomask defining the shape and size of the electrodes was used to apply an etch-resist layer, and then the excess metal was removed. The configuration of the microelectrode chip is schematically shown in Figure 1(a) (see supplementary material for additional details41).
FIG. 1.
(a) Design of the glass chip with the micropatterned interdigitated electrodes (not drawn to scale). The dotted line delineates the area covered by the microfluidic chamber attached on the chip for encapsulating the cell sample. (b) Micrograph of the interdigitated electrode array with cells sedimented on top. The cell density in the suspension media is 200 × 105 cells/ml. (c) Circuit diagram of the experimental setup. The dotted red line represents the chip consisting of cells sedimented over the microelectrode array. The voltage waveform across the chip is monitored using a digital oscilloscope. (d) A typical oscilloscope signal snapshot illustrating the input voltage V1 (square wave of 600 mVpp at 20 Hz) and the voltage waveform across the chip. (e) A plot of the voltage waveform across the chip as a function of time, based on the oscilloscope display. V2 is the steady-state value of the voltage across the chip (before it switches its direction).
C. Experimental apparatus and data acquisition
Microfluidic flow chambers (9.8 mm × 20 mm × 0.25 mm, Grace BioLabs, OR) were sealed on the chip, thus enclosing the microelectrode array (Figure 1(a)). The cell suspension was injected into the inlet port of the microfluidic chamber with a micro-pipette, after which the ports were sealed with tape and the cells were allowed sufficient time to form an equilibrated sedimentation layer. The sedimentation time of the cells was calculated to be ∼40 s (see calculations in the supplementary material41). A density of 200 × 105 cells/ml (adjusted using the procedure described in the Sample Preparation sub-section) in the suspension media above the chip corresponds to a monolayer of cells (see Figure 1(b) and supplementary material41). We did not observe any direct (microscopy) or indirect (electrical signal) indication of any adhesion or spreading of the cells on the chip/electrode surface.
A square wave AC signal of 600 mV peak-to-peak at 20 Hz from the output of Agilent 33220A function generator was applied across the circuit with the interdigitated microelectrodes after ∼5 min of cell injection when a constant sedimentation layer has formed. The circuit diagram of the experimental setup is shown in Figure 1(c). We monitored the change in the dielectric properties of the cells, sedimented over the interdigitated electrodes of our chip, by recording the signal shape across the chip using Agilent DSO3202A digital oscilloscope and analyzing it on the basis of the equivalent circuit (Figure 1(d)). The low frequency of the signal was chosen to let the voltage waveform across the chip attain a steady-state value. The voltage waveforms across the chip were recorded for cell samples at various Cedex viabilities (Figure 1(e)). The steady-state value of the voltage across the chip, V2, in the circuit can be estimated as
| (1) |
where V1 is the applied input voltage, |Z| is the overall circuit impedance (a comprehensive parameter accounting for both the resistance and capacitance of the circuit), and R1 is the known resistor connected in series with the chip (Figure 1(c)). By using the steady-state value, V2, obtained from the voltage waveforms (Figure 1(e)) in Eq. (1), we calculated |Z| for cell samples at different viabilities. Since all other parameters were maintained constant, the change in |Z| reflects the changes in the dielectric properties of the cells, which in turn are correlated to their viability. The measurement and data processing for one |Z| data point from the signal recorded across the chip using the procedure described here typically took ∼5–10 min.
III. RESULTS
The initial short-term viability, viable cell density, total cell density, and diameter of the cultured cells centrifuged and resuspended in the sugar buffer as a function of time are shown in Fig. 2. Typical data obtained by conventional methods for the long-time profiles of culture density and viability of NS0 cells grown in shake flasks are shown in Fig. 3. The viable cell density data were normalized by their initial value. The cells begin growing and multiplying; however, after some time, this is followed by slow death of an increasing fraction of the culture. The onset of the decrease in cell viability was clearly registered after day 3 when the normalized vcd reached a value of ∼12. Interestingly, from this point further the rate of decrease of cell viability remained constant at 1.2% h−1. A similar cell growth crash profile was also observed in a large scale bioreactor (data not shown), where the pH and the dissolved oxygen were precisely controlled. We believe that the viability drop was associated with nutrient depletion. Samples of cells at different stages of growth and degrees of viability were then analyzed by the microsensor device introduced here and by assays for cell specific oxygen uptake rate and compared to data by commercial bioreactor capacitance sensor.
FIG. 2.
Plots of (a) initial cell viability and diameter, (b) vcd and tcd in the sugar buffer as a function of time. These data were obtained by analyzing healthy cells (viability >95%) using Cedex instrument based on dye exclusion test.
FIG. 3.
Plots of (a) viability and normalized viable cell density as a function of time and (b) cell diameter as a function of Cedex viability for the NS0 murine myeloma cells in the basal media (the diverging data point at ∼10% viability is likely an artifact of the optical readout due to the emergence of very small vesicles near the end of the cell life cycle registered as “cells” by the Cedex analyzer). The viable cell density in (a) was normalized by the initial value on the first day. The data in (a) and (b) were obtained by analyzing the cells samples using the dye exclusion Cedex instrument.
We measured the circuit impedance with only sugar buffer contained in the microfluidic chamber on the chip, in order to determine the contribution of the media alone in the absence of suspended cells. This background media circuit impedance, |Z|, measured using only sugar buffer was ∼170 kΩ. We then introduced in the microchamber a big range of cell samples suspended in sugar buffer (cell density maintained at 200 × 105 cells/ml to obtain a monolayer as described in Methodology section), having different dye exclusion test based viabilities. The obtained circuit impedance data are plotted in Figure 4. At higher Cedex viabilities, the chip circuit impedance decreases linearly with decreasing Cedex viability. However, the impedance remains nearly constant once the Cedex-measured cell viability decreases to ∼60%–70%. The impedance data for the cells suspended in culture supernatant followed the same trend as those obtained using sugar buffer as cell media (Figure 4). The offset between the impedance data in sugar buffer and culture supernatant is most likely due to the difference between the electrical conductivities of the two media, which were measured to be 9.28 mS/cm and 10.90 mS/cm, respectively. We expect that higher media conductivity would result in lower circuit impedance, which indeed is the case in Figure 4.
FIG. 4.
Plot of circuit impedance as a function of dye exclusion test based viability (obtained with Cedex assay) for cells suspended on top of the microelectrode chip in sugar buffer or culture supernatant. Cell density is 200 × 105 cells/ml in the suspension media.
In a control experiment, when the cell density above the microelectrode array was increased fourfold, to 800 × 105 cells/ml, a multilayer of sedimented cells was formed above the chip. The impedance data at cell densities of 200 × 105 cells/ml and 800 × 105 cells/ml are compared in Figure 5. The two data sets followed similar trend as a function of Cedex viability, except that the impedance values were slightly higher at higher cell densities. The observed rise in circuit impedance with increasing cell density may be attributed to the higher impedance offered by the more dense (and ion-rich) multilayer of cells relative to a monolayer. Overall, these experiments lead to the important conclusion that the thickness of the multilayer cell sediment does not have a major impact on the viability data estimates (see supplementary material for additional data41). This can be attributed to the short-range, near-surface penetration of the electrical field by the interdigitated micron-scale electrodes, which detects the viability status of the first cell layer, with little interference from any overlying material. The effective height of the field penetration is comparable to the distance between the microelectrodes, which in our case is comparable to the size of one cell (layer). A similar advantage of high near-surface sensitivity, unaffected by the thickness of the overlying sedimented layer, was also conclusively proven in our earlier study of immunoassay on-chip sensing where the aggregation state in a sedimented layer of bioagglutinated particles could be reliably detected by impedance measurement through interdigitated microelectrodes.18
FIG. 5.
Plot of circuit impedance as a function of dye exclusion test based viability (obtained with Cedex) for monolayer and multilayer of cells suspended on top of the chip in culture supernatant at densities of 200 × 105 cells/ml or 800 × 105 cells/ml correspondingly.
All of these data sets are unified by one important feature—the similarity in their trends. We normalized all impedance data sets in Figures 4 and 5 with their respective values at 95% viability. All combined normalized data are plotted in aggregate in Figure 6. We found that irrespective of the type of cell suspension media (sugar buffer or culture supernatant) and the cell density (monolayer or multilayer of cells above chip), the circuit impedance was approximately linearly correlated with the Cedex data at higher viabilities—when the Cedex viability decreased from 95% to 60% the impedance linearly decreased by ∼60%. However, when the measured Cedex viability decreased below 60%, the circuit impedance remained approximately constant at its value at 60% Cedex viability. The normalized impedance trend indicates that by using the impedance of healthy cells at high viability as reference, we can predict the viability of any cell sample by measuring its relative impedance at the same cell density and calculating the percentage decrease in impedance relative to reference.
FIG. 6.
Plot, compiled from Figs. 4 and 5, of normalized circuit impedance as a function of dye exclusion test based viability (obtained with Cedex) for cells suspended on top of the chip in sugar buffer or culture supernatant at densities of 200 × 105 cells/ml or 800 × 105 cells/ml. The data were normalized with their respective values at ∼95% viability. The trends in the correlation of the results of the two assays are universal across all types of media and cell culture samples studied.
The reason behind the observed disagreement between the viability estimates based on Cedex and impedance measurements is likely that the criteria for cell death evaluated by these two techniques are different as they follow different events in cell physiological devolution. The Cedex technique determines viability based on the staining of cells with a dye. Such staining due to dye penetration occurs when the cell membrane is significantly compromised, which constitutes one of the final stages of the process of cell death.42 However, the cell viability and metabolic activity decrease even while the cell membrane is intact. For example, the onset of apoptosis, which is one of the earliest events during the process of cell death, is characterized by molecular level changes in the symmetry and composition of the cell membrane. This event may not be recognized by the dye exclusion test as the membrane remains intact.37,43 However, techniques based on the AC polarization and electrokinetics measure changes in the overall dielectric properties of the cells. The results show that unlike the dye exclusion based techniques, AC field techniques can determine the changes and differences in the physiological state of the cells even when their membrane integrity is not compromised.
The impedance data values below 60% Cedex viability remain approximately constant (Figure 6). By correlating the impedance data to cell viability, we conclude that the dielectric properties of the cells reveal that they are already “unhealthy” by the time Cedex viability reaches 60%. Discrepancies between dye exclusion and electrical properties based viability assays have been reported and analyzed by other groups as well, where the electrical technique based viability decreases at a faster rate than the dye exclusion test based viability.38,39 The rapid decrease in cell viability measured by our technique, relative to the dye exclusion test, suggests that the detected changes in the cell polarization response capture the transition to a suboptimal physiological state occurring during the initial stages of cell death. NS0 cells, analyzed by us in this study, have been previously reported to be susceptible to their environment and their apoptosis can be triggered relatively easily compared to other industrial cell lines.44 We believe that by the time the Cedex viability decreases to 60%, most of the NS0 cells are already in one or the other stages of cell death. In this light, we conclude that the AC-based on-chip technique measures more precisely the metabolic conditions that are important from the perspective of using the cells in bioreactor operations.
Our study is based on commercial cell lines and processes, using real large-scale bioreactor operations for therapeutic protein manufacturing; however, the consistency of industrial cell culture processes varies from batch to batch. Due to the natural characteristics of these cells in the reactor batch, and the multi-day process duration, cell parameters can potentially have significant variations even under strictly maintained operational conditions. Variation can also be introduced through analytical instruments. For example, Cedex viability readings sometimes show variation as high as 10%–20% (data shown in the supplementary material41). The extent of variability in our on-chip impedance data (Fig. 6) is of the same order. In spite of that variability, we have consistently obtained exactly the same dependence for many diverse cell samples. These samples include multiple measurements on cell cultures suspended in three different media (Fig. 4) and cells of different thickness above the interdigitated electrodes (Fig. 5, also illustrating the robustness of the measurement and its low sensitivity to the thickness of the sedimented layer). When the data are normalized, they readily fall on a single master curve, Fig. 6. Accounting for this variability in the data, we believe that the trendline in Fig. 6 does reliably represent the average data trend. In summary, even though the specificity of the sample could lead to larger error and we were only able to collect a limited number of samples, the independent observation of the same correlation for more than 6 different samples could not be considered coincidental. As a yet another alternative approach to viability evaluation using the dielectric properties of cells, we optically monitored the movement of cells on chip due to dielectrophoresis under the influence of applied AC fields to determine their response at crossover frequency. The viability trend measured by this method corresponds to the trend with the impedance shown in Figure 6. The details of this experiment and the results obtained are included in the supplementary material.41
The above conclusions can be correlated to other cell parameters. Interestingly, the experimental impedance trend (Figure 6) resembles the trend of the cell diameter as a function of Cedex viability (Figure 3(b)). Cell diameter initially decreases linearly but does not change significantly once the Cedex viability decreases to ∼60%, although the rate of decrease of cell diameter during the viability drop from 90% to 60% is not as high as the measured rate of decrease of impedance. One simple, but unlikely, hypothesis for the correlation of the size to the measured impedance is that the impedance signal captures directly the change in cell size as a change in the electrical properties of the system. It is much more likely, however, that both the changes in impedance and size are derivatives of the ionic and osmotic equilibria in cells at changing viabilities. The changes in both cell diameter and dielectric properties are manifestation of the physiological changes during the process of cell death. The drastic decrease in cell diameter at low viability (last data point in Figure 3(b)) is likely an artifact of the optical method readout due to the emergence of very small vesicles registered as “cells” by the Cedex analyzer.
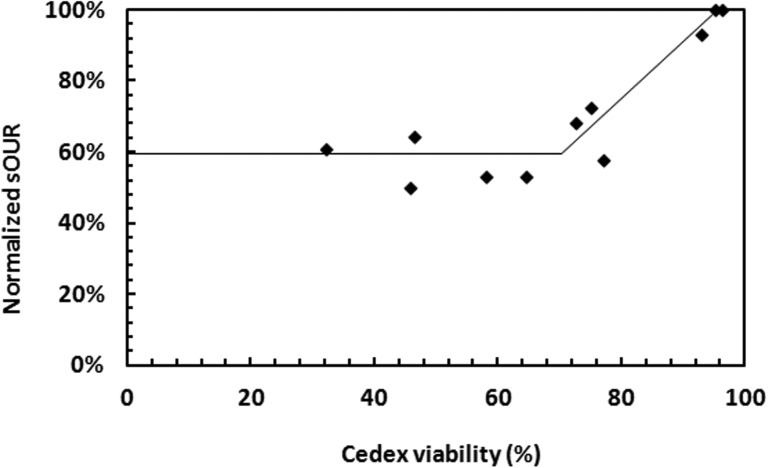
Further confirmation of our findings about the cell viability status was achieved by monitoring the cell specific oxygen uptake rate (sOUR), which is another measure of the cell metabolic activity, using the protocol described in Ref. 45. When cells are “unhealthy,” they consume less oxygen on a normalized cell basis. Cells may exhibit a finite sOUR or metabolic activity even after the initiation of apoptosis;32 therefore, it is crucial to evaluate the relative change in sOUR to determine a change in the cell viability status. We found that the trend of the cell sOUR is in correspondence with the impedance trend reported in Figure 6. Similarly to the impedance data, when the Cedex based cell viability dropped from >90% to 40%–70% range, sOUR decreased to ∼60% of its value at >90% viability (Figure 7). sOUR did not change significantly within the range of 40%–70% viability.
FIG. 7.
Plot of normalized sOUR as a function of dye exclusion test based viability (obtained with Cedex).
These results make possible to compare the mechanisms and provide an interpretation of the observed disparity between the viable cell density data obtained using dye exclusion test based Cedex and commercial online capacitance probes installed in the bioreactors. This problem, exemplified in Figure 8, is of immense impact when compounded over the scale and cost of modern biotechnology operations. Compared to off-line Cedex measurement, capacitance probes can provide continuous online data which are very useful for process monitoring and real-time feedback control. In Fig. 8, the viable cell density measured using capacitance probes decreases at a much smaller rate compared to that measured using Cedex (see supplementary material for the procedure used to collect capacitance probe data41). On the contrary, the rate of decrease of cell viability evaluated by our on-chip impedance measurement technique was higher in comparison to that based on Cedex. Differences between cell viability characterization using capacitance probes and on-chip impedance measurement have been tabulated in the supplementary material.41 Capacitance probes estimate viable cell density by correlating the electrical capacitance data of the bulk cell suspension to the viable biomass as routinely done in the literature.21,32,34 The small rate of viability drop measured by the capacitance probes relative to Cedex, as well as to our impedance based technique, is most likely because the dense background suspension of unhealthy or damaged cells (especially during the initial stages of cell death) may also offer a finite capacitance,46 and therefore wrongly read as viable by the bioreactor probe. Moreover, the bioreactor sensor capacitance data are processed to obtain the viable cell density on the basis on a simplified model that involves various assumptions regarding the cell physiology (such as small membrane conductivity, constant membrane capacitance, and constant cell radius), which do not hold true once the cell viability begins to decrease.3,32 Thus, the impedance measuring chips with interdigitated electrodes investigated here may eventually find application as better, smaller, and more precise sensing units in commercial bioreactors.
FIG. 8.
An example of the discrepancy observed in the vcd data obtained using the dye exclusion test (Cedex) and the AC electrokinetics based online capacitance probes (Futura, Aber Instruments) installed in the industrial bioreactors. These data are obtained from a fed-batch mammalian cell culture run in a large-scale bioreactor. The capacitance data were converted to vcd (adjusted capacitance data) by an average coefficient obtained through regression analysis of historical data from day 0 to peak vcd (i.e., day 9). Cedex and capacitance probe based data were normalized by day 0 vcd obtained using Cedex.
IV. CONCLUSIONS
The in-situ characterization and monitoring of cell physiological status and death are an important but challenging task, especially given the inconsistencies among the data from different viability assays. We present a simple approach of applying low-frequency AC fields for direct and precise, albeit non-continuous, on-chip impedance characterization of the physiological condition of cells sedimented over an array of interdigitated microelectrodes. These results were correlated to the common viability assays based on dye exclusion tests. A linear correlation between the two techniques was observed at high cell viabilities (95%–60%), demonstrating that the technology developed in this study is capable of detecting the initial phases of the cell death in a bioreactor cell culture cycle. The impedance data, however, showed little change after the dye exclusion test based viability reached ∼60%. This trend suggests that the cells attain a suboptimal metabolic state in their viability status when the Cedex viability decreases below 60%. We hypothesize that intensive physiological changes take place in the cells during the early phases of death even though their membrane remains intact. While the dye exclusion tests monitor the integrity of the cell membrane, our on-chip technique monitors the overall impedance offered by the cells to AC electric fields and appears more sensitive to the physiologic changes taking place inside the cells (even before the membrane integrity is compromised). The later stage discrepancy between the dye exclusion test and our technique is probably because unhealthy cells with intact outer membranes are counted as viable by the dye exclusion test. Additionally, we observed a correlation between the impedance data obtained with our chips and the data on cell diameter and sOUR. Variability observed in the impedance data was of the same order as that observed in commercial viability assays such as Cedex. Such data inconsistencies have negative impact on manufacturing decisions, and better understanding, monitoring, and control of cell death processes are necessary to address this limitation in viability characterization techniques.
We believe that after further improvements, the microsensor chips with interdigitated microelectrodes presented here can potentially supplement the existing capacitance probes used in the bioreactors for characterizing the cell physiology through continuous impedance measurement. The chip (and its microfluidic sample enclosure) can be potentially integrated into high throughput automation systems for cell line selection.
ACKNOWLEDGMENTS
We are thankful to Dr. Sonia Grego from Research Triangle Institute International for fabricating the microelectrode chips. The financial support of this study by Biogen Idec, Research Triangle Park, NC was gratefully acknowledged.
References
- 1.Schwartz S. M. and Bennett M. R., Am. J. Pathol. 147, 229 (1995). [PMC free article] [PubMed] [Google Scholar]
- 2.Fink S. L. and Cookson B. T., Infect. Immun. 73, 1907 (2005). 10.1128/IAI.73.4.1907-1916.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel P. and Markx G. H., Enzyme Microb. Technol. 43, 463 (2008). 10.1016/j.enzmictec.2008.09.005 [DOI] [Google Scholar]
- 4.Strober W., “Trypan blue exclusion test of cell viability,” in Current Protocols in Immunology, edited by Coligan J. E. and Kruisbeek A. M. (Wiley-Greene, New York, 1994), pp. A.3.3–A.3.4 [Google Scholar]
- 5.Black L. and Berenbaum M. C., Exp. Cell Res. 35, 9 (1964). 10.1016/0014-4827(64)90066-7 [DOI] [PubMed] [Google Scholar]
- 6.Bhuyan B. K., Loughman B. E., Fraser T. J., and Day K. J., Exp. Cell Res. 97, 275 (1976). 10.1016/0014-4827(76)90617-0 [DOI] [PubMed] [Google Scholar]
- 7.Hoskins J. M., Meynell G. G., and Sanders F. K., Exp. Cell Res. 11, 297 (1956). 10.1016/0014-4827(56)90105-7 [DOI] [PubMed] [Google Scholar]
- 8.Kaltenbach J. P., Kaltenbach M. H., and Lyons W. B., Exp. Cell Res. 15, 112 (1958). 10.1016/0014-4827(58)90067-3 [DOI] [PubMed] [Google Scholar]
- 9.Phillips H. J. and Terryberry J. E., Exp. Cell Res. 13, 341 (1957). 10.1016/0014-4827(57)90013-7 [DOI] [PubMed] [Google Scholar]
- 10.Schrek R., Am. J. Cancer 28, 389 (1936). 10.1158/ajc.1936.389 [DOI] [Google Scholar]
- 11.Flanagan L. A., Lu J., Wang L., Marchenko S. A., Jeon N. L., Lee A. P., and Monuki E. S., Stem Cells 26, 656 (2008). 10.1634/stemcells.2007-0810 [DOI] [PubMed] [Google Scholar]
- 12.Gascoyne P. R., Noshari J., Anderson T. J., and Becker F. F., Electrophoresis 30, 1388 (2009). 10.1002/elps.200800373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L. and Bashir R., Biotechnol. Adv. 26, 135 (2008). 10.1016/j.biotechadv.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 14.Li H. and Bashir R., Sens. Actuators, B 86, 215 (2002). 10.1016/S0925-4005(02)00172-7 [DOI] [Google Scholar]
- 15.Auerswald J. and Knapp H. F., Microelectron. Eng. 67–68, 879 (2003). 10.1016/S0167-9317(03)00150-3 [DOI] [Google Scholar]
- 16.Gagnon Z., Mazur J., and Chang H. C., Lab Chip 10, 718 (2010). 10.1039/b917220c [DOI] [PubMed] [Google Scholar]
- 17.Huang Y., Holzel R., Pethig R., and Wang X. B., Phys. Med. Biol. 37, 1499 (1992). 10.1088/0031-9155/37/7/003 [DOI] [PubMed] [Google Scholar]
- 18.Gupta S., Kilpatrick P. K., Melvin E., and Velev O. D., Lab Chip 12, 4279 (2012). 10.1039/c2lc40127d [DOI] [PubMed] [Google Scholar]
- 19.Labeed F. H., Coley H. M., Thomas H., and Hughes M. P., Biophys. J. 85, 2028 (2003). 10.1016/S0006-3495(03)74630-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labeed F. H., Coley H. M., and Hughes M. P., Biochim. Biophys. Acta. 1760, 922 (2006). 10.1016/j.bbagen.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 21.Patel P. M., Bhat A., and Markx G. H., Enzyme Microb. Technol. 43, 523 (2008). 10.1016/j.enzmictec.2008.09.006 [DOI] [Google Scholar]
- 22.Markx G. H., Talary M. S., and Pethig R., J. Biotechnol. 32, 29 (1994). 10.1016/0168-1656(94)90117-1 [DOI] [PubMed] [Google Scholar]
- 23.Ratanachoo K., Gascoyne P. R. C., and Ruchirawat M., Biochim. Biophys. Acta. 1564, 449 (2002). 10.1016/S0005-2736(02)00494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung C., Waterfall M., Pells S., Menachery A., Smith S., and Pethig R., J. Electr. Bioimp. 2, 64 (2011). 10.5617/jeb.196 [DOI] [Google Scholar]
- 25.Castellarnau M., Errachid A., Madrid C., Juarez A., and Samitier J., Biophys. J. 91, 3937 (2006). 10.1529/biophysj.106.088534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demierre N., Braschler T., Muller R., and Renaud P., Sens. Actuators, B 132, 388 (2008). 10.1016/j.snb.2007.09.078 [DOI] [Google Scholar]
- 27.Pethig R., Crit. Rev. Biotechnol. 16, 331 (1996). 10.3109/07388559609147425 [DOI] [Google Scholar]
- 28.Suehiro J., Yatsunami R., Hamada R., and Hara M., J. Phys. D: Appl. Phys. 32, 2814 (1999). 10.1088/0022-3727/32/21/316 [DOI] [Google Scholar]
- 29.Suehiro J., Hamada R., Noutomi D., Shutou M., and Hara M., J. Electrostat. 57, 157 (2003). 10.1016/S0304-3886(02)00124-9 [DOI] [Google Scholar]
- 30.Unni H. N., Hartono D., Yung L. Y. L., Ng M. M. L., Lee H. P., and Khoo B. C., Biomicrofluidics 6, 012805 (2012). 10.1063/1.3671065 [DOI] [Google Scholar]
- 31.Melvin E. M., Moore B. R., Gilchrist K. H., Grego S., and Velev O. D., Biomicrofluidics 5, 034113 (2011). 10.1063/1.3620419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opel C. F., Li J., and Amanullah A., Biotechnol. Prog. 26, 1187 (2010). 10.1002/btpr.425 [DOI] [PubMed] [Google Scholar]
- 33.Gimsa J., Marszalek P., Loewe U., and Tsong T. Y., Biophys. J. 60, 749 (1991). 10.1016/S0006-3495(91)82109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabros M., Dennewald D., Currie D. J., Lee M. H., Todd R. W., Marison I. W., and von Stockar U., Bioprocess Biosyst. Eng. 32, 161 (2009). 10.1007/s00449-008-0234-4 [DOI] [PubMed] [Google Scholar]
- 35.Pethig R. and Talary M. S., IET Nanobiotechnol. 1, 2 (2007). 10.1049/iet-nbt:20060018 [DOI] [PubMed] [Google Scholar]
- 36.Thakur M., Mergel K., Weng A., Frech S., Gilabert-Oriol R., Bachran D., Melzig M. F., and Fuchs H., Biosens. Bioelectron. 35, 503 (2012). 10.1016/j.bios.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Becker F. F., and Gascoyne P. R., Biochim. Biophys. Acta 1564, 412 (2002). 10.1016/S0005-2736(02)00495-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolic-Jaric M., Cabel T., Salimi E., Bhide A., Braasch K., Butler M., Bridges G. E., and Thomson D. J., Biomicrofluidics 7, 024101 (2013). 10.1063/1.4793223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braasch K., Nikolic-Jaric M., Cabel T., Salimi E., Bridges G. E., Thomson D. J., and Butler M., Biotechnol. Bioeng. 110, 2902 (2013). 10.1002/bit.24976 [DOI] [PubMed] [Google Scholar]
- 40.Barnes L. M., Bentley C. M., and Dickson A. J., Cytotechnology 32, 109 (2000). 10.1023/A:1008170710003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.See supplementary material at http://dx.doi.org/10.1063/1.4895564E-BIOMGB-8-009405 for details on patterned electrode fabrication, calculation of sedimentation time of a cell on chip, calculation of cell density needed to obtain a monolayer on chip, viability estimation based on the cell movement on chip due to dielectrophoresis, procedure for collecting capacitance probe data used to calculate viable cell density, a table comparing cell viability characterization using capacitance probes and on-chip impedance measurement and plots showing variability in Cedex data and impedance trend as a function of cell density.
- 42.Hughes J. F. M., Bortner C. D., Purdy G. D., and Cidlowski J. A., J. Biol. Chem. 272, 30567 (1997). 10.1074/jbc.272.48.30567 [DOI] [PubMed] [Google Scholar]
- 43.Vermes I., Haanen C., Steffens-Nakken H., and Reutelingsperger C., J. Immunol. Methods 184, 39 (1995). 10.1016/0022-1759(95)00072-I [DOI] [PubMed] [Google Scholar]
- 44.Li J., Gu W., Edmondson D. G., Lu C., Vijayasankaran N., Figueroa B., Stevenson D., Ryll T., and Li F., Biotechnol. Bioeng. 109, 1685 (2012). 10.1002/bit.24450 [DOI] [PubMed] [Google Scholar]
- 45.Jorjani P. and Ozturk S. S., Biotechnol. Bioeng. 64, 349 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Asami K., J. Phys. D: Appl. Phys. 39, 4656 (2006). 10.1088/0022-3727/39/21/023 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4895564E-BIOMGB-8-009405 for details on patterned electrode fabrication, calculation of sedimentation time of a cell on chip, calculation of cell density needed to obtain a monolayer on chip, viability estimation based on the cell movement on chip due to dielectrophoresis, procedure for collecting capacitance probe data used to calculate viable cell density, a table comparing cell viability characterization using capacitance probes and on-chip impedance measurement and plots showing variability in Cedex data and impedance trend as a function of cell density.