Abstract
Objectives.
As the “fourth-age” conception suggests that the adaptability of psychosocial capabilities is disrupted at old-old age due to failures in maintaining balanced functions, this study examines the predictability of subjective wellness outcomes by factual dysfunction markers of health among old-old people across 12 years.
Method.
Participants were self-respondents in a 3-wave survey that sampled the older (age 75–94) Jewish population in Israel. Wave 1 (N = 1,369, mean age = 83.5) preceded Wave 2 (N = 687, mean age = 85.4) by 4 years and Wave 3 (N = 164, mean age = 91.6) by 12 years.
Results.
The dysfunction markers (comorbidity, medication consumption, doctor’s visits, and difficulties in activities of daily living [ADL]) predicted subjective wellness by relating to an increase in depressive symptoms, as well as to a decrease in life evaluation and self-rated health, beyond adjustment for sociodemographics. However, in most cases, an interaction finding indicated that dysfunction markers were weaker predictors of age-related change in subjective wellness among older participants.
Discussion.
At old-old age, the results point to reduced predictability of subjective wellness by factual dysfunction. This finding supports the fourth-age model. Still, researchers should consider an alternative interpretation, by which increasing independence between factual and subjective indicators is protective, rather than debilitating, among old-old people.
Key Words: Aging, Dysfunction markers, Fourth age, Old-old, Subjective wellness.
Adaptation to old-old age entails an intriguing intertwinement of resilience and vulnerability. A long-lived life attests to constitutional and psychological fortitude, but it simultaneously imposes imminent dangers of deterioration and death. Living in such dialectics of survival and finitude demands tenacious adaptation systems. This study addresses this tenacity by examining the predictability of subjective wellness, referring to self-perceptions, or self-evaluations, of one’s physical and mental integrity. This focus is guided by the assumption that the manner whereby old-old individuals maintain their integrity is reflected by the responsiveness of their subjective functions to the intensifying biological declines.
The issue of predictability at old-old age is pertinent to the conception of the “fourth age” (Baltes & Smith, 1999, 2003) as based on Baltes’ (1997) theory of the incomplete architecture of human ontology. This theory asserts that human development is not programmed to function optimally at the fourth age (about age 85 and older) because the individual’s psychosocial resources (e.g., knowledge, skills, coping strategies), which are acquired and regulated in cultural contexts, become inefficient at this age to fulfill their life-long role of compensating for the natural constraints and omissions of biological functioning. Therefore, old-old individuals are pushed into the limits of their psychological capacity, and essential balances between gains and losses may not be sustained any more.
The notions of the third and the fourth age gained popularity in scholarly discourse (Carr & Komp, 2011; Laslett, 1991). The rationale for this division of old age largely dwelt on Neugarten’s (1974) distinction between the “young-old” and “old-old” individuals. Thus, although young-old individuals still maintain personal fulfillment and social engagement in a relatively good health, old-old individuals are mostly characterized by disability and dependency. In this frame, the aforementioned contribution of Baltes and Smith (1999, 2003) derives from their evidence-based explication of the developmental process underlying the transition from the third to the fourth age. Illustrating this process, Gilleard and Higgs (2010) depict the fourth age as a “black hole” that negates the individual’s agency in life through the collapse of capabilities such as choice, autonomy, and self-expression. Further illustrative terms are offered by the gerodynamics model (Schroots, 1995), which regards very old individuals as far-from-equilibrium systems. Such systems are driven to lower order transformations that produce more disorder than order until the system’s death.
Taking this conceptual viewpoint one step further, it may be inferred that the capability of old-old individuals to preserve subjective self-coherence may be jeopardized by unpredictable changes in their destabilized adaptation systems. Such a condition may hamper the predictability (i.e., the feasibility of being forecast by earlier data) of self-perceptions and self-evaluations at this age. Presumably, the ability of earlier data to predict later functioning shrinks not only when aging processes lead into conspicuously different modes of existence (e.g., dementia) but also when normative, continuous trajectories of decline take place (Gerstorf, Smith, & Baltes, 2006).
The current conceptual framework gives precedence to the deterioration of physical health in explaining the possibly loosening coherence of subjective wellness. Thus, the predictability of subjective wellness outcomes would preferably be tested by objective health-status markers, which do not share subjective elements with the predicted outcomes. However, epidemiological surveys typically assess health by self-report queries that contain subjective ingredients ensuing from verbal difficulties, memory biases, and self-presentational motives. To minimize this confounding, this study used factual health-status queries, whereby respondents reported straightforward facts supposedly accessible to them, such as diseases they suffered from, medications they took, their recent visits to the doctor, and their difficulty in ADL. Although such factual self-reports still suffer from the aforementioned confounders, researchers usually treat them as valid, objective-like markers (Young, Boyd, Guralnik, & Fried, 2010). As wellness at old-old age is usually on the decline, this study presents these factual predictors as dysfunction markers.
By incorporating the currently examined outcomes into subjective wellness, we dwell on previous conceptions of wellness that emphasized the subjective, or self-perceived, experience of health (Adams, Bezner, & Steinhardt, 1997; Jensen & Allen, 1994). Most conceptions of wellness refer to a synergistic construct consisting of various dimensions, such as physical, cognitive, emotional, and social (Roscoe, 2009). Yet, despite this multiplicity, evidence shows that subjective wellness has an underlying, unified dimension (Adams et al., 1997; Harari, Waehler, & Rogers, 2005). This study presents subjective wellness by a parsimonious set of indices, including depressive symptoms (self-perceptions typifying mental wellness), personal life evaluation (one’s rough evaluation of how well one lives), and self-rated health (a self-perception of global physical wellness). Although these indices were interrelated (see Results), they were not combined into a composite score in order to keep track on their respective facets.
Longitudinal studies that focused on predicting subjective wellness outcomes in old-old populations showed diversity over the salience of physical health predictors versus psychosocial predictors. For example, poor daily functioning in an 85-year-old cohort sample predicted depression for a 4-year period (Stek et al., 2006). Also, chronic illness, difficulties in ADL, and cognitive decline among individuals with a mean age 77, independently predicted depressive symptoms for a 2-year period (Turvey, Schultz, Beglinger, & Klein, 2009). Turning to an example of psychosocially based prediction, social support appeared the strongest predictor of change in depressive symptoms for an 8-year period among individuals with a mean age 86 who were in a process of disablement (Fauth, Gerstorf, Ram, & Malmberg, 2012).
When examining life satisfaction as an outcome, physical symptoms and impaired ADL among individuals aged 78–93 predicted a decline in life satisfaction for a 3-year period (Enkvist, Ekström, & Elmståhl, 2012). Another study highlighted the loss of a spouse and stronger depressive symptoms among individuals aged 80 and older as predictors of decline in life satisfaction for a 6-year period (Berg, Hoffan, Hassing, McClearn, & Johansson, 2009). When examining subjective health as an outcome, Galenkamp, Braam, Huisman, and Deeg (2013) found in a 17-year long study on individuals aged 60–85 that poor self-rated health was decreasingly determined by chronic diseases and increasingly determined by severe disability. Another study showed that a psychological index of personality predicted self-rated health among octogenarians and nonagenarians for a 2-year period, regardless physical health effects (Wu & Schimmele, 2006).
The aforementioned studies indicate that subjective wellness outcomes can be soundly predicted at old-old age by earlier data, but the question of how solid this predictability is, compared with younger age, still needs an answer. As argued previously, this study attempts to provide such an answer by restricting the examined predictors into the factual, health-status domain. This examination adjusts for potential biases of sociodemographic covariates (gender, origin, education, and marital status), which may represent confounding effects of age-related resources and vulnerabilities. Thus, although sociodemographic characteristics are often consistent concomitants of subjective wellness (Pinquart & Sorensen, 2000), their statistical control is required for exposing the strength of major predictors, such as disability, at old age (Fauth et al., 2012). Evidently, beyond the scope of this study, further research should address the intricate paths, and possibly loops, of predictability where subjectivity prevails not only in the predicted outcomes but also in predictors coming from psychosocial domains.
In conclusion, the predictability of subjective wellness outcomes at old-old age bears a conceptual implication, notably for the fourth-age model (Baltes & Smith, 1999). Following this model, we hypothesized that the ability to predict subjective wellness outcomes by factual dysfunction markers would diminish at old-old age compared with younger age. Employing multilevel modeling (MLM), we tested this hypothesis in panel data of a nationwide survey specifically designed for the older (75+) population.
Method
Database and Participants
The Cross-Sectional and Longitudinal Aging Study (CALAS) is a three-wave survey designed to assess the health, mental, and social status of Israelis aged 75 and over. It conducted a random sampling of the old Jewish population in Israel stratified by age group (75–79, 80–84, 85–89, and 90–94), gender, and place of birth (Israel, Europe/America, and Mideast/North Africa). The initial sample was drawn on January 1, 1989, from the Israel National Population Registry (a listing updated for births, deaths, and in and out migration).
Wave 1 (W1) interviews were conducted during 1989–1992. Out of the 2,400 sampled individuals (100 in each of the 24 strata), 15.7% had died before the sampling day or were not located, and 8.5% refused to be interviewed. In the 1820 interviews that took place, 1,369 individuals were self-respondents, whereas 451 were interviewed by proxy (either because they were incapable of responding or had died between the sampling day and the interview date).
Wave 2 (W2) interviews were conducted in 1993–1994 with an average interval of nearly 4 years from W1. Of the original participants, 1,455 were interviewed: 697 as self-respondents and 758 by proxy (either because they were incapable of responding or had died between W1 and W2). Wave 3 (W3) interviews were conducted in 2001–2002, with an average interval of nearly 12 years from W1. As no proxy interviews were planned at W3, there were 325 potential participants, out of the 1,369 self-respondents at W1, who were recorded alive in the National Population Registry as of February 2000. In this group, 25.5% had died until the interview date, 9.2% were not located, 6.5% were incapable of responding, 5.2% refused, and 3.1% were only partly interviewed. The final W3 sample consisted of 164 participants at the age range 86–105.
Included in the current analyses are participants who were self-respondents at the waves they took part in: N = 1,369 at W1, 697 at W2, and 164 at W3. The random stratified design of the initial sampling ensured comparable numbers of participants within the age range (75–94) as well as across gender and place-of-birth categories. Table 1 presents background characteristics of the samples at the three waves. As seen, W2 participants still approximated the original stratified distribution of gender, but the higher longevity of women was apparent at W3. Also expected at W3 were the fewer married participants due to widowhood.
Table 1.
Descriptive Statistics of the Study Variables
| Variable | Wave 1 | Wave 2 | Wave 3 |
|---|---|---|---|
| 1989–1992 | 1993–1994 | 2001–2002 | |
| N = 1,369 | N = 687 | N = 164 | |
| Age | |||
| Mean | 83.52 | 85.37 | 91.57 |
| SD | 5.42 | 5.14 | 4.39 |
| Gender | |||
| Women (%) | 46.3 | 46.4 | 57.3 |
| Place of birth | |||
| Israeli-born (%) | 31.8 | 33.9 | 36.0 |
| Education (school years) | |||
| Mean | 7.79 | 8.26 | 7.86 |
| SD | 5.54 | 5.53 | 5.66 |
| Marital status | |||
| Married (%) | 42.7 | 39.4 | 26.1 |
| Depressive symptoms | |||
| Mean | 0.75 | 0.81 | 0.86 |
| SD | 0.44 | 0.46 | 0.45 |
| Life evaluation | |||
| Mean | 2.80 | 2.75 | 2.77 |
| SD | 0.98 | 0.92 | 1.02 |
| Self-rated health | |||
| Mean | 1.98 | 1.93 | 2.06 |
| SD | 0.85 | 0.77 | 0.88 |
| Number of diseases | |||
| Mean | 3.67 | 4.15 | 4.02 |
| SD | 2.47 | 2.55 | 2.77 |
| Number of medications | |||
| Mean | 3.02 | 3.32 | 2.68 |
| SD | 2.27 | 2.31 | 1.96 |
| Doctor’s visits | |||
| Mean | 1.03 | 0.93 | 0.81 |
| SD | 0.94 | 0.90 | 0.86 |
| Difficulties in ADL | |||
| Mean | 1.59 | 2.07 | 5.39 |
| SD | 3.59 | 3.78 | 6.47 |
Note. ADL, activities of daily living.
Measures
The CALAS measures were adapted from U.S. multidimensional surveys of old populations, from which questions were adapted to the Israeli context.
Depressive symptoms were measured by the Center for Epidemiological Studies-Depression scale (CESD; Radloff, 1977). The Hebrew adaptation of the CESD in the CALAS was validated by Ruskin et al. (1996). Respondents were asked to rate the frequency they had experienced each of 20 depressive symptoms in the past month on a scale of not at all (0), sometimes (1), most of the time (2), and almost every day (3). The items expressed negative affects, lack of well-being, psychosomatic reactions, and interpersonal distress. The score was the respondent’s mean rating after reversing four positive items. Cronbach’s alpha coefficients were .88, .88, and .85 at W1, W2, and W3, respectively.
Life evaluation was measured by asking the respondents to rate their lives today on a scale ranging from 1 (very difficult) to 4 (good).
Self-rated health was measured by asking respondents to rate their current health on a scale ranging from 1 (bad) to 4 (excellent).
Number of diseases (comorbidity) was measured on a checklist of 26 major diseases and medical conditions (e.g., heart disease, cancer, arthritis, diabetes) that the respondent ever suffered from or was ever informed by a physician of having them.
Number of medications was measured by the count of the respondent’s current medications. The information was collected by asking the respondents to display the containers of all medications they were currently taking and recording them.
Doctor’s visits included the reported number of visits to the doctor in the last month (0 = 0 visits, 1 = 1 visit, 2 = 2–4 visits, 3 = 5 or more visits).
Difficulties in ADL were measured by Katz, Downs, Cash, and Grotz’s (1970) scale with Branch, Katz, Kniepmann, and Papsidero’s (1984) addition. This measure consisted of seven items, each rating the difficulty in performing a certain activity (crossing a room, washing, dressing, eating, grooming, transferring, and toileting) on a scale of no difficulty (0), some difficulty (1), severe difficulty (2), and complete disability (3). The sum score ranged from 0 to 21. Alpha coefficients were .89, .86, and .92 at W1, W2, and W3, respectively.
Procedure
The participants were interviewed in their residence (home or institution). The interviewers read aloud the questions and recorded the participants’ responses. The interviews were usually conducted in Hebrew, but the interviewers were multilingual and trained to interview, when necessary, in the respondent’s native language (occasionally with a translator). The interview lasted approximately 2hr. The study was approved according to Helsinki Committee’s ethical requirements for treating research participants. Participants signed an informed consent and their confidentiality was secured. Further details on the CALAS are presented in various publications (e.g., Benyamini, Blumstein, Lusky, & Modan, 2003; Blumstein, Shmotkin, Eyal, Shorek, & Lerner-Geva, 2008; Cohen-Mansfield, Shmotkin, & Hazan, 2010; Palgi et al., 2010; Shmotkin et al., 2010; Shrira, Palgi, Ben-Ezra, & Shmotkin, 2011).
Data Analysis
The current longitudinal data were analyzed by MLM, which can simultaneously consider interindividual differences and intraindividual changes, thus addressing differential patterns and degrees of changes in the variables along time. Moreover, MLM handles prevalent difficulties in longitudinal data, such as dropout of respondents along waves and varying intervals between waves (Mroczek, Almeida, Spiro, & Pafford, 2006).
In order to examine how dysfunction markers co-vary over time with each of the dependents (depressive symptoms, life evaluation, and self-rated health), we first fitted separate MLMs to each of the dysfunction markers (number of diseases, number of medications, doctor’s visits, and difficulties in ADL), generating altogether 12 models. The dependents were standardized to a T metric. Assuming a value of depressive symptoms/life evaluation/self-rated health for person i at time t, the models were parameterized as Subit = L i + S i (timeit) + e it, where Subit is a function of an individual-specific intercept parameter, L i is an individual-specific slope parameter, S i indicates change over the selected time dimension (with each model including one of the four dysfunction markers), and e it is a residual error. The interindividual differences in level, L i, and slope, S i, are assumed to be normally distributed around group means, correlated with each other, and uncorrelated with the residual error, e it. Models were fitted to the data using SPSS 17 (Peugh & Enders, 2005), with incomplete data being treated as missing at random (Little & Rubin, 2002). We used the Δ pseudo-R 2 index (Snijders & Bosker, 1999) to assess the relative proportion of explained variance for each model. By including age (centered at the grand mean age at W1), gender (coded as man = 0, woman = 1), place of birth (two dummy variables, respectively, coded as Israeli-born = 0, Europe/America or Mideast/North Africa = 1), education (measured as years of schooling), and marital status (coded as currently unmarried = 0, married = 1) as covariates into our models, we examined the extent that these variables accounted for interindividual differences in the dependents’ level and their covariance with the dysfunction markers.
Following these basic models, additional growth models examined whether interindividual differences in the respective level of depressive symptoms/life evaluation/self-rated health and their covariance with dysfunction markers changed as a function of age (centered at the grand mean age at W1). These models were parameterized as Subit = L i + Agei (centered) + S i (timeit) + Agei (centered)*S i (timeit) + e it. In these models, gender, place of birth, education, and marital status were included as covariates.
Results
Descriptive Statistics
Table 1 presents the means and standard deviations of the study’s variables. Significance tests for differences between waves had to avoid the mixture of dependent and independent observations as respectively produced by surviving and non-surviving participants. Hence, a cautious inspection (also considering paired t tests that respectively compared survivors to W2 and W3 with their preceding measurements) shows that at W2, relative to W1, levels of subjective wellness tended to decline and levels of dysfunction, except for doctor’s visits, rose (these differences were stronger and significant at the .001 level in testing only the 687 survivors to W2). This trend was only partially replicated at W3 when, relative to W2, only one of three indices indicated a decline in subjective wellness (more depressive symptoms; difference significant at .001 in testing only the 164 survivors to W3), and only one of four markers indicated a rise in dysfunction (more difficulties in ADL; difference significant at .001 in testing only those 164).
Table 2 presents correlations between the study variables at W1. The subjective wellness indices (depressive symptoms, life evaluation, and self-rated health) were substantially intercorrelated (r sizes ranging 0.44–0.52). They yielded one factor in a principal-component factor analysis, explaining 65.6% of the total variance (Kaiser–Meyer–Olkin measure of sampling adequacy was 0.68; Bartlett test of sphericity was 840.4, df = 3, p < .001). Depressive symptoms were correlated with higher impairment on all dysfunction markers (r sizes ranging 0.24–0.34). Life evaluation and self-rated health were correlated with lower impairment on all dysfunction markers (r sizes ranging 0.16–0.41). The dysfunction markers showed low to moderate intercorrelations (r sizes ranging 0.17–0.54). All the aforementioned correlations were significant at .001 and yielded a similar pattern at W2 and W3.
Table 2.
Bivariate Correlations Between the Study Variables at Wave 1
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Depressive symptoms | — | ||||||
| 2. Life evaluation | −.49*** | — | |||||
| 3. Self-rated health | −.44*** | .52*** | — | ||||
| 4. Number of diseases | .34*** | −.25*** | −.41*** | — | |||
| 5. Number of medications | .26*** | −.19*** | −.30*** | .54*** | — | ||
| 6. Doctor’s visits | .24*** | −.16*** | −.21*** | .26*** | .29*** | — | |
| 7. Difficulties in ADL | .29*** | −.22*** | −.22*** | .21*** | .17*** | .19*** | — |
| 8. Age | .05* | −.09** | −.03 | .01 | .06* | .09** | .21*** |
| 9. Gendera | .24*** | −.11*** | −.16*** | .19*** | .16*** | .12*** | .10*** |
| 10. Place of birthb | .16*** | .05 | .09** | −.07* | −.04 | .00 | −.01 |
| 11. Education | −.21*** | .15*** | .16*** | −.04 | .01 | −.05 | −.09** |
| 12. Marital statusc | −.24*** | .13*** | .10*** | −.10*** | −.08** | −.10*** | −.12*** |
Notes. N = 1,369. ADL, activities of daily living.
aCoded 0 = man; 1 = woman.
bCoded 0 = Israeli-born; 1 = born outside Israel.
cCoded 0 = currently unmarried; 1 = married.
*p < .05. **p < .01. ***p < .001
Multilevel Models
First, we estimated the relative amount of between-person and within-person variance in subjective wellness indices by considering a model that allowed random effects for only the intercept. The intraclass correlation was 0.43, 0.35, and 0.41 for depressive symptoms, life evaluation, and self-rated health, respectively, suggesting that 43%, 35%, and 41% of the total variation in each respective index were of between-persons, and that the remainder was within-person variation. This result indicated substantial variability within persons over time. Given this indication, we proceeded to evaluate the different dysfunction markers as dimensions to model within-person change in depressive symptoms, life evaluation, and self-rated health over time.
Dysfunction markers were significantly related to an increase in depressive symptoms when models, respectively, included number of diseases (slope = 1.27, SE = 0.08, p < .0001, Δ pseudo-R 2 = .072), number of medications (slope = 0.97, SE = 0.09, p < .0001, Δ pseudo-R 2 = .024), doctor’s visits (slope = 1.65, SE = 0.23, p < .0001, Δ pseudo-R 2 = .015), or difficulties in ADL (slope = 0.76, SE = 0.06, p < .0001, Δ pseudo-R 2 = .088). When sociodemographic covariates were added to the models, the relationships between all dysfunction markers and depressive symptoms remained significant (for number of diseases: slope = 1.14, SE = 0.10, p < .0001, Δ pseudo-R 2 = .200; for number of medications: slope = 0.88, SE = 0.09, p < .0001, Δ pseudo-R 2 = .069; for doctor’s visits: slope = 1.41, SE = 0.23, p < .0001, Δ pseudo-R 2 = .078; for difficulties in ADL: slope = 0.66, SE = 0.06, p < .0001, Δ pseudo-R 2 = .100). All covariates were associated with depressive symptoms: Positive coefficients emerged for age, gender (higher depressive symptoms for women) and the two dichotomized place-of-birth variables (higher depressive symptoms for those born in Europe/America or in Mideast/North Africa vs. Israeli-born), whereas negative coefficients emerged for education and marital status (higher depressive symptoms for unmarried).
Dysfunction markers were significantly related to a decrease in life evaluation when models, respectively, included number of diseases (slope = −0.99, SE = 0.08, p < .0001, Δ pseudo-R 2 = .058), number of medications (slope = −0.73, SE = 0.09, p < .0001, Δ pseudo-R 2 = .023), doctor’s visits (slope = −1.39, SE = 0.22, p < .0001, Δ pseudo-R 2 = .012), or difficulties in ADL (slope = −0.55, SE = 0.05, p < .0001, Δ pseudo-R 2 = .098). When sociodemographic covariates were added to the models, the relationships between all dysfunction markers and life evaluation remained significant (for number of diseases: slope = −0.91, SE = 0.08, p < .0001, Δ pseudo-R 2 = .059; for number of medications: slope = −0.72, SE = 0.09, p < .0001, Δ pseudo-R 2 = .028; for doctor’s visits: slope = −1.23, SE = 0.22, p < .0001, Δ pseudo-R 2 = .010; for difficulties in ADL: slope = −0.48, SE = 0.06, p < .0001, Δ pseudo-R 2 = .100). Of the covariates, age was negatively associated with life evaluation, whereas education and marital status were positively associated (higher life evaluation for married).
Dysfunction markers were also significantly related to a decrease in self-rated health when models, respectively, included number of diseases (slope = −1.42, SE = 0.07, p < .0001, Δ pseudo-R 2 = .052), number of medications (slope = −1.15, SE = 0.09, p < .0001, Δ pseudo-R 2 = .092), doctor’s visits (slope = −1.99, SE = 0.21, p < .0001, Δ pseudo-R 2 = .008), or difficulties in ADL (slope = −0.51, SE = 0.04, p < .0001, Δ pseudo-R 2 = .060). When sociodemographic covariates were added to the models, the relationships between all dysfunction markers and self-rated health remained significant (for number of diseases: slope = −1.35, SE = 0.07, p < .0001, Δ pseudo-R 2 = .064; for number of medications: slope = −1.09, SE = 0.09, p < .0001, Δ pseudo-R 2 = .111; for doctor’s visits: slope = −1.80, SE = 0.22, p < .0001, Δ pseudo-R 2 = .033; for difficulties in ADL: slope = −0.47, SE = 0.05, p < .0001, Δ pseudo-R 2 = .060). Of the covariates, a negative coefficient emerged for gender (lower self-rated health for women), and a positive coefficient for education.
Table 3 presents the estimates of the model that also included, besides the covariates, the interaction terms between age and the respective dysfunction marker. As seen, when depressive symptoms were the dependent, except for the age × number of diseases, all the interactions were significant in their respective models. When life evaluation was the dependent, both the age × number of medications and age × doctor’s visits interactions were significant. When self-rated health was the dependent, all the interactions were significant.
Table 3.
Growth Models for Depressive Symptoms, Life Evaluation, and Self-Rated Health Examining the Interaction Between Age and the Respective Dysfunction Marker
| Model | Depressive symptoms | Life evaluation | Self-rated health | |||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| Age | 0.11 | 0.06 | −0.14* | 0.06 | −0.15* | 0.06 |
| Gender | 2.18*** | 0.60 | −0.06 | 0.53 | −0.60 | 0.51 |
| Place of birth (west) | 2.25*** | 0.61 | −0.51 | 0.54 | −1.00* | 0.51 |
| Place of birth (east) | 2.81*** | 0.70 | −0.72 | 0.61 | −0.73 | 0.59 |
| Education | −0.21*** | 0.05 | 0.26*** | 0.04 | 0.23*** | 0.04 |
| Marital status | −2.70*** | 0.55 | 1.43** | 0.52 | 0.47 | 0.49 |
| Number of diseases slopea | 1.14*** | 0.10 | −0.91*** | 0.08 | −1.35*** | 0.07 |
| Age × slope | 0.00 | 0.01 | 0.01 | 0.01 | 0.02* | 0.01 |
| Age | 0.19** | 0.05 | −0.22*** | 0.06 | −0.19** | 0.06 |
| Gender | 2.47*** | 0.52 | −0.39 | 0.55 | −1.36* | 0.53 |
| Place of birth (west) | 1.85*** | 0.53 | −0.35 | 0.55 | −0.74 | 0.53 |
| Place of birth (east) | 3.15*** | 0.60 | −0.80 | 0.63 | −0.80 | 0.62 |
| Education | −0.23*** | 0.04 | 0.25*** | 0.04 | 0.25*** | 0.04 |
| Marital status | −2.96*** | 0.50 | 1.57** | 0.53 | 0.53 | 0.51 |
| Number of medications slopea | 0.88*** | 0.09 | −0.71*** | 0.09 | −1.09*** | 0.09 |
| Age × slope | −0.03* | 0.01 | 0.04* | 0.01 | 0.04** | 0.01 |
| Age | 0.20*** | 0.05 | −0.18** | 0.05 | −0.19** | 0.05 |
| Gender | 2.94*** | 0.54 | −0.84 | 0.55 | −1.96*** | 0.54 |
| Place of birth (west) | 2.18*** | 0.54 | −0.65 | 0.56 | −1.30* | 0.55 |
| Place of birth (east) | 2.92*** | 0.62 | −0.82 | 0.64 | −0.83 | 0.63 |
| Education | −0.21*** | 0.04 | 0.24*** | 0.04 | 0.22*** | 0.04 |
| Marital status | −2.93*** | 0.51 | 1.43** | 0.53 | 0.44 | 0.52 |
| Doctor’s visits slopea | 1.42*** | 0.22 | −1.23*** | 0.22 | −1.83*** | 0.22 |
| Age × slope | −0.10* | 0.03 | 0.08* | 0.03 | 0.11** | 0.03 |
| Age | 0.06 | 0.04 | −0.05 | 0.04 | −0.03 | 0.04 |
| Gender | 2.57*** | 0.52 | −0.45 | 0.55 | −1.59** | 0.55 |
| Place of birth (west) | 2.45*** | 0.52 | −0.76 | 0.55 | −1.55** | 0.55 |
| Place of birth (east) | 2.92*** | 0.61 | −0.58 | 0.64 | −0.40 | 0.63 |
| Education | −0.20*** | 0.04 | 0.24*** | 0.04 | 0.23*** | 0.04 |
| Marital status | −2.79*** | 0.50 | 1.49** | 0.53 | 0.57 | 0.53 |
| Difficulties in ADL slopea | 0.74*** | 0.06 | −0.53*** | 0.07 | −0.55*** | 0.05 |
| Age × slope | −0.03** | 0.01 | 0.01+ | 0.01 | 0.02** | 0.00 |
Notes. N = 1,369 participants who provided 2,220 observations. ADL, activities of daily living. Gender is coded 0 = man, 1 = woman; marital status is coded 0 = currently unmarried; 1 = married.
aSlope refers to the relationship between the specific dysfunction marker entered to the model and the dependent indicated in the column (dependents were T-score standardized).
+ p = .07. *p < .05. **p < .01. ***p < .001.
Following Aiken and West (1991), the interactions were probed by separately calculating simple slopes for those whose age was 1 SD less than the mean, at the mean, and 1 SD more than the mean. As illustrated by the regression lines in Figure 1a, difficulties in ADL were a weaker predictor of age-related change in depressive symptoms among older participants. In Figure 1b, number of medications was a weaker predictor of age-related change in life evaluation among older participants. In Figure 1c, doctor’s visits were a weaker predictor of age-related change in self-rated health among older participants. The same trend was found when plotting the other significant interactions.
Figure 1.
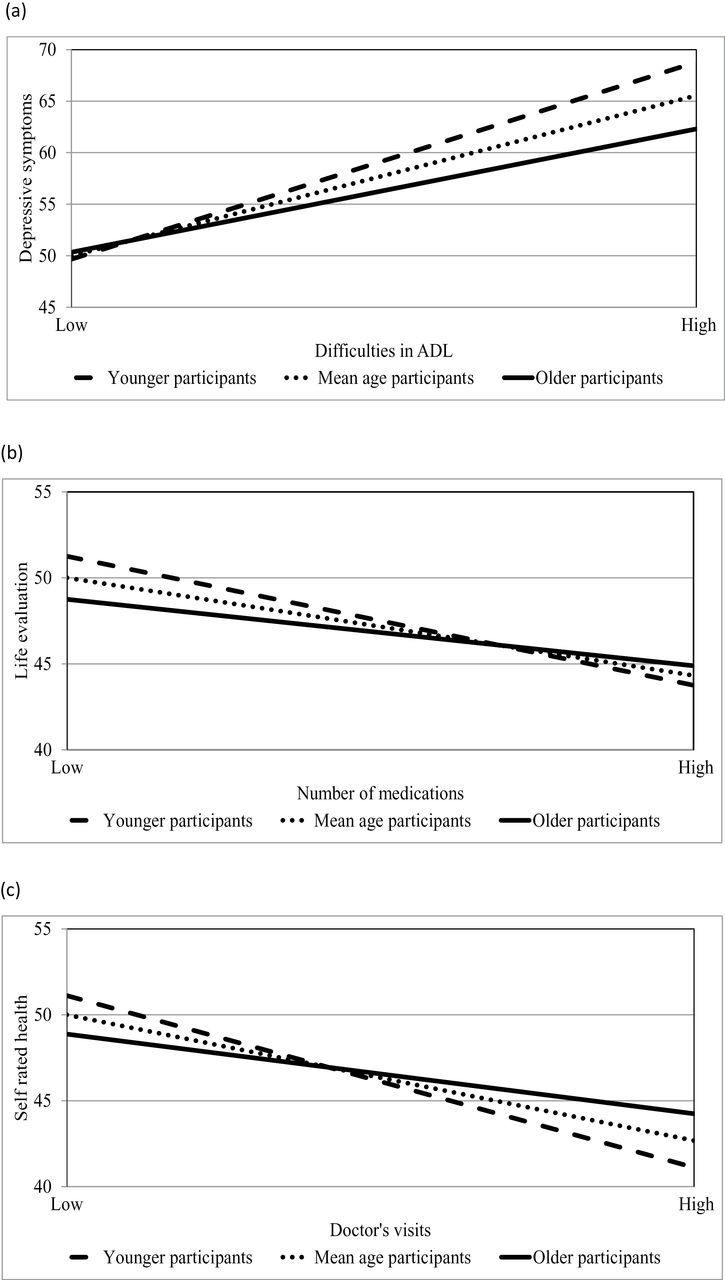
The relationships between dysfunction markers and (a) depressive symptoms, (b) life evaluation, and (c) self-rated health (in T scores) as a function of age (younger and older participants are defined as being 1 SD less than and more than the mean age, respectively).
Discussion
Old-old people present a paradigmatic position by which their survival indicates resilience, whereas their proximity to death implies vulnerability. This paradox possibly points to a qualitatively distinct constellation of fourth-age functioning (Baltes & Smith, 1999). In this context, we studied the predictability of subjective wellness outcomes, namely self-perceptions and self-evaluations about one’s mental and physical status, among old-old Israelis over a 12-year follow-up. Our results seemingly support the fourth-age reasoning of a loosening structure of functions among the old-old. Thus, using factual dysfunction markers as predictors, we found that the ability of those markers to predict later manifestations of subjective wellness was progressively weakened at old-old age. Next, we discuss the current findings and the caution required in their interpretation.
The old-old participants (mostly in their 90s) who survived to W3 in the current longitudinal study plausibly represented more resilient people than those who either had passed away prior to W3 or were then unavailable for reasons such as disability and refusal. This resilience is implied by the finding that certain dysfunction markers (number of diseases, number of medications, and doctor’s visits) did not increase at W3, relative to W2. Yet, this finding might have explanations other than resilience, such as the effect of lower mobility on reducing doctor’s visits and the effect of medical care policies toward old-old people on reducing the number of targeted diseases and related medications (Blumstein et al., 2008). Generally, W3 participants still represented a great variability of well-functioning versus ill-functioning people. Thus, with respect to the subjective wellness outcomes and most dysfunction markers, W3 yielded similar, or even higher, variances relative to W1 and W2 (see the respective SDs in Table 1).
The MLM analysis indicated a reasonable balance of between-person variance (35%–43%) and within-person variation over time (57%–65%). In this frame, an increase in each of the dysfunction markers was associated with the expected increase, or decrease, in each of the subjective wellness outcomes over time. These associations remained significant after adjustment for sociodemographic variables.
With the indication that dysfunction markers predicted subjective wellness in late life, it is important to understand the additional interaction whereby the predictability of subjective wellness was conditioned upon age. Briefly, this predictability was attenuated at old-old age. More specifically, our growth curve models indicated that dysfunctional markers, at the within-person level, were less predictive of age-related change in subjective wellness among the old-old. Thus, out of four predictors, two (number of medications and doctor’s visits) interacted with age in predicting all three indices of subjective wellness; one (difficulties in ADL) interacted with age in predicting two indices (depressive symptoms and self-rated health); and one (number of diseases) interacted with age in predicting one index (self-rated health).
Notably, the data eliminate certain explanations that appear relevant to this kind of age-based interaction. First, as argued previously, the attenuated predictability of subjective wellness did not appear to result from curtailed variability at old-old, relative to younger, age. Second, this attenuation did not appear a confounded result because it remained consistent beyond adjustment for sociodemographics. Lastly, the finding did not appear to involve lower measurement reliability among the old-old, as multi-item measures (depressive symptoms and ADL) presented similarly high Cronbach’s alpha coefficients of internal consistency across all waves.
In view of the earlier considerations, the current findings seemingly corroborate Baltes’s (1997) theory of the incomplete architecture of human ontology. Thus, the reduced predictability of subjective wellness conforms to the assertion that culturally regulated resources, including self-conceived knowledge about oneself, become at the fourth age less adaptive due to a shaky balance with the worsening biological functioning. Rather than reflecting a methodological artifact, these findings suggest a systemic weakening of functional relations, such as the current ones between factual and subjective indicators of adaptation. This process does not imply that individual differences among the old-old should shrink, but rather that they become less relevant to the individual’s functioning (a point stressing the necessity of within-person, rather than between-person, analytic approach).
The phenomenon of reduced predictability still involves certain complexities within the fourth-age conception. First, due to blurred boundaries between the third and fourth ages (George, 2011), this phenomenon may already start among the young-old. For example, analysis of U.S. nationwide data revealed that higher levels and positive changes in perceived control predicted survival more strongly at midlife than at older age (Infurna, Ram, & Gerstorf, 2012). Second, reduced predictability at old-old age may be differential across domains. For example, strengthening factors, such as social relatedness and support, may counter dysfunction by increasing their associations with subjective wellness in later life (Charles & Carstensen, 2009; Pinquart & Sorensen, 2000). Also, crucial within-person changes in mental outcomes during late phases of life may be effectively predicted by newly pertinent factors, such as distance to death and distance to (or from) disability, rather than by more conventional predictors (Gerstorf, Ram, Röcke, Lindenberger, & Smith, 2008; Palgi et al., 2010; Ram, Gerstorf, Fauth, Zarit, & Malmberg, 2010).
In contrast to the fourth-age conception of a downslope in adaptability, and hence in predictability, at very late life, there is still an option to understand the relative independence of subjective wellness from dysfunction markers as a strategy of adaptation to old-old age. This latter interpretation may derive its rationale from Shmotkin’s (2005, 2011) model on the pursuit of happiness in the face of adversity. According to this model, the contesting systems of subjective well-being (currently represented by subjective wellness) and the hostile-world scenario (currently represented by the imminent threats posed by the dysfunction markers) reciprocally regulate each other’s activation. Thus, the role of subjective well-being is to ensure one’s functioning within a favorable psychological environment, whereas the role of the hostile-world scenario is to scan for actual or potential threats to one’s life or integrity. For old-old people, however, it may be more adaptive for the subjective well-being system to constitute a reasonably favorable psychological environment in partial disconnection with the threats imposed by critically impaired functions. Hence, by implementing this strategy at old-old age, subjective wellness becomes less efficient in indexing factual health, thus gaining flexibility in exercising protective psychological mechanisms (e.g., self-serving appraisals, emotional regulation) necessary to maintain well-being. Accordingly, the possibility that the effect of health decrements on subjective wellness becomes weaker at old-old age may actually indicate a well-functioning, self-regulatory system.
The previous interpretation may further explain various cross-sectional findings that the usually expected associations of disability and chronic stresses with subjective wellness are lower among old-old, than among younger, people (Jang, Poon, & Martin, 2004; Mehta et al., 2008; Palgi, 2012) and that these relatively lower associations indicate better functioning (Hong, Zarit, & Malmberg, 2004). In this vein, Shmotkin and colleagues (in press) found old-old participants to hold a surprisingly favorable evaluation of their present memory capability despite an objective decline in their cognitive functioning along preceding years. Also, Bodner, Palgi, and Kaveh (2012) found that positive affect was more independent of negative affect among old-old participants, and that such independence was related to higher self-esteem. The indications of these studies still need further longitudinal data confirming that decreasing predictability of self-evaluations in the realm of wellness may actually reflect a protective, rather than debilitating, mode of adaptation to old-old age.
The current results should be considered with caution in view of certain limitations. First, although our three-wave design allowed for MLM, testing changes in strength of relationships along time requires more measurement points so that a correlation between predictors and outcomes can be established at the within-person level. Second, the generalizability of the results is dubious with regard to physically and mentally frail old-old people, who are largely inaccessible to surveys such as the current one. Third, the resort to single-item measures in this study appears problematic, mainly when subjective evaluations are involved. Nevertheless, single-item measures proved efficient in countless studies. An evident example is our single-item measure of self-rated health, whose validity is strongly established in epidemiological literature (e.g., Benyamini et al., 2003; Jylhä, 2009; Latham & Peek, 2013). Fourth, this study examined the predictability of subjective wellness through a sample starting at age 75. From a developmental perspective (Martin & Martin, 2002), a better design should span not only proximal predictors pertaining to later life but also distal predictors of earlier life. Lastly, there may be a cultural bias inherent in the current participants, originally drawn from a national sample of the Jewish, older Israeli population. Thus, data showed that the likelihood of clinical depression among this population was twice as high as expected among counterpart populations in the United States and Europe (Ruskin et al., 1996; Shmotkin & Litwin, 2009). Possibly, this evidence presents the burden of past traumas and stress over older Israelis in ways that could affect subjective wellness.
In conclusion, the dynamics of subjective wellness at old-old age is still a largely uncharted area. In front of critical health declines and impending death, people at the fourth age face the challenge to maintain a coherent self-view that serves their adaptability. Accordingly, our data showed that subjective wellness could be predicted among the old-old over a period of 12 years. Nevertheless, we also found that markers of health dysfunction, based on factual self-reports, became less predictive as age advanced. This study presents a dilemma whether to interpret the weakened relations between factual and subjective indicators as a sign that functional structures at old-old age get looser, or as a mechanism that suits adaptational needs at this age. Notably, both interpretations have conceptual frameworks to rely on. Future research should address this dilemma further, thus making the study of old-old age a catalyst for understanding how psychological integration may still be sustained even when life is close to disintegration.
Funding
The first two waves of the CALAS were funded by grants from the U.S. National Institute on Aging (R01-5885-03 and R01-5885-06) and conducted by the Department of Clinical Epidemiology at the Chaim Sheba Medical Center. The third wave of data collection and continued work were conducted by the Herczeg Institute on Aging at Tel Aviv University, supported by the Israel Academy of Science (1041–541), the Israel National Institute for Health Policy (R/17/2001), and a donation from Ellern Foundation. The presently reported study was also supported by the Basic Research Fund at Tel Aviv University.
Acknowledgments
We would like to thank Adrian Walter-Ginzburg, Carmel Kahana-Morgenstern, Menachem Ben-Ezra, Yaron Sela, Aviad Orbach, and Ben Bulmash.
References
- Adams T., Bezner J., Steinhardt M. (1997). The conceptualization and measurement of perceived wellness: Integrating balance across and within dimensions. American Journal of Health Promotion, 11, 208–218. 10.4278/0890-1171-11.3.208 [DOI] [PubMed] [Google Scholar]
- Aiken L. S., West S. G. (1991). Multiple regression: Testing and interpreting interaction. Newbury Park, CA: Sage; [Google Scholar]
- Baltes P. B. (1997). On the incomplete architecture of human ontogeny. Selection, optimization, and compensation as foundation of developmental theory. The American Psychologist, 52, 366–380. 10.1037/0003-066X.52.4.366 [DOI] [PubMed] [Google Scholar]
- Baltes P. B., Smith J. (1999). Multilevel and systemic analyses of old age: Theoretical and empirical evidence for a fourth age. In Bengtson V. L., Schaie K. W. (Eds.), Handbook of theories of aging (pp. 153–173). New York, NY: Springer; [Google Scholar]
- Baltes P. B., Smith J. (2003). New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology, 49, 123–135. 10.1159/000067946 [DOI] [PubMed] [Google Scholar]
- Benyamini Y., Blumstein T., Lusky A., Modan B. (2003). Gender differences in the self-rated health-mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? The Gerontologist, 43, 396–405. 10.1093/geront/43.3.396 [DOI] [PubMed] [Google Scholar]
- Berg A. I., Hoffman L., Hassing L. B., McClearn G. E., Johansson B. (2009). What matters, and what matters most, for change in life satisfaction in the oldest-old? A study over 6 years among individuals 80+. Aging and Mental Health, 13, 191–201. 10.1080/ 13607860802342227 [DOI] [PubMed] [Google Scholar]
- Blumstein T., Shmotkin D., Eyal N., Shorek A., Lerner-Geva L. (2008). A longitudinal evaluation of medication use among the old-old population in Israel. Research on Aging, 30, 55–73. 10.1177/0164027507307920 [Google Scholar]
- Bodner E., Palgi Y., Kaveh D. (2012). Does the relationship between affect complexity and self-esteem differ in young-old and old-old participants? The Journals of Gerontology, Series B: Psychological Sciences and Social SciencesAdvance online publication. 10.1093/geronb/gbs095 [DOI] [PubMed] [Google Scholar]
- Branch L. G., Katz S., Kniepmann K., Papsidero J. A. (1984). A prospective study of functional status among community elders. American Journal of Public Health, 74, 266–268. 10.2105/AJPH.74.3.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D. C., Komp K. (Eds.). (2011). Gerontology in the era of the third age: Implications and next steps. New York, NY: Springer; [Google Scholar]
- Charles S. T., Carstensen L. L. (2009). Social and emotional aging. Annual Review of Psychology, 61, 383–409. 10.1146/annurev.psych.093008.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J., Shmotkin D., Hazan H. (2010). The effect of homebound status on older persons. Journal of the American Geriatrics Society, 58, 2358–2362. 10.1111/j.1532-5415.2010.03172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist A., Ekström H., Elmståhl S. (2012). What factors affect life satisfaction among the oldest-old? Archives of Gerontology and Geriatrics, 54, 140–145. 10.1016/j.archger.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Fauth E. B., Gerstorf D., Ram N., Malmberg B. (2012). Changes in depressive symptoms in the context of disablement processes: Role of demographic characteristics, cognitive function, health, and social support. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67, 167–177. 10.1093/geronb/gbr078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galenkamp H., Braam A. W., Huisman M., Deeg D. J. H. (2013). Seventeen-year time trend in poor self-rated health in older adults: Changing contributions of chronic diseases and disability. The European Journal of Public Health, 23, 511–517. 10.1093/eurpub/cks031 [DOI] [PubMed] [Google Scholar]
- George L. K. (2011). The third age: Fact or fiction—or does it matter?In Carr D. C., Komp K. (Eds.), Gerontology in the era of the third age (pp. 245–259). New York, NY: Springer; [Google Scholar]
- Gerstorf D., Ram N., Röcke C., Lindenberger U., Smith J. (2008). Decline in life satisfaction in old age: Longitudinal evidence for links to distance-to-death. Psychology and Aging, 23, 154–168. 10.1037/0882-7974.23.1.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D., Smith J., Baltes P. B. (2006). A systemic-wholistic approach to differential aging: Longitudinal findings from the Berlin Aging Study. Psychology and Aging, 21, 645–663. 10.1037/ 0882-7974.21.4.645 [DOI] [PubMed] [Google Scholar]
- Gilleard C., Higgs P. (2010). Aging without agency: Theorizing the fourth age. Aging and Mental Health, 14, 121–128. 10.1080/ 13607860903228762 [DOI] [PubMed] [Google Scholar]
- Harari M. J., Waehler C. A., Rogers J. R. (2005). An empirical investigation of a theoretically based measure of perceived wellness. Journal of Counseling Psychology, 52, 93–103. 10.1037/ 0022-0167.52.1.93 [Google Scholar]
- Hong T. B., Zarit S. H., Malmberg B. (2004). The role of health congruence in functional status and depression. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 59, P151–P157. 10.1093/geronb/59.4.P151 [DOI] [PubMed] [Google Scholar]
- Infurna F. J., Ram N., Gerstorf D. (2012). Level and change in perceived control predict 19-year mortality: Findings from the Americans’ Changing Lives Study. Developmental Psychology. Advance online publication. 10.1037/a0031041 [DOI] [PubMed] [Google Scholar]
- Jang Y., Poon L. W., Martin P. (2004). Individual differences in the effects of disease and disability on depressive symptoms: The role of age and subjective health. International Journal of Aging and Human Development, 59, 125–137. 10.2190/RT1W-2HD7- KG5X-K1FB [DOI] [PubMed] [Google Scholar]
- Jensen L. A., Allen M. N. (1994). A synthesis of qualitative research on wellness-illness. Qualitative Health Research, 4, 349–369. 10.1177/104973239400400402 [Google Scholar]
- Jylhä M. (2009). What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science and Medicine, 69, 307–316. 10.1016/j.socscimed.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Katz S., Downs T. D., Cash H. R., Grotz R. C. (1970). Progress in development of the index of ADL. The Gerontologist, 10, 20–30. 10.1093/geront/10.1_Part_1.20 [DOI] [PubMed] [Google Scholar]
- Laslett P. (1991). A fresh map of life: The emergence of the third age. Cambridge, MA: Harvard University Press; [Google Scholar]
- Latham K., Peek C. W. (2013). Self-rated health and morbidity onset among late midlife U.S. adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 68, 107–116. 10.1093/geronb/gbs104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. J. A., Rubin D. B. (2002). Statistical analysis with missing data. New York, NY: Wiley; [Google Scholar]
- Martin P., Martin M. (2002). Proximal and distal influences on development: The model of developmental adaptation. Developmental Review, 22, 78–96. 10.1006/drev.2001.0538 [Google Scholar]
- Mehta M., Whyte E., Lenze E., Hardy S., Roumani Y., Subashan P., … Studenski S. (2008). Depressive symptoms in late life: Associations with apathy, resilience and disability vary between young-old and old-old. International Journal of Geriatric Psychiatry, 23, 238–243. 10.1002/gps.1868 [DOI] [PubMed] [Google Scholar]
- Mroczek D. K., Almeida D. M., Spiro A., III, Pafford C. (2006). Modeling intraindividual stability and change in personality. In Mroczek D. K., Little T. D. (Eds.), Handbook of personality development (pp. 163–180). Mahwah, NJ: Lawrence Erlbaum; [Google Scholar]
- Neugarten B. L. (1974). Age groups in American society and the rise of the young-old. Annals of the American Academy of Politics and Social Sciences, 415, 187–198. 10.1177/000271627441500114 [Google Scholar]
- Palgi Y. (2012). Ongoing cumulative chronic stressors as predictors of well-being in the second half of life. Journal of Happiness Studies. Advance online publication. 10.1007/s10902-012-9371-1 [Google Scholar]
- Palgi Y., Shrira A., Ben-Ezra M., Spalter T., Shmotkin D., Kavé G. (2010). Delineating terminal change in subjective well-being and subjective health. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 61–64. 10.1093/geronb/gbp095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peugh J. L., Enders C. K. (2005). Using the SPSS mixed procedure to fit cross-sectional and longitudinal multilevel models. Educational and Psychological Measurement, 65, 717–741. 10.1177/ 0013164405278558 [Google Scholar]
- Pinquart M., Sorensen S. (2000). Influences of socioeconomic status, social network, and competence on subjective well-being in later life: A meta-analysis. Psychology and Aging, 15, 187–224. 10.1037/0882-7974.15.2.187 [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [Google Scholar]
- Ram N., Gerstorf D., Fauth E., Zarit S., Malmberg B. (2010). Aging, disablement, and dying: Using time-as-process and time-as-resources metrics to chart late-life change. Research in Human Development, 7, 27–44. 10.1080/15427600903578151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe L. J. (2009). Wellness: A review of theory and measurement for counselors. Journal of Counseling and Development, 87, 216–226. 10.1002/j.1556–6678.2009.tb00570.x [Google Scholar]
- Ruskin P. E., Blumstein Z., Walter-Ginzburg A., Fuchs Z., Lusky A., Novikov I., Modan B. (1996). Depressive symptoms among community-dwelling oldest-old residents in Israel. American Journal of Geriatric Psychiatry, 4, 208–217. 10.1097/00019442-199622430-00004 [DOI] [PubMed] [Google Scholar]
- Schroots J. J. F. (1995). Gerodynamics: Toward a branching theory of aging. Canadian Journal on Aging, 14, 74–81. 10.1017/S0714980800010515 [Google Scholar]
- Shmotkin D. (2005). Happiness in face of adversity: Reformulating the dynamic and modular bases of subjective well-being. Review of General Psychology, 9, 291–325. 10.1037/1089-2680.9.4.291 [Google Scholar]
- Shmotkin D. (2011). The pursuit of happiness: Alternative conceptions of subjective well-beingIn Poon L. W., Cohen-Mansfield J. (Eds.), Understanding well-being in the oldest old (pp. 27–45). New York, NY: Cambridge University Press; [Google Scholar]
- Shmotkin D., Litwin H. (2009). Cumulative adversity and depressive symptoms among older adults in Israel: The differential roles of self-oriented versus other-oriented events of potential trauma. Social Psychiatry and Psychiatric Epidemiology, 44, 989–997. 10.1007/s00127-009-0020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmotkin D., Eyal N., Hazan H., Shkolnik T., Shorek A., Cohen-Mansfield J. (in press). Between the subjective and the objective: How informative is subjective evaluation of memory among the old-old? Clinical Gerontologist. 10.1080/07317115.2013.788115 [Google Scholar]
- Shmotkin D., Lerner-Geva L., Cohen-Mansfield J., Blumstein T., Eyal N., Shorek A., … Hazan H. (2010). Profiles of functioning as predictors of mortality in old age: The advantage of a configurative approach. Archives of Gerontology and Geriatrics, 51, 68–75. 10.1016/j.archger.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Shrira A., Palgi Y., Ben-Ezra M., Shmotkin D. (2011). Functioning and mortality of Holocaust survivors: Physical resilience and psychosocial vulnerabilities. Journal of Loss and Trauma, 16, 67–83. 10.1080/15325024.2010.519297 [Google Scholar]
- Snijders T. A. B., Bosker R. J. (1999). Multilevel analysis: An introduction to basic and advanced multilevel modeling. London, UK: Sage; [Google Scholar]
- Stek M. L., Vinkers D. J., Gussekloo J., van der Mast R. C., Beekman A. T., Westendorp R. G. (2006). Natural history of depression in the oldest old: Population-based prospective study. The British Journal of Psychiatry: The Journal of Mental Science, 188, 65–69. 10.1192/bjp.188.1.65 [DOI] [PubMed] [Google Scholar]
- Turvey C. L., Schultz S. K., Beglinger L., Klein D. M. (2009). A longitudinal community-based study of chronic illness, cognitive and physical function, and depression. The American Journal of Geriatric Psychiatry, 17, 632–641. 10.1097/JGP.0b013e31819c498c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Schimmele C. M. (2006). Psychological disposition and self-reported health among the ‘oldest-old’ in China. Ageing and Society, 26, 135–151. 10.1017/S0144686X0500423X [Google Scholar]
- Young Y., Boyd C. M., Guralnik J. M., Fried L. P. (2010). Does self-reported function correspond to objective measures of functional impairment? Journal of the American Medical Directors Association, 11, 645–653. 10.1016/j.jamda.2009.12.084 [DOI] [PMC free article] [PubMed] [Google Scholar]


