Supplemental Digital Content is available in the text
Keywords: chronic kidney disease, renin–angiotensin–aldosterone system-blockade, sodium restriction, volume status
Abstract
Purpose of review
Restriction of dietary sodium is recommended at a population level as well as for groups at high cardiovascular risk, and chronic kidney disease (CKD). This review addresses recent evidence for the protective effect of dietary sodium restriction in CKD patients specifically.
Recent findings
Sodium intake in CKD populations is generally high, and often above population average. Recent data demonstrated that moderately lower sodium intake in CKD patients is associated with substantially better long-term outcome of renin–angiotensin–aldosterone system (RAAS)-blockade, in diabetic and nondiabetic CKD, related to better effects of RAAS-blockade on proteinuria, independent of blood pressure. This is in line with better short-term efficacy of RAAS-blockade during moderate sodium restriction in diabetic and nondiabetic CKD. This effect of sodium restriction is likely mediated by its effects on volume status. Sustainable sodium restriction can be achieved by approaches on the basis of behavioral sciences.
Summary
Moderate restriction of dietary sodium can substantially improve the protective effects of RAAS-blockade in CKD, by specific renal effects apparent from proteinuria reduction. The latter precludes straightforward extrapolation of data from nonrenal populations to CKD. Concerns regarding the adverse effects of a very low sodium intake should not distract from the protective effects of moderate sodium restriction. Prospective studies should assess the efficacy and sustainability of different strategies to target high sodium intake in CKD, along with measures at population level.
Video abstract
INTRODUCTION
Restriction of dietary sodium to a maximum of 5 g of salt (sodium chloride) daily for an adult, corresponding to ca. 2000 mg sodium, is among the top priorities of the WHO for the combat of chronic noncommunicable diseases [1]. In line with this, for chronic kidney disease (CKD) patients, the 2012 Kidney Disease Improving Global Outcomes guideline recommends the reduction of daily sodium intake to less than 2000 mg/90 mmol per day [2]. Salt intake varies widely between different countries, but generally exceeds the recommended amount in most communities where data are available. Excess sodium intake is associated with considerable morbidity and mortality, and, hence, substantial costs in terms of health expenditure. It has been estimated that a 3-g reduction of salt intake would reduce healthcare costs by $10–24 billion per year [3]. Of note, these estimates were based on cardiovascular disease only: inclusion of the costs related to sodium-related morbidity and mortality in CKD would have increased these figures even further.
Box 1.

no caption available
SODIUM INTAKE IN CHRONIC KIDNEY DISEASE PATIENTS
Among the many studies on CKD patients in the literature, only a minority reports 24-h sodium excretion. In these studies, average 24-h sodium excretion in CKD patients is usually in the range between 160 and 240 mmol [4,5▪▪,6,7]. This is in the same range as in the general population, or even higher. This is remarkable, considering the fact that these patients were under dedicated nephrology care. It could indicate either neglect of sodium status, or failure of current strategies in renal care to achieve sustained reduction of dietary sodium, or reflect the association between risk behavior and risk to develop CKD.
EFFECT OF DIETARY SODIUM RESTRICTION IN CHRONIC KIDNEY DISEASE PATIENTS
In CKD, blood pressure is usually sodium sensitive. Moreover, renal protein loss is reduced by dietary sodium restriction. This has been shown for sodium restriction as a single measure, as well as for sodium restriction as an add-on to antihypertensive treatment [8,9▪]. Interestingly, proteinuria reduction by sodium restriction remains significant after adjustment for the fall in blood pressure, suggesting an independent renoprotective effect of sodium restriction, both as a single measure and in combination with blockade of the renin–angiotensin–aldosterone system (RAAS) [8]. A blood pressure-independent effect of dietary sodium on the kidney is substantiated by data in healthy volunteers, in which dietary sodium restriction reduces albuminuria within the normal range, without a detectable effect on blood pressure [10].
INTERACTION BETWEEN SODIUM INTAKE AND EFFECTS OF RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM-BLOCKADE
In CKD, RAAS-blockers are first-line therapy for the treatment of hypertension and proteinuria, and accordingly the vast majority of CKD patients are on maintenance treatment with either an angiotensin-converting-enzyme (ACE) inhibitor or an angiotensin-receptor blocker (ARB). It has been known for over 2 decades that the effects of RAAS-blockers are blunted by high sodium intake [8,11,12]. Of note, by interfering with the buffering action of the RAAS on the hemodynamic consequences of altered sodium status, RAAS-blockade renders blood pressure sodium-sensitive, thus creating better therapeutic opportunities for sodium restriction, even in previously sodium-resistant patients. Sodium restriction increases the top of the dose response of RAAS-blockade for both blood pressure and proteinuria [13]. The effect of moderate sodium restriction during RAAS-blockade on blood pressure and proteinuria is approximately similar to the effect of adding a diuretic, whereas the maximum effect is achieved by their combination in nondiabetic [8] as well as diabetic CKD (Fig. 1) [5▪▪]. The reduction in blood pressure and proteinuria is accompanied by a slight decrease in renal function.
FIGURE 1.

Sodium excretion (upper panel) during four different 6-week treatment periods by a rotation schedule in patients with diabetes and CKD on ACE-inhibitor therapy. NS intake was very high and accordingly also during SR without and with hydrochlorothiazide, sodium intake remained above recommended levels. Nevertheless, proteinuria (lower panel) was reduced significantly, as was blood pressure (data not shown). CKD, chronic kidney disease; HCT, hydrochlorothiazide; NS, normal sodium; SR, sodium restriction. Adapted from [5▪▪].
Of note, in the latter study, habitual sodium intake was very high, that is an average of 224 mmol Na+/day. During intervention, sodium intake decreased to 148 mmol/day. Although this was still substantially above the recommended levels, nevertheless, it was associated with a significant reduction in blood pressure and proteinuria.
This study also allows inferences on the clinical relevance of the so-called ‘sodium paradox’ in patients with diabetes and CKD. The ‘sodium paradox’ refers to the rise in glomerular filtration rate and filtration fraction that has been reported during low sodium diet in uncomplicated type I diabetes and experimental diabetes. It is partly attributed to excess intrarenal RAAS activity. Concerns regarding the possible adverse renal effects of hyperfiltration may have contributed to the underrated role of sodium restriction in patients with diabetes and CKD, but the current data do not support the relevance of the sodium paradox for the clinical condition of diabetes with CKD during RAAS-blockade.
In a head-to-head comparison, moderate dietary sodium restriction during monotherapy ACE-inhibitor (ACEi) more effectively reduced blood pressure and proteinuria than adding an ARB [12]. Dual RAAS-blockade has now been deemed obsolete, due to worse renal outcome in long-term studies [14–16] related to increased risk for acute renal function deterioration. The current data show that moderate dietary sodium restriction as add-on to monotherapy RAAS-blockade provides a more effective alternative to dual blockade. Of note, this study also contained a dual blockade-sodium restriction arm. This regimen had potent effects on blood pressure and proteinuria, accompanied by a substantial reduction in renal function. The latter was much more pronounced than the mild decrease in renal function during single blockade at similar sodium restriction. The decrease in renal function was fully reversible after changing the regimen, demonstrating its hemodynamic nature, but illustrates that dual RAAS-blockade severely compromises the kidneys’ capacity to maintain glomerular filtration during sodium restriction, which may explain the increased risk for acute kidney injury associated with dual blockade in long-term studies.
INTERACTION BETWEEN SODIUM INTAKE AND LONG-TERM OUTCOME OF RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM-BLOCKADE
Recently, the effect of sodium intake on the long-term outcome of RAAS-blockade was analyzed in data sets from the large intervention trials that provided the empirical basis for our current RAAS-blockade-based treatment regimens in CKD. Data from the ACE-inhibitor arm from the Ramipril Efficacy In Nephropathy (REIN) trial were analyzed by tertiles of sodium intake, assessed as urinary sodium excretion throughout the study [6]. By design, blood pressure was titrated to less than 140/90 mmHg in all patients by adding antihypertensives on top of the ACEi, with a diuretic as the first titration step. Accordingly, blood pressure was not different by sodium intake. However, proteinuria reduction was less effective in the higher tertiles of sodium intake and was associated with a substantially worse long-term renal outcome, with 60% of the patients in the upper tertile reaching the renal end point versus 20% in the lowest tertile (Fig. 2).
FIGURE 2.
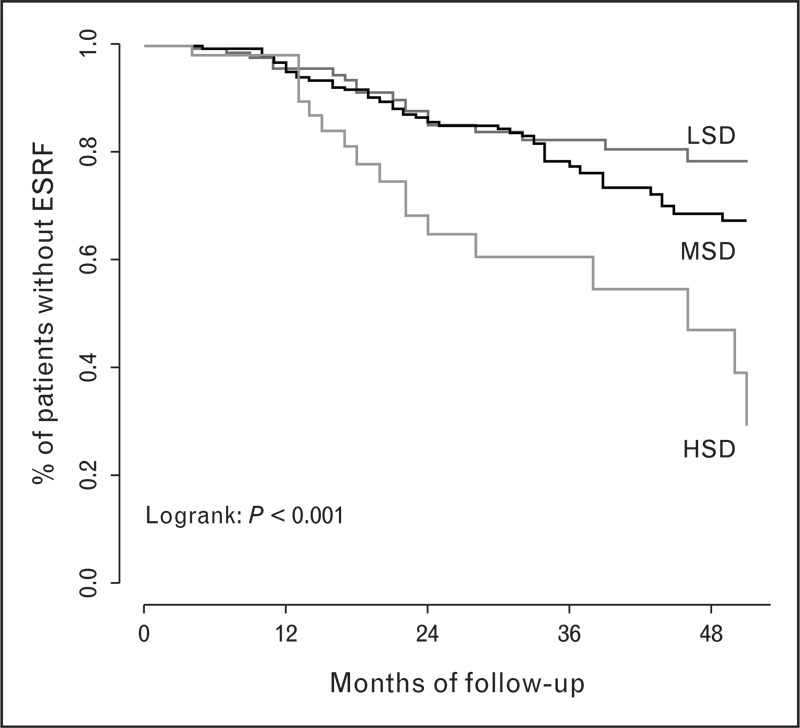
Renal survival in patients on ACEi by tertile of salt intake LSD 7.1 g/day, MSD 10.8 g/day, and HSD 14.2 g/day. ESRF, end stage renal failure; HSD, high sodium diet; LSD, low sodium diet; MSD, medium sodium diet. Adapted from [6].
In the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan Study (RENAAL)/Irbesartan Diabetic Nephropathy Trial (IDNT) data set the conventional treatment arm was also analyzed. In these studies, blood pressure was also titrated by design. With conventional treatment, overall outcome was worse, but not different by sodium intake. In the ARB arm, however, the risk to reach a renal or cardiovascular end point in patients in the highest tertile was approximately two-fold higher than in the lowest tertile. Accordingly, the treatment benefit from the ARB over conventional antihypertensives was present in the lower tertile only (Fig. 3, [4]). In line with the REIN data, the lack of protective action of RAAS-blockade in those ingesting excessive sodium was associated with lack of antiproteinuric effect, despite similar blood pressure.
FIGURE 3.
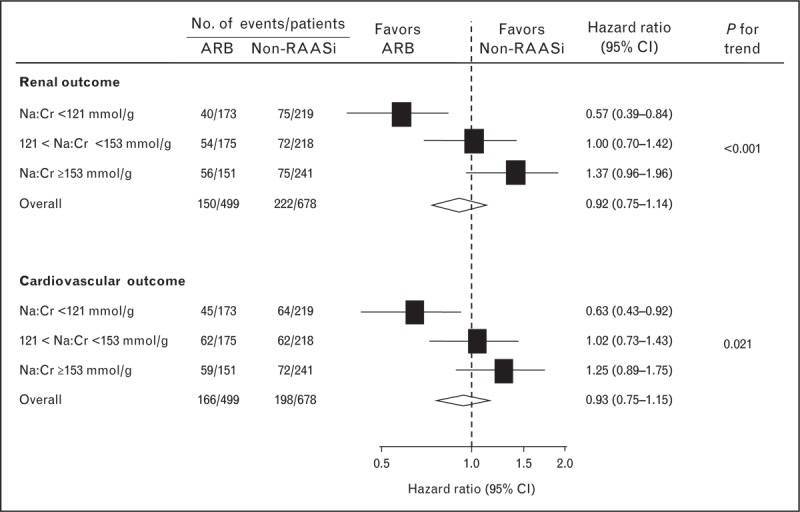
Effect of sodium intake by tertile of urinary Na/creatinine (Cr) on the treatment benefit of ARB for renal (upper panels) and cardiovascular (lower panels) outcome in patients with type 2 diabetes and nephropathy. Urinary sodium excretion in the subsequent tertiles corresponded to a dietary salt intake of 8.9, 10.9, and 12.2 g, respectively. ARB, angiotensin-receptor blocker; RAAS, renin–angiotensin–aldosterone system. Adapted from [4].
Thus, in CKD patients on RAAS-blockade, a modestly lower dietary sodium intake, in the range recommended for the general population, is associated with substantial benefits regarding renal and cardiovascular outcome. This difference in outcome occurs despite adequate blood pressure control in those on the highest sodium intake and is associated with persistence of proteinuria. Of note, in these studies, the titration schedule led to more diuretic use in patients who ingested excess sodium. Apparently, this did not sufficiently prevent the adverse effect of excessive sodium intake on long-term outcome. This is somewhat disconcerting, considering the similarity of the short-term effects of sodium restriction and diuretics. It could be that control of volume overload was still insufficient, as high sodium intake is associated with diuretic resistance [17] or, the other way round, that diuretic use is associated with side-effects that adversely affect long-term outcome, such as for instance potassium depletion or hyperuricemia [18].
CAN WE GO TOO LOW?
Observational data from various studies, showing a J-curve between sodium intake and renal and cardiovascular outcome, have raised concern on the safety of rigorous sodium restriction [19–21]. These observational data should be interpreted with caution, as a habitual salt intake below 5 g daily is a rarity in the outpatient population, and may well be an indicator of concomitant conditions compromising nutritional status as well as outcome. Moreover, quantification of sodium intake was questionable in some of the studies, by lack of 24-h urine data on sodium intake. This may have contributed to the substantial differences in the level of the nadir of the J-curve. Obviously, this debate is seriously hampered by a lack of prospective long-term sodium intervention data in CKD. Nevertheless, the presence of a J-curve has been reported in several independent studies (albeit not all [22▪▪]) and should be given serious consideration. In experimental renal disease, we previously found that a regimen of ACEi and rigorous sodium restriction reduced blood pressure, proteinuria, and glomerular damage, but unexpectedly aggravated tubulo-interstitial damage [23]. This was also found in healthy rats, so, it is not a particularity of the model but a generalizable adverse effect of the combination of RAAS-blockade or rigorous sodium restriction. This is disconcerting, as, moreover, the interstitial damage was not readily apparent from noninvasive parameters, so that if this occurred in patients it would go unnoticed. This once more underlines the need for better noninvasive markers of tubulo-interstitial damage. Considering the consistent association of interstitial damage with long-term renal outcome, this could provide a potential explanation for the association of very low sodium intake with worse renal outcome. Its mechanisms deserve further explanation, but could include intrarenal ischemia, excess reactive renin or aldosterone activation, or other causes.
MECHANISMS UNDERLYING THE INTERACTION OF SODIUM INTAKE WITH RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM-BLOCKADE EFFICACY
Several mechanisms could explain the interaction between sodium intake and the effects of RAAS-blockade. First, high sodium intake suppresses RAAS-activity in the circulation, and blocking a suppressed cascade cannot be expected to have much effect. For tissue RAAS-activity other mechanisms may be relevant as high sodium increases tissue conversion of angiotensin (ang) I [24] and annihilates the effects of ACEi on tissue ang I conversion [25]. Moreover, low sodium potentiates the ACEi-induced increase of vasodilator and antifibrotic substances such as ang 1–7 [26] and AcSDKP [27]. Finally, it is important to realize that high sodium intake is often associated with other nutritional factors that can affect renal function and the response to RAAS-blockade by themselves, such as for instance protein intake [10,12,28].
SODIUM INTAKE AND VOLUME STATUS AND THEIR EFFECTS ON RESPONSE TO RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM-BLOCKADE
Sodium intake is generally recognized as an important determinant of the extracellular volume (ECV), and it is plausible that the effect of high sodium intake on the response to RAAS-blockade is to an important extent determined by its effect on ECV. This is supported by the similarity of the effects of diuretic and dietary sodium restriction on RAAS-blockade efficacy (at least on short term) [5▪▪,8,29] and by the predictive effect of volume markers on the effect of sodium restriction and/or diuretic on the efficacy of RAAS-blockade [30], as outlined in more detail in the next paragraph.
In this respect, it is important to realize for a given sodium intake that volume status can be very different between patients, depending on the avidity of sodium retaining mechanisms. The latter can vary by renal condition, with more avid retention in proteinuric patients [31▪▪], in diabetes, and overweight or obesity [32]. Accordingly, volume overload can be present even if sodium intake is adequately restricted, as occurs in severely nephrotic patients as an extreme example. Conversely, volume expansion can be absent despite a high sodium intake, due to highly efficient sodium excretion, or a highly effective nonosmotic storage mechanism [33]. Recent data suggest that fibroblast growth factor 23 (FGF23) is involved in renal sodium retention [34▪] and volume status [35], thus providing a mechanistic link between the adverse effects of high sodium intake and high phosphate levels [36] on therapy response to RAAS-blockade. In line with this assumption, high FGF23 was associated with an impaired response to low sodium diet on top of ACEi (Humalda, Am J Kidney Dis, in press).
TITRATING SODIUM AND VOLUME STATUS IN CHRONIC KIDNEY DISEASE
Assessment of volume status may be useful to guide therapy in CKD patients on RAAS-blockade. In patients with elevated N-terminal pro-brain natriuretic peptide (NT-proBNP), as a marker for volume expansion, sodium intervention by diet, diuretic, or both, reduces blood pressure and proteinuria, whereas a normal NT-proBNP predicts a minor or nonsignificant effect (Fig. 4) [30]. Apparently, in the absence of cardiac disease, mild elevation of NT-proBNP indicates subclinical volume expansion as a suitable target for intervention. Accordingly, the volume intervention reduces NT-proBNP. If it normalizes, further volume targeting does not result in a clinical response; if it is still above normal, additional volume targeting (e.g., adding diuretic to the diet) results in a clinical response. These data in proteinuric patients suggest that it could be relevant to not only assess sodium intake, but also corresponding volume status, as this could potentially prevent overzealous sodium restriction or diuretic treatment, and its adverse consequences in CKD patients.
FIGURE 4.
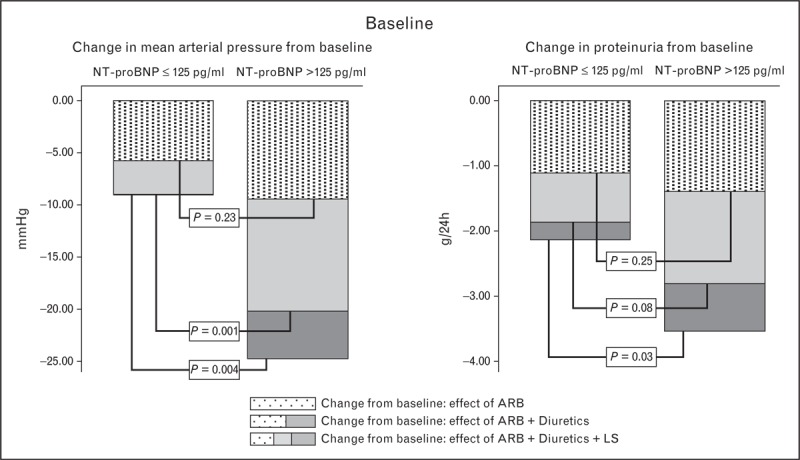
Predictive value of NT-proBNP at baseline (untreated) for the responses of blood pressure (left panel) and proteinuria (right panel) to ARB, add-on sodium restriction, and add-on sodium restriction plus diuretic. NT-proBNP above the upper level of normal (>125 pg/ml) was not associated with the response to ARB, but predicted a more pronounced response to volume intervention by sodium restriction and thiazide. A similar predictive value of NT-proBNP for clinical efficacy of volume intervention was found for NT-proBNP during monotherapy ARB (not shown). ARB, angiotensin-receptor blocker; LS, low sodium. Adapted from [32].
Quantification of ECV is not part of the clinical routine in CKD, and validation of the available volume markers such as NT-proBNP will probably be cumbersome, due to interference of cardiac functional status. Yet, it might be worthwhile to put more effort in assessing volume status per se, not only for its role in modifying the response to RAAS-blockade per se, but also as a possible independent cardiovascular risk factor in CKD patients [37▪].
MANAGING SODIUM INTAKE IN CHRONIC KIDNEY DISEASE: IMPORTANCE OF BEHAVIORAL APPROACHES
How should sodium status be targeted in the clinic? It is increasingly recognized that current strategies to change dietary habits are ineffective, as illustrated by data from the Masterplan study, in which support by trained nurses was effective in improving compliance with pharmacological guidelines, but not in improving compliance with lifestyle measures [7]. There is compelling evidence from behavioral sciences that sustained lifestyle changes require a dedicated, behavioral approach [38–40]. Such approaches are not yet part of the clinical routine in renal care, but are being tested currently (SUBLIME, ClinicalTrials.gov identifier NCT02132013).
MONITORING SODIUM INTAKE FROM 24-H URINE
The gold standard for the assessment of sodium intake is from well collected 24-h urine, as dietary recall and food frequency questionnaires are notoriously unreliable for the assessment of sodium intake. This relates to the fact that only 15% of the sodium ingested is added during cooking or during meals, whereas the remainder is present in the food in hidden form, as additives in processed foods [41▪]. As collection of 24-h urine is considered cumbersome by many, and moreover prone to collection errors, these data are not routinely available in many centers. However, assessment of 24-h creatinine excretion allows detection of collection errors by testing observed creatinine excretion versus creatinine excretion expected from anthropometric data [42]. Moreover, additional nutritional factors relevant to outcome CKD can also be reliably assessed from 24-h urine, such as phosphate intake, protein intake, potassium [43], magnesium [44], sulphate [45], and finally, the absolute value of 24-h creatinine excretion, by its association with muscle mass, is a robust marker of physical fitness and predictor or mortality risk [46]. Thus, 24-h urine can provide a multidimensional nutrition and fitness profile relevant to CKD patients that renders the investment in terms of patient instruction and urine collection even more worthwhile.
CONCLUSION
Control of sodium and volume status is crucial in the management of diabetic and nondiabetic CKD patients for control of blood pressure and proteinuria, and eventually prevention of progressive renal function loss and its complications. This is particularly so in patients on RAAS-blockade, as sodium overload interferes with its therapeutic efficacy, as apparent from persistence of proteinuria, even when blood pressure is well controlled. In most CKD patients habitual sodium intake is too high, despite medical supervision. In both diabetic and nondiabetic CKD a moderately lower dietary sodium, even at levels substantially above the recommended amount, is associated with a substantially better response to RAAS-blockade in short-term interventions, and a substantially better renal and cardiovascular outcome in post-hoc analyses of hard end point studies. Concerns have been raised on the safety of rigorous sodium restriction on the basis of a J-curve for sodium intake and outcome, with higher risk not only at higher sodium intakes, but also at the lower end. Safety concerns on rigorous sodium restriction should not distract from the considerable potential benefits of moderate sodium restriction in the vast majority of CKD patients, in whom sodium intake is high or very high. Population measures, including the action of government and industry, are important to facilitate reduction of sodium intake [3]. Moreover, it is crucial to develop better strategies for lifestyle management in CKD patients. This should include monitoring of dietary sodium (as well as other relevant dietary factors) from 24-h urine, as well as integration of behavioral approaches into regular care.
Acknowledgements
None.
Conflicts of interest
Disclosure of funding: G.N. is advisor for Astra Zeneca. J.K.H. has nothing to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.co-nephrolhypertens.com).
REFERENCES
- 1.Beaglehole R, Bonita R, Horton R, et al. Priority actions for the noncommunicable disease crisis. Lancet 2011; 377:1438–1447 [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3:1–150 [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 2012; 82:330–337 [DOI] [PubMed] [Google Scholar]
- 5▪▪.Kwakernaak AJ, Krikken JA, Binnenmars SH, et al. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol 2014; 2:385–395 [DOI] [PubMed] [Google Scholar]; First intervention trial showing the effects of sodium restriction, diuretic, and their combination in patients with diabetes and nephropathy on RAAS-blockade. Moderate dietary sodium restriction considerably increased the efficacy of RAAS-blockade. This article also reports on sodium excretion in the unselected recruitment population, showing that sodium intake in this outpatient population of patients with diabetes and nephropathy was considerably above the population average in The Netherlands.
- 6.Vegter S, Perna A, Postma MJ, et al. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 2012; 23:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zuilen AD, Bots ML, Dulger A, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int 2012; 82:710–717 [DOI] [PubMed] [Google Scholar]
- 8.Vogt L, Waanders F, Boomsma F, et al. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 2013; 24:2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]; Elegant study demonstrating salt sensitivity in CKD patients as sodium restriction lowered blood pressure, proteinuria, and ECV.
- 10.Krikken JA, Lely AT, Bakker SJ, Navis G. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int 2007; 71:260–265 [DOI] [PubMed] [Google Scholar]
- 11.Navis G, de Jong PE, Donker AJ, et al. Moderate sodium restriction in hypertensive subjects: renal effects of ACE-inhibition. Kidney Int 1987; 31:815–819 [DOI] [PubMed] [Google Scholar]
- 12.Slagman MC, Waanders F, Hemmelder MH, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 2011; 343:d4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wapstra FH, Van Goor H, Navis G, et al. Antiproteinuric effect predicts renal protection by angiotensin-converting enzyme inhibition in rats with established adriamycin nephrosis. Clin Sci (Lond) 1996; 90:393–401 [DOI] [PubMed] [Google Scholar]
- 14.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553 [DOI] [PubMed] [Google Scholar]
- 15.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 16.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213 [DOI] [PubMed] [Google Scholar]
- 17.Wilcox CS, Mitch WE, Kelly RA, et al. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med 1983; 102:450–458 [PubMed] [Google Scholar]
- 18.Bellomo G. Uric acid and chronic kidney disease: a time to act? World J Nephrol 2013; 2:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas MC, Moran J, Forsblom C, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011; 34:861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011; 34:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011; 306:2229–2238 [DOI] [PubMed] [Google Scholar]
- 22▪▪.McQuarrie EP, Traynor JP, Taylor AH, et al. Association between urinary sodium, creatinine, albumin, and long-term survival in chronic kidney disease. Hypertension 2014; 64:111–117 [DOI] [PubMed] [Google Scholar]; This study, with 8.5 years of follow-up, unveils that the relation between sodium intake and adverse renal outcome is independent from, and additive to, proteinuria, moreover, they could not discern a J-curve effect.
- 23.Hamming I, Navis G, Kocks MJ, van Goor H. ACE inhibition has adverse renal effects during dietary sodium restriction in proteinuric and healthy rats. J Pathol 2006; 209:129–139 [DOI] [PubMed] [Google Scholar]
- 24.Boddi M, Poggesi L, Coppo M, et al. Human vascular renin-angiotensin system and its functional changes in relation to different sodium intakes. Hypertension 1998; 31:836–842 [DOI] [PubMed] [Google Scholar]
- 25.Kocks MJ, Buikema H, Gschwend S, et al. High dietary sodium blunts affects of angiotensin-converting enzyme inhibition on vascular angiotensin I-to-angiotensin II conversion in rats. J Cardiovasc Pharmacol 2003; 42:601–606 [DOI] [PubMed] [Google Scholar]
- 26.Kocks MJ, Lely AT, Boomsma F, et al. Sodium status and angiotensin-converting enzyme inhibition: effects on plasma angiotensin-(1-7) in healthy man. J Hypertens 2005; 23:597–602 [DOI] [PubMed] [Google Scholar]
- 27.Kwakernaak AJ, Waanders F, Slagman MC, et al. Sodium restriction on top of renin-angiotensin-aldosterone system blockade increases circulating levels of N-acetyl-seryl-aspartyl-lysyl-proline in chronic kidney disease patients. J Hypertens 2013; 31:2425–2432 [DOI] [PubMed] [Google Scholar]
- 28.Bellizzi V, Di Iorio BR, De Nicola L, et al. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int 2007; 71:245–251 [DOI] [PubMed] [Google Scholar]
- 29.Ekinci EI, Thomas G, Thomas D, et al. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care 2009; 32:1398–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slagman MC, Waanders F, Vogt L, et al. Elevated N-terminal pro-brain natriuretic peptide levels predict an enhanced antihypertensive and antiproteinuric benefit of dietary sodium restriction and diuretics, but not angiotensin receptor blockade, in proteinuric renal patients. Nephrol Dial Transplant 2012; 27:983–990 [DOI] [PubMed] [Google Scholar]
- 31▪▪.Svenningsen P, Friis UG, Versland JB, et al. Mechanisms of renal NaCl retention in proteinuric disease. Acta Physiol (Oxf) 2013; 207:536–545 [DOI] [PubMed] [Google Scholar]; Excellent review on the mechanisms of sodium retention, for example, activation of ENaC by urinary plasmin in nephrotic syndromes.
- 32.Visser FW, Krikken JA, Muntinga JH, et al. Rise in extracellular fluid volume during high sodium depends on BMI in healthy men. Obesity (Silver Spring) 2009; 17:1684–1688 [DOI] [PubMed] [Google Scholar]
- 33.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009; 15:545–552 [DOI] [PubMed] [Google Scholar]
- 34▪.Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 2014; 6:744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]; First article to demonstrate the involvement of bone-mineral disease key-hormone FGF23 to upregulation of NCC and thus fluid retention.
- 35.Baia LC, Humalda JK, Vervloet MG, et al. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol 2013; 8:1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoccali C, Ruggenenti P, Perna A, et al. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 2011; 22:1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Hung SC, Kuo KL, Peng CH, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 2014; 85:703–709 [DOI] [PubMed] [Google Scholar]; In this article, volume overload was assessed by bioimpedance in predialysis CKD patients and found to correlate with proteinuria, blood pressure, and higher antihypertensive medication use. This article demonstrates that volume status might be objectified by bedside measurements and may have clinical consequences.
- 38.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007; 334:885–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robare JF, Bayles CM, Newman AB, et al. The ‘10 keys’ to healthy aging: 24-month follow-up results from an innovative community-based prevention program. Health Educ Behav 2011; 38:379–388 [DOI] [PubMed] [Google Scholar]
- 40.Zhang SX, Guo HW, Wan WT, Xue K. Nutrition education guided by Dietary Guidelines for Chinese Residents on metabolic syndrome characteristics, adipokines and inflammatory markers. Asia Pac J Clin Nutr 2011; 20:77–86 [PubMed] [Google Scholar]
- 41▪.Carrigan A, Klinger A, Choquette SS, et al. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J Ren Nutr 2014; 24:13–1919e1 [DOI] [PMC free article] [PubMed] [Google Scholar]; Elegant study quantifying the abundance of sodium in food additives, and thus enforcing the need for structured interventions and aid for CKD patients in order to reduce sodium intake.
- 42.Ix JH, Wassel CL, Stevens LA, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol 2011; 6:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth A, Dunkler D, Gao P, et al. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int 2014; doi: 10.1038/ki.2014.214. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Joosten MM, Gansevoort RT, Mukamal KJ, et al. Urinary and plasma magnesium and risk of ischemic heart disease. Am J Clin Nutr 2013; 97:1299–1306 [DOI] [PubMed] [Google Scholar]
- 45.van den Berg E, Pasch A, Westendorp WH, et al. Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol 2014; 25:1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinkeler SJ, Kwakernaak AJ, Bakker SJ, et al. Creatinine excretion rate and mortality in type 2 diabetes and nephropathy. Diabetes Care 2013; 36:1489–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]


