Abstract
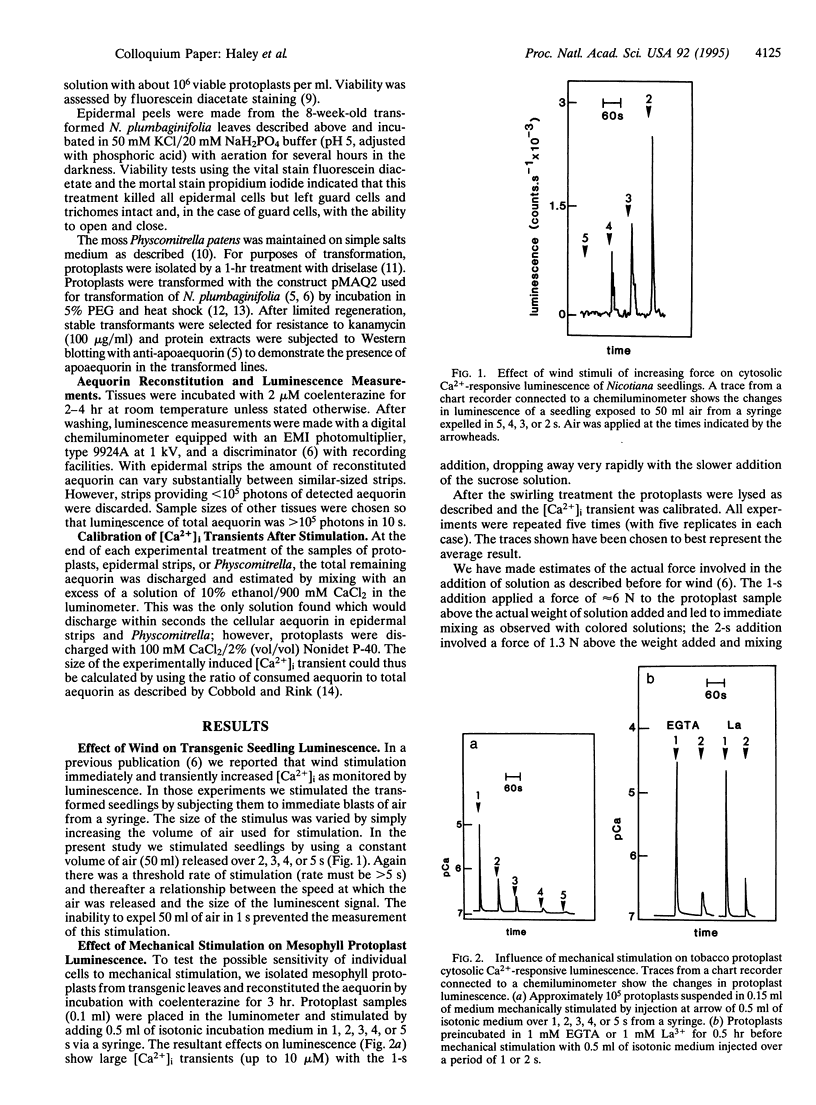
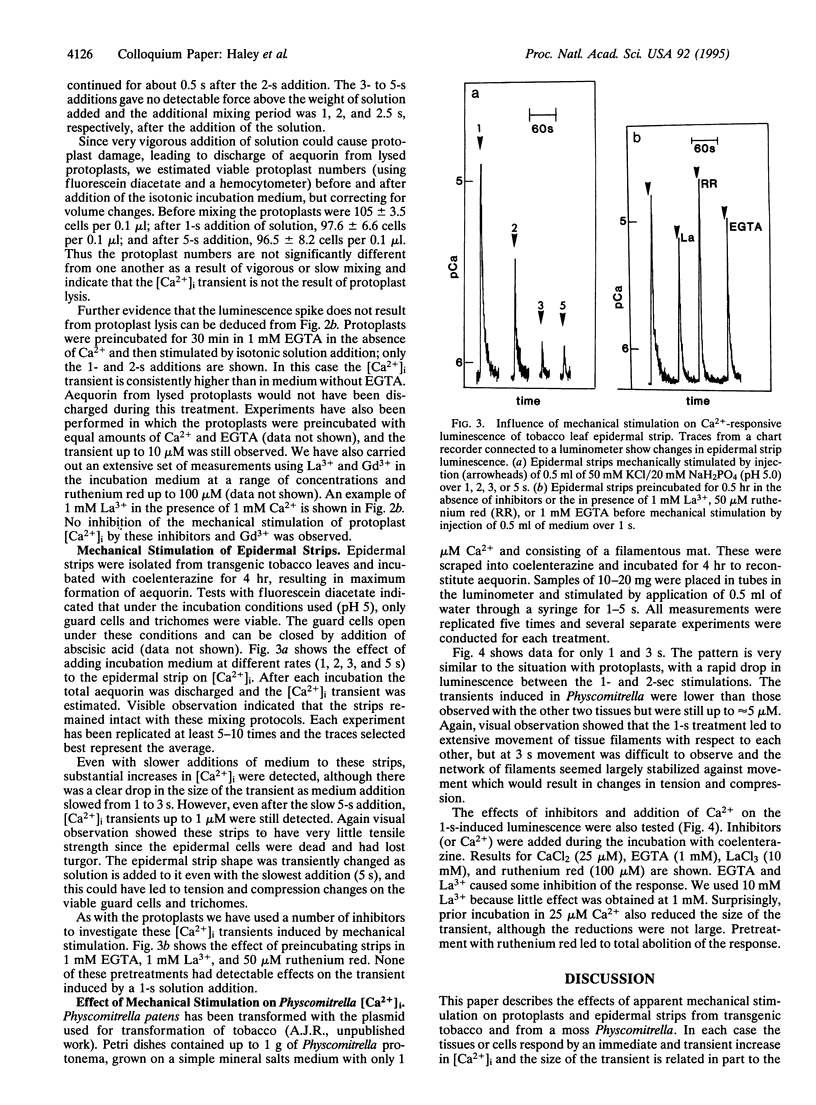
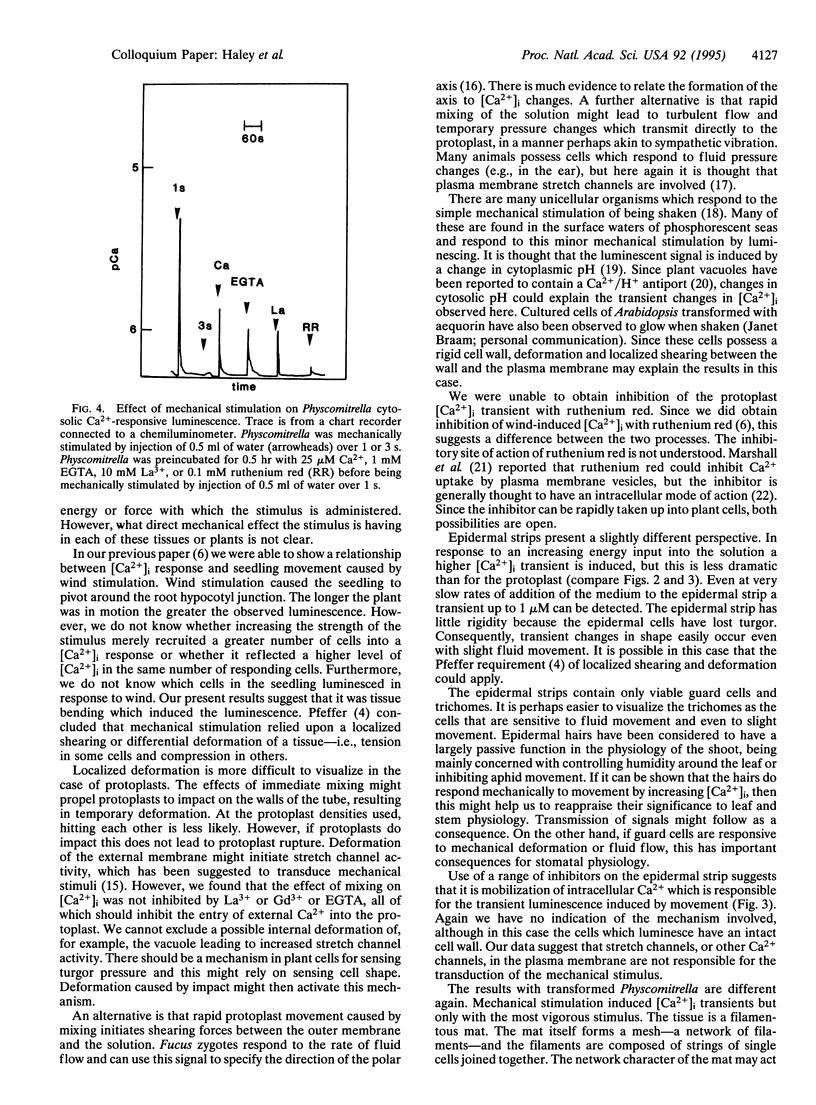
Mechanical signals are important influences on the development and morphology of higher plants. Using tobacco transformed with the Ca(2+)-sensitive luminescent protein aequorin, we recently reported the effects of mechanical signals of touch and wind on the luminescence and thus intracellular calcium of young seedlings. When mesophyll protoplasts are isolated from these transgenic tobacco plants and mechanically stimulated by swirling them in solution, cytoplasmic Ca2+ increases immediately and transiently up to 10 microM, and these transients are unaffected by an excess of EGTA in the medium. The size of the transient effect is related to the strength of swirling. Epidermal strips isolated from transgenic tobacco leaves and containing only viable guard cells and trichomes also respond to the strength of swirling in solution and can increase their cytoplasmic Ca2+ transiently up to 10 microM. Finally, the moss Physcomitrella patens containing recombinant aequorin exhibits transient increases in cytoplasmic Ca2+ up to 5 microM when swirled in solution. This effect is strongly inhibited by ruthenium red. Our data indicate that the effect of mechanical stimulation can be found in a number of different cell types and in a lower plant as well as tobacco and suggest that mechanoperception and the resulting increase in cytoplasmic Ca2+ may be widespread.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. J., Sanders D. Two Voltage-Gated, Calcium Release Channels Coreside in the Vacuolar Membrane of Broad Bean Guard Cells. Plant Cell. 1994 May;6(5):685–694. doi: 10.1105/tpc.6.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Davis R. W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990 Feb 9;60(3):357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J Mol Evol. 1983;19(5):309–321. doi: 10.1007/BF02101634. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jenkins G. I., Courtice G. R., Cove D. J. Gravitropic responses of wild-type and mutant strains of the moss Physcomitrella patens. Plant Cell Environ. 1986;9(8):637–644. doi: 10.1111/j.1365-3040.1986.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Knight C. D., Futers T. S., Cove D. J. Genetic analysis of a mutant class of Physcomitrella patens in which the polarity of gravitropism is reversed. Mol Gen Genet. 1991 Nov;230(1-2):12–16. doi: 10.1007/BF00290643. [DOI] [PubMed] [Google Scholar]
- Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991 Aug 8;352(6335):524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Knight M. R., Read N. D., Campbell A. K., Trewavas A. J. Imaging calcium dynamics in living plants using semi-synthetic recombinant aequorins. J Cell Biol. 1993 Apr;121(1):83–90. doi: 10.1083/jcb.121.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M. R., Smith S. M., Trewavas A. J. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16(2):141–169. [PubMed] [Google Scholar]



