Abstract
Background
The aim of this study was to investigate the sensitivity to rapamycin of endometrial cancer cells with different phosphatase and tensin homologue (PTEN) expression to understand the mechanism of resistance to mammalian target of rapamycin (mTOR) inhibitors in the treatment of endometrial cancer.
Material/Methods
Twenty specific pathogen-free female BALB/c mice received transplants of either HEC-1A (PTEN-positive) or Ishikawa (PTEN-negative) cells. Mice in the treatment group were injected intraperitoneally once a week for 4 consecutive weeks. The control group was injected weekly with phosphate buffer saline (PBS) for 4 consecutive weeks. Tumor volume, tumor mass, growth curves, and inhibition rate were measured, after which the mice were killed.
Results
Both tumor growth rate and size were slower in the treatment group than in the control group for all mice that received transplants of either HEC-1A or Ishikawa cells. The tumor inhibition rates in the treatment group were 48.1% and 67.1% in mice transplanted with HEC-1A and Ishikawa cells, respectively.
Conclusions
The inhibitory effects of rapamycin were enhanced in PTEN-negative Ishikawa tumor cells compared with PTEN-positive HEC-1A cells, which could explain the reduced effect of rapalogues in some endometrial cancer patients and help to understand the mechanism of resistance to this drug.
MeSH Keywords: Endometrial Neoplasms, PTEN Phosphohydrolase, Receptor, Epidermal Growth Factor, TOR Serine-Threonine Kinases
Background
Endometrial cancers are one of the most common gynecological cancers in many developed countries [1], which has promoted the importance of early detection and treatment to the general public. The 5-year survival rates are now approximately 90% for patients who are diagnosed at stage I [2]. Endometrial cancers are caused by many risk factors, including diabetes, hypertension, postmenopausal status, infertility (particularly in the presence of polycystic ovarian syndrome), early menarche and late menopause, radiation exposure, a family history of endometrial cancer, and long-term use of estrogens. Moreover, increased life expectancy and a high body mass index may also explain the increased incidence of this disease [3]. Many studies have reported that obesity affects multiple signalling pathways, including those related to endometrial cancers. One of the most representative is the activation of the PI3K/Akt pathway. Severe obesity can highly activate the mammalian target of rapamycin (mTOR), which is down-stream of PI3K/Akt [4].
In terms of clinical, pathological, and molecular features, 2 main categories of endometrial cancer exist. Type I, which is also known as endometrioid adenocarcinoma, is the most common and accounts for about 80% of all endometrial carcinomas [5]. It is usually estrogen-related and is typically a low-grade adenocarcinoma. Type II (serous carcinoma) is not hormone-dependent and is usually considered to be high-grade and more aggressive [6]. Type I patients can generally be treated more effectively and have a more favorable prognosis. Type I and II involve different kinds and degrees of genetic alternations; type I frequently shows microsatellite instability and mutations of the PIK3CA, PIK3R1, K-Ras and β-catenin genes, whereas type II is characterized by p53 mutations and chromosomal instability [7]. In both types, mutations of the PTEN genes may occur and the prevalence in type I and II is 83% and <10%, respectively. Therefore, investigation on the effect of treatment with variable PTEN expression status may assist in the selection of appropriate medication [8].
The increasing incidence rate of endometrial cancer has attracted intensive research that has mainly focused on the methods of diagnosis and treatment. At present, surgery is still the primary treatment for endometrial cancer, generally comprising a laparotomy, extrafascial total hysterectomy, and bilateral salpingo-oophorectomy. The role of adjuvant therapies, such as radiotherapy and medical therapy, is somewhat controversial [9,10]. For example, some studies have suggested that post-operative external pelvic irradiation can reduce the loco-regional recurrence rate; however, complication rates were also increased and no significant improvements in the overall survival rates were observed [11,12]. Some authors have suggested that adjuvant radiotherapy may be more valuable in patients with high-grade disease, but the role of radiotherapy is still debatable [13].
Medical therapies for endometrial cancers can be categorized as hormonal therapy, chemotherapy, and molecular target therapy. Progestin and anti-estrogens have been used as palliative treatments, mainly for advanced or recurrent endometrial cancer. Some studies have shown that progestin does not improve either the risk of recurrence or the survival rate in patients with early-stage cancer [14]. Chemotherapy, such as a combination of doxorubicin, cisplatin, and paclitaxel, has shown promising responses and survival rates for advanced or recurrent endometrial cancer [15]. However, the toxicity and adverse effects of chemotherapy are still a concern that scientists continually seek to improve [16,17].
The unsatisfactory effectiveness and adverse effects obtained from conventional pharmacological treatments have promoted research into the molecular target therapies that are aimed at specific cell targets and pathways in endometrial carcinogenesis. The most widely accepted therapies are the tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin inhibitors (mTORIs). mTOR is a serine kinase in the phosphatidylinositol-3 kinase family. The mTOR pathway involves various elements that have been shown to be associated with human cancers, such as the PI3K mutation and protein kinase B (AKT) overexpression. The signalling of PI3K and AKT can be deregulated by the overexpression of EGFR [18], a cell surface receptor that is correlated with endometrial cancer [19]. Therefore, the dysregulation of mTOR signalling could enhance the incidence of cancer and the inhibition of mTOR could reduce carcinogenesis. mTOR inhibitors have been reported to inhibit cell proliferation in various cell lines derived from small-cell lung cancer [20], breast cancer [21], pancreatic cancer [22], and prostate cancer [23]. Rapamycin (also known as sirolimus) is the main mTOR inhibitor and, similar to some of its analogues such as deforolimus, everolimus, and temsirolimus, can affect the downstream pathway and form the complexes mTORC1 and mTORC2 [24]. mTORC1 functions as a sensor to control protein synthesis, whereas mTORC2 is insensitive to rapamycin and functions as a regulator of the cytoskeleton [25].
Several mTORIs, such as rapamycin and its associated analogues (temsirolimus, deforolimus, and zotarolimus) have shown promising results in the treatment of cancer in both animal and human studies [26–29]. However, the resistance of these therapeutic agents has caused numerous treatment failures [30]. For example, rapamycin has shown resistance to lung cancer, renal cancer, breast cancer, and endometrial cancer [31–34]. The details of the mechanism of this resistance remain unknown, but the most recent and significant studies have suggested several of them: the downstream effectors of the PI3K/AKT/mTOR pathway; activation of HIF, PTEN expression; elevated superoxide levels; stimulation of autophagy; immune cell response; and ERK/MAPK, Notch, and Aurora signaling pathways [35]. Studies have also suggested that rapamycin sensitivity is dependent on the degree of PTEN expression on chromosome 10 [36] and the quality of mTORC1 phosphorylation sites [37]. This study used nude mice injected with endometrial cancer cells with different PTEN status to investigate the inhibitory effect of rapamycin.
Material and Methods
Experimental animals
Twenty specific pathogen-free female BALB/c nude mice aged 4–6 weeks with a mean body mass of 16–18 g were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. All animals were handled and cared for in accordance with Chinese national and institutional guidelines under licence number SCXY (Beijing) 2006–2009. The specific pathogen-free barrier systems with a certificate of conformity number SYXK (Guangdong) 2007-0083 were used to rear and maintain all mice. They were examined daily and monitored for weight, physical activity, and signs of distress. The IVIS® Lumina II animal in-vivo imaging system from Xenogen was used to examine all mice.
Ten nude mice were randomly allocated to the HEC-1A (PTEN-positive) cell group and the Ishikawa (PTEN-negative) cell group, and were then evenly subdivided into the treatment and control groups. All mice in the treatment groups were injected intraperitoneally once a week with 15 mg/kg rapamycin (LC Laboratories®, USA) for 4 consecutive weeks. The control groups were injected once a week with phosphate buffer saline (PBS) for 4 consecutive weeks.
The single-cell suspensions were prepared using 0.25% of trypsin digestion on the stable Ishikawa and HEC-1A cells at the logarithmic growth phase. Subcutaneous injections of 0.2 mL (3×107 cells/mL) of the suspensions were made into the right hip of the mice. Drug treatments began when the diameter of the transplanted tumor reached 0.5 cm. The mice were placed in the imaging system for in-vivo observation 2 weeks before and 1 week after the drug treatment. Tumor volume (V) was measured and calculated weekly by the equation:
| (1) |
where a and b are the long- and short-axis diameter, respectively. The mice were killed after 1 week of drug treatment and the tumor mass, growth curves, and inhibition rate (I,%) were then calculated by:
| (2) |
Cell culture and transfection
The human endometrial carcinoma cell lines HEC-1A and Ishikawa were obtained from the Third Affiliated Sun Yat-sen University Hospital of Gynecology. The cells were grown in DMEM culture medium (Gibco®, Life Technologies™, USA) supplemented with 10% fetal bovine serum (Thermo Scientific™ HyClone™, USA), 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere with 5% carbon dioxide.
Ishikawa and HEC-1A cells in good condition at the logarithmic growth phase were selected. The cells were seeded in 6-well plates at 5×104/well for 1 day before the experiment. Infection of the virus particles with green fluorescent protein-lentiviral vector (GeneChem®, China) was performed at a cell fusion rate of 30–50% with a complex infection index (multiplicity of infection, MOI) of 50. Polybrene was added to the medium until a final concentration of 5 μg/mL was reached. Fresh medium was replaced every 8~12 h and fluorescence expression was observed after 3~4 days of infection.
Green fluorescent protein (GFP) was used for imaging the endometrial tumors of the nude mice. GFP is one of the most commonly used fluorescent proteins and the fluorescence reaction does not require any additional substrates or cofactors; green fluorescence is emitted after blue light excitation and no damage to growing cells or tissues occurs [38].
Histopathological analysis
All harvested tumor tissues were fixed in formalin and embedded in paraffin for standard hematoxylin and eosin analysis.
Statistical analysis
Data are presented as the mean ± the standard deviation (SD). SPSS 11.5 was used for statistical analysis using the Student’s t test assuming equal variance. A 2-tailed test was used to calculate the P value, which was considered to be statistically significant when less than 0.05.
Results
Fluorescence microscopic observation of transfected GFP-endometrial cancer cell lines
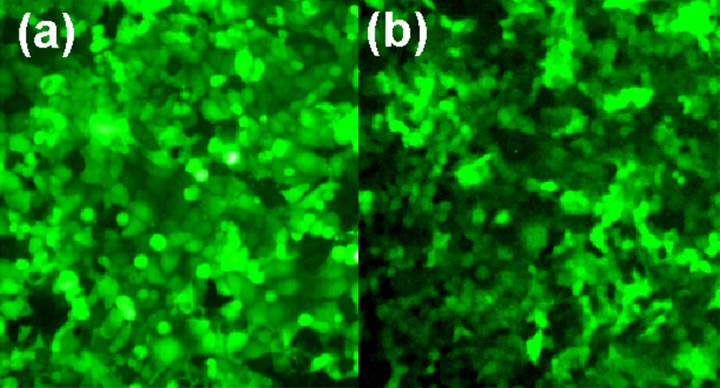
The fluorescence of the transfected GFP-HEC-1A and Ishikawa cells was distributed uniformly over the entire cell, with strong fluorescent signal intensity. The transfection efficiency was close to 100% (Figure 1A, 1B).
Figure 1.
Green fluorescent images (×200) of HEC-1A cells (A) and Ishikawa cells (B).
Inhibitory effect of rapamycin on HEC-1A and Ishikawa cells in nude mice
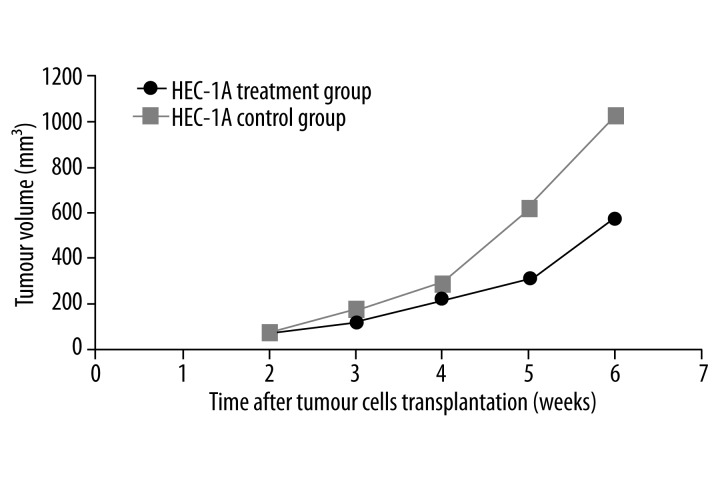
The tumor growth rate was slower in the treatment group than in the control group of mice that were transplanted with HEC-1A cells. The differences in tumor volume were statistically significant after 3 doses of rapamycin (P<0.05; Figure 2). The treatment and control groups had a mean tumour volume of (0.40±0.09) g and (0.77±0.19) g, respectively. The inhibition rate was 48.1%.
Figure 2.
Comparison of tumor growth curves between HEC-1A treatment and control groups. * indicates P<0.05 and ** indicates P<0.01.
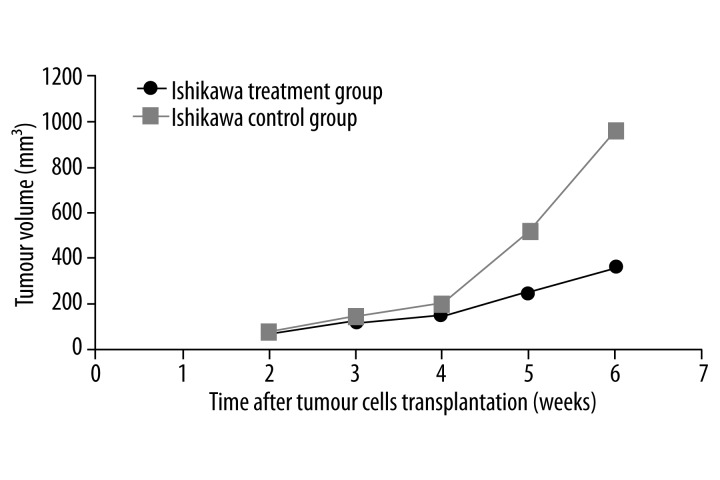
In the nude mice injected with Ishikawa cells, the tumor growth was slower in the treatment group than in the control group. The differences in tumor volume were statistically significant after 2 doses of rapamycin (P<0.05; Figure 3). The treatment and control groups had a mean tumor volume of (0.24±0.10) g and (0.73±0.24) g, respectively (p<0.01). The inhibition rate was 67.1%, and was higher than that of the HEC-1A cell group.
Figure 3.
Comparison of tumor growth curves between Ishikawa treatment and control groups. * indicates P<0.05 and ** indicates P<0.01.
Tumor growth of nude mice before and after transplantation of endometrial cancer cells
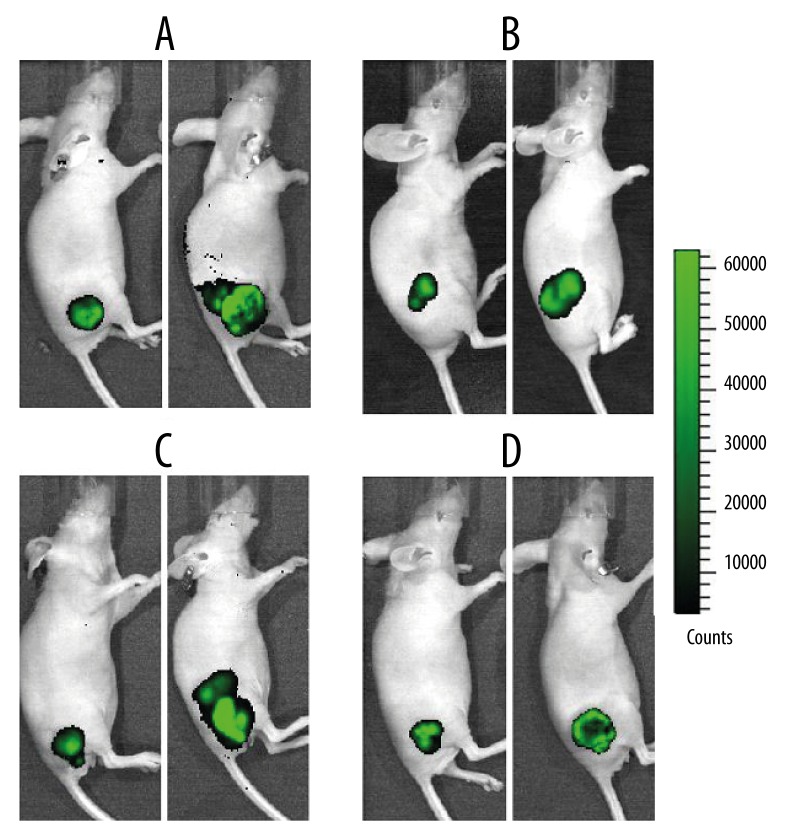
Tumor growth was observed using the IVIS® Lumina II in-vivo imaging system. Stable expression of the GFP was detected in the nude mice 7 days after transplantation, but a vernier calliper could not be used for accurate measurement. The tumor volume in all groups was increased 6 weeks after the inoculation of cells. The fluorescence intensity of the 2 control groups had increased significantly, indicating that the tumor size had also increased significantly. In contrast, the fluorescence intensity of both treatment groups had decreased significantly. The intensity in the Ishikawa cell group was significantly lower than that in the HEC-1A cell group, and the intensity in the center of the Ishikawa cell tumor appeared weakened, indicating that tumor tissue necrosis had begun in this area (Figure 4).
Figure 4.
Bioluminescence images of the HEC-1A control group (A), the HEC-1A treatment group (B), the Ishikawa control group (C), and the Ishikawa treatment group (D).
Effect of rapamycin on the organizational structure of endometrial cancer cells with different PTEN expression
The layer distribution of tumor tissue in the histopathological analysis helped to visualize the PBS group of HEC-1A and Ishikawa cells. For both types of cell, cell nuclear atypia, nuclear membrane thickening, coarse nuclear chromatin, prominent nucleoli, and comparatively less tumor necrosis were observed. In the treatment groups, inflammatory cell infiltration, tumor cell nucleus fragmentation and disappearance, enhanced eosinophilic cytoplasm, and large areas of tumor necrosis were observed.
Discussion
Endometrial cancer is one of the most common female genital tract malignancies, and affects around 81 500 women, mainly those over 50 years of age, every year in the European Union [2]. Loss or mutation of the PTEN gene is common in endometrial carcinoma, occurring in 60–80% of cases, which is a much higher rate than that seen in other common gynecological malignancies [39]. This loss of PTEN function by mutational mechanisms mostly occurs at the onset of endometrial tumorigenesis. Some studies have therefore suggested using this information as a diagnostic marker for early endometrial precancers [40,41]. In general, PTEN operates as a tumor suppressor gene, and prevents cells from growing and dividing too rapidly. Therefore, it is reasonable to hypothesize that PTEN expression can influence the development of endometrial cancers.
In different types of cancer, the degree of PTEN expression is associated with metastatic behavior and can affect treatment outcomes. For example, the loss of PTEN in breast cancer has been shown to be associated with disease-related death and lymph node metastasis, but not to tumor grade or disease recurrence [42]. The loss of PTEN expression was also associated with non-responsiveness to treatment with cetuximab in metastatic colorectal cancer patients, and the authors of the study suggested that PTEN expression may predict the efficacy of certain drug treatments for colorectal cancer [43]. Furthermore, a recent study suggested that the level of PTEN expression affects the sensitivity of poly-ADP ribose polymerase (PARP) inhibitors in the treatment of endometrioid endometrial adenocarcinomas [44]. However, the role of PTEN alterations and the associated outcome of treatment with one of the most important molecular target therapies, mTOR, in endometrial carcinoma are not well established. Here, we found that rapamycin has an advanced inhibitory effect on PTEN-negative cancer in nude mice.
Rapamycin was the first mTOR inhibitor to demonstrate anti-tumor activity. Clinical trials showed an acceptable level of effectiveness of rapamycin and its analogues (rapalogues) in the treatment of endometrial cancer, breast cancer, pancreatic cancer and hematological tumors [45]. However, resistance is becoming a growing concern. This study found that the sensitivity of rapamycin differs in PTEN-positive HEC-1A cells and PTEN-negative Ishikawa cells. The tumor volume of nude mice injected with the Ishikawa cells was reduced after the second dose of rapamycin and the inhibition rate after 4 doses was 67.1%. In contrast, the tumor volume of nude mice injected with HEC-1A cells was reduced after the third dose of rapamycin and the inhibition rate after 4 doses was 48.1%. These results indicate that rapamycin can significantly inhibit both PTEN-positive HEC-1A and PTEN-negative Ishikawa tumor cells, and that the effect on the latter was more significant (Figure 4).
Studies have shown PTEN expression can affect the sensitivity of signal transduction inhibitors that target the PI3K/AKT/mTOR pathway in endometrial carcinoma cells [46]. This may be caused by the PTEN-negative regulation of AKT and phosphor-mTOR. It has also been shown that PTEN can counteract the activity of the PI3K/AKT/mTOR pathway and that the loss of PTEN can activate the PI3K/AKT/mTOR pathway. Therefore, with the loss of PTEN, pharmacological inhibitors of this pathway may further mimic the effects that are rescued by the restoration of PTEN [36]. This may explain the mechanism of the enhanced inhibitory effect of rapamycin in PTEN-negative tumor cells compared with its effect in PTEN-positive cells.
Conclusions
The results of this study clearly show that rapamycin has an enhanced inhibitory effect in PTEN-negative tumour cells in nude mice. We propose that the level of PTEN expression may affect the clinical response to rapamycin and possibly other mTOR inhibitors. This effect should be considered during the selection of drug treatments for endometrial cancer patients. Results of this study also help to explain the reduced effect of rapalogues in some patients with endometrial cancer and to understand the mechanism of this drug resistance.
Footnotes
Conflict of interest statement
The Authors declare that they have no conflicts of interest to disclose.
Source of support: This research was supported financially by the Science and Technology Development Fund of the Macao Special Administrative Region (028/2011/A2) and Macau Polytechnic Institute Fund (RP/ESS-05/2013)
References
- 1.Jamison PM, Noone AM, Ries LA, et al. Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22:233–41. doi: 10.1158/1055-9965.EPI-12-0996. [DOI] [PubMed] [Google Scholar]
- 2.Plataniotis G, Castiglione M. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v41–45. doi: 10.1093/annonc/mdq245. [DOI] [PubMed] [Google Scholar]
- 3.Ali AT. Risk factors for endometrial cancer. Ceska Gynekol. 2013;78:448–59. [PubMed] [Google Scholar]
- 4.Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063–70. doi: 10.1111/j.1467-789X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 5.Shang C, Lu YM, Meng LR. MicroRNA-125b down-regulation mediates endometrial cancer invasion by targeting ERBB2. Med Sci Monit. 2012;18(4):BR149–55. doi: 10.12659/MSM.882617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black JD, English DP, Roque DM, Santin AD. Targeted therapy in uterine serous carcinoma: an aggressive variant of endometrial cancer. Womens Health (Lond Engl) 2014;10:45–57. doi: 10.2217/whe.13.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;24(Suppl 6):vi33–38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 8.Weigelt B, Banerjee S. Molecular targets and targeted therapeutics in endometrial cancer. Curr Opin Oncol. 2012;24:554–63. doi: 10.1097/CCO.0b013e328354e585. [DOI] [PubMed] [Google Scholar]
- 9.Zips D, Krause M, Yaromina A, et al. Epidermal growth factor receptor inhibitors for radiotherapy: biological rationale and preclinical results. J Pharm Pharmacol. 2008;60:1019–28. doi: 10.1211/jpp.60.8.0008. [DOI] [PubMed] [Google Scholar]
- 10.Falcone F, Balbi G, Di Martino L, et al. Surgical management of early endometrial cancer: an update and proposal of a therapeutic algorithm. Med Sci Monit. 2014;20:1298–313. doi: 10.12659/MSM.890478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 12.Kong A, Johnson N, Cornes P, et al. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev. 2007;18(2):CD003916. doi: 10.1002/14651858.CD003916.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Gadducci A, Greco C. The evolving role of adjuvant therapy in endometrial cancer. Crit Rev Oncol Hematol. 2011;78:79–91. doi: 10.1016/j.critrevonc.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Hirsch PL, Jarvis G, Kitchener H, Lilford R. Progestagens for endometrial cancer. Cochrane Database Syst Rev. 2000;2:CD001040. doi: 10.1002/14651858.CD001040. [DOI] [PubMed] [Google Scholar]
- 15.Aapro MS, van Wijk FH, Bolis G, et al. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003;14:441–48. doi: 10.1093/annonc/mdg112. [DOI] [PubMed] [Google Scholar]
- 16.Indumathy S, Dass CR. Finding chemo: the search for marine-based pharmaceutical drugs active against cancer. J Pharm Pharmacol. 2013;65:1280–301. doi: 10.1111/jphp.12097. [DOI] [PubMed] [Google Scholar]
- 17.Tacar O, Dass CR. Doxorubicin-induced death in tumour cells and cardiomyocytes: is autophagy the key to improving future clinical outcomes? J Pharm Pharmacol. 2013;65:1577–89. doi: 10.1111/jphp.12144. [DOI] [PubMed] [Google Scholar]
- 18.Fasolo A, Sessa C. Targeting mTOR pathways in human malignancies. Curr Pharm Des. 2012;18:2766–77. doi: 10.2174/138161212800626210. [DOI] [PubMed] [Google Scholar]
- 19.Scambia G, Benedetti Panici P, Ferrandina G, et al. Significance of epidermal growth factor receptor expression in primary human endometrial cancer. Int J Cancer. 1994;56:26–30. doi: 10.1002/ijc.2910560106. [DOI] [PubMed] [Google Scholar]
- 20.Seufferlein T, Rozengurt E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer Res. 1996;56:3895–97. [PubMed] [Google Scholar]
- 21.van der Poel HG, Hanrahan C, Zhong H, Simons JW. Rapamycin induces Smad activity in prostate cancer cell lines. Urol Res. 2003;30:380–86. doi: 10.1007/s00240-002-0282-1. [DOI] [PubMed] [Google Scholar]
- 22.Grewe M, Gansauge F, Schmid RM, et al. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999;59:3581–87. [PubMed] [Google Scholar]
- 23.Pang H, Faber LE. Estrogen and rapamycin effects on cell cycle progression in T47D breast cancer cells. Breast Cancer Res Treat. 2001;70:21–26. doi: 10.1023/a:1012570204923. [DOI] [PubMed] [Google Scholar]
- 24.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Gadducci A, Tana R, Cosio S, et al. Molecular target therapies in endometrial cancer: from the basic research to the clinic. Gynecol Endocrinol. 2008;24:239–49. doi: 10.1080/09513590801953556. [DOI] [PubMed] [Google Scholar]
- 27.Roque DM, Santin AD. Updates in therapy for uterine serous carcinoma. Curr Opin Obstet Gynecol. 2012;25:29–37. doi: 10.1097/GCO.0b013e32835af98d. [DOI] [PubMed] [Google Scholar]
- 28.Suh DH, Kim JW, Kim K, et al. Major clinical research advances in gynecologic cancer in 2012. J Gynecol Oncol. 2013;24:66–82. doi: 10.3802/jgo.2013.24.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med Chem. 2010;10:571–81. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–19. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruppuso PA, Boylan JM, Sanders JA. The physiology and pathophysiology of rapamycin resistance: implications for cancer. Cell Cycle. 2011;10:1050–58. doi: 10.4161/cc.10.7.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Sun SY, Owonikoko TK, et al. Rapamycin induces Bad phosphorylation in association with its resistance to human lung cancer cells. Mol Cancer Ther. 2012;11:45–56. doi: 10.1158/1535-7163.MCT-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner-Kubath C, Shabbir W, Saferding V, et al. The PI3 kinase/mTOR blocker NVP-BEZ235 overrides resistance against irreversible ErbB inhibitors in breast cancer cells. Breast Cancer Res Treat. 2011;129:387–400. doi: 10.1007/s10549-010-1232-1. [DOI] [PubMed] [Google Scholar]
- 34.Carew JS, Kelly KR, Nawrocki ST. Mechanisms of mTOR inhibitor resistance in cancer therapy. Target Oncol. 2011;6:17–27. doi: 10.1007/s11523-011-0167-8. [DOI] [PubMed] [Google Scholar]
- 35.Santoni M, Pantano F, Amantini C, et al. Emerging strategies to overcome the resistance to current mTOR inhibitors in renal cell carcinoma. Biochim Biophys Acta. 2014;1845:221–31. doi: 10.1016/j.bbcan.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Li T, Yang Y, Li X, et al. EGFR- and AKT-mediated reduction in PTEN expression contributes to tyrphostin resistance and is reversed by mTOR inhibition in endometrial cancer cells. Mol Cell Biochem. 2012;361:19–29. doi: 10.1007/s11010-011-1082-0. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SO, Roux PP. Rapamycin resistance: mTORC1 substrates hold some of the answers. Curr Biol. 2013;23:R880–83. doi: 10.1016/j.cub.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Baranov E, Jiang P, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci USA. 2000;97:1206–11. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tashiro H, Blazes MS, Wu R, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–40. [PubMed] [Google Scholar]
- 40.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 41.Djordjevic B, Hennessy BT, Li J, et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol. 2012;25:699–708. doi: 10.1038/modpathol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–76. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 43.Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97:1139–45. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:53ra75. doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 45.Bae-Jump VL, Zhou C, Boggess JF, et al. Rapamycin inhibits cell proliferation in type I and type II endometrial carcinomas: a search for biomarkers of sensitivity to treatment. Gynecol Oncol. 2010;119:579–85. doi: 10.1016/j.ygyno.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao L, Yang YB, Li XM, et al. Differential sensitivity of human endometrial carcinoma cells with different PTEN expression to mitogen-activated protein kinase signaling inhibits and implications for therapy. J Cancer Res Clin Oncol. 2010;136:1089–99. doi: 10.1007/s00432-009-0756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]