Abstract
Visceral leishmaniasis (VL) is a chronic and fatal disease caused by Leishmania infantum in Brazil. Leukocyte recruitment to infected tissue is a crucial event for the control of infections such as VL. Leucotriens are lipid mediators synthesized by 5-lipoxygenase (5-LO) and they display a protective role against protozoan parasites by inducing several functions in leucocytes. We determined the role of 5-LO activity in parasite control, focusing on the inflammatory immune response against Leishmania infantum infection. LTB4 is released during in vitro infection. The genetic ablation of 5-LO promoted susceptibility in highly resistant mice strains, harboring more parasites into target organs. The susceptibility was related to the failure of neutrophil migration to the infectious foci. Investigating the neutrophil failure, there was a reduction of proinflammatory cytokines involved in the related Th17 axis released into the organs. Genetic ablation of 5-LO reduced the CD4+T cells producing IL-17, without interfering in Th1 subset. L. infantum failed to activate DC from 5-LO−/−, showing reduced surface costimulatory molecule expression and proinflammatory cytokines involved in Th17 differentiation. BLT1 blockage with selective antagonist interferes with DC maturation and proinflammatory cytokines release. Thus, 5-LO activation coordinates the inflammatory immune response involved in the control of VL.
1. Introduction
Visceral leishmaniasis (VL) is one of the most severe clinical manifestations of infection with Leishmania parasites and it is a major cause of human mortality and morbidity globally; VL is caused by Leishmania donovani and Leishmania infantum (World Health Organization, 2010).
The host protective response against Leishmania spp. is predominantly mediated by cellular immunity mechanisms, which are critical for parasite replication control and disease resolution. Initially, during infection, activated dendritic cells (DCs) modulate inflammatory leucocyte recruitment to the infection foci [1] and the development of the T CD4+ lymphocyte response characterized by robust IFN-γ and IL-17 production [2, 3]. The immune cell recruitment to Leishmania infection foci is managed by inflammatory mediators. Chemokines and cytokines have crucial roles in determining the outcome of leishmaniasis [4, 5]. Lipid mediators such as leukotrienes (LTs) are another class of molecules involved in host defense [6].
LTs are generated from the membrane phospholipids of activated innate immune cells, arachidonic acid (AA), through activation of the 5-lipoxygenase (5-LO) enzymes. 5-LO catalyzes oxidation of AA to intermediate 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which is enzymatically reduced by 5-LO to the unstable epoxide A4 leukotriene (LTA4). LTA4 could be hydrolyzed to form leukotriene B4 (LTB4), which is a potent effector of leukocyte chemotaxis and activation through the BLT1/BLT2 G-protein coupled receptors [7]. Inflammatory disease such as asthma [8], allergic rhinitis [9], and rheumatoid arthritis [10] are associated with increased levels of LTB4.
Studies have demonstrated that LTB4 is a potent leukotropic, proinflammatory, and immunoregulator mediator [11, 12]. These molecules are involved in the control of infectious diseases, including viral [13, 14], bacterial [15–17], fungal [18–20], and protozoan infections such as those caused by T. gondii [21] and T. cruzi [22] and nematode infections [23].
Regarding leishmaniasis, LTB4 displays leishmanicidal activity on macrophages [24] and neutrophils [25] during in vitro infection with L. amazonensis, through mechanisms dependent on nitric oxide (NO) and reactive oxygen species (ROS), respectively. In addition, the inhibition of the 5-LO pathway promoted high susceptibility to L. amazonensis infection, increasing footpad swelling and harbored more parasites in resistant and susceptible infected mice [26]. In in vitro macrophage infection with L. donovani parasites, the 5-LO enzymatic activity is enhanced, leading to increased amounts of arachidonic acid metabolites [27], and in vivo, L. donovani infection promotes an increase of cyclooxygenase and lipoxygenase activities in spleen cells [28]. It was recently reported that L. infantum in vitro infection inhibits the LTB4 signaling pathway dependent on homologous DCSIGN (SIGNR3) during parasite recognition by macrophages [29], suggesting a protective role of LTB4 during VL induced by L. infantum. Their potential in the recruitment of leukocytes that might be involved in parasite restriction is less well understood. We investigated the role of 5-LO activity in the control of experimental VL induced by L. infantum, focusing on the inflammatory immune response. We demonstrated that mice lacking 5-LO signaling displayed high susceptibility to L. infantum infection because of a commitment on the related Th17 axis released by CD4 T lymphocytes and neutrophil migration to the infection foci.
2. Material and Methods
2.1. Mice
Female wild-type 129/SvEv (WT) mice or mice genetically deficient in 5-LO (129/SvEv-5-LO−/−), 18–22 g in weight, were housed in the animal facility of the Department of Biochemistry and Immunology of the School of Medicine of Ribeirão Preto at the University of São Paulo (Brazil) in temperature-controlled rooms (22–25°C); the mice received water and food ad libitum. The experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines on the welfare of experimental animals and with the approval of the Ethics Committee of the School of Medicine of Ribeirão Preto.
2.2. Parasite Culture, Infection, and Parasite Load Estimation
L. infantum (isolate HU-UFS14) was cultured in Schneider medium with 20% heat-inactivated fetal bovine serum, 5% penicillin and streptomycin (from Sigma-Aldrich, Saint Louis, MO, USA), and 2% male human urine. The parasite virulence was maintained by serial passages in BALB/c mice. The mice were injected in the retroorbital plexus with 107 stationary-phase L. infantum promastigotes in 100 μL PBS. The hepatic and splenic parasite burdens were determined using a quantitative limiting dilution assay.
2.3. DC Generation and Infection
Generation of bone marrow-derived dendritic cells (BMDC) was performed as previously described [30]. The BMDCs (1 × 106/mL) cultured in RPMI-1640 supplemented with 10% FBS were infected with L. infantum promastigote forms at a 1 : 5 ratio (cells/parasites) for 12, 24, 36, and 48 h. The supernatants were collected to measure LTB4 by ELISA (BiotrakTm, Amersham Pharmacia Biotech, UK). In some wells, LPS (200 ng/mL) was added to the BMDC culture as the positive control group. The cells were harvested and their surface expression characterized by flow cytometry using antibodies against CD11c, MHC class-II, CD86, and CD40 conjugated to APC, FITC, PECy7, PerCP, and Alexa700, respectively, as well as the control isotypes. The cytokine releases were measured into the supernatant culture using commercial ELISA kits, according to the manufacturer's instructions (BD Biosciences, R&D Systems, Minneapolis, MN, USA). In some experiments, selective BLT1 leukotriene B4 receptor antagonist (U-75302, Sigma-Adrich) (10 μM) was added 12 h before L. infantum infection.
2.4. Cytokine Release
To assess the influence of LTB4 on cytokine production, the liver tissue samples were harvested by a tissue trimmer, weighed, and tittered in 0.5 mL of PBS Complete (Roche Diagnostics, Mannheim, Germany) containing protease inhibitor cocktail. The levels of IFN-γ, IL-17, TNF-α, IL-12p40, IL-23, IL-6, TGF-β, and IL-1β were determined using commercial ELISA kits.
2.5. Cell Culture and Inflammatory Cells Phenotype
Single-cell suspensions of spleen tissue samples from the 5-LO−/− or WT mice at 6th wpi were aseptically prepared, diluted to a concentration of 2 × 106 cells/mL, and dispensed into 48-well plates in a total volume of 500 μL of complete RPMI-1640 medium (1 × 106 cells/well; Gibco) with or without soluble Leishmania Ag (5 μg/mL). The cell culture supernatants were harvested after 72 h of culture at 37°C in 5% CO2, and the cytokine levels in the supernatants were determined by ELISA with commercial kits (BD Biosciences and R&D Systems). For the leukocyte identification, the inflammatory cells were gated based on their characteristic size (FSC) and granularity (SSC), and the T lymphocytes (CD4+CD3+), dendritic cell activation markers (CD11chighCD40+, CD11chighCD86+, and CD11chighMHC-II+), and neutrophils subsets: activated (Ly6GhighCD11bhigh) or inactivated neutrophils (Ly6GintCD11bint) were identified individually. For the intracellular staining, the cells were previously cultured with PMA (50 ng/mL) and ionomycin for 4 h in order to obtain the maximum of cytokine production and permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's guidelines and stained with anti-IFN-γ or anti-IL-17 conjugated to APC-Cy7 and Alexa700 and with anti-CD3 and anti-CD4 for surface staining with FITC and PerCP, respectively. Rat IgG2b and rat IgG2a were used as the isotype controls. All the antibodies were supplied from BD Biosciences and eBiosciences (San Diego, CA, USA). The cell acquisition was performed using a FACSort flow cytometer. The data were plotted and analyzed using the FlowJo software (Tree Star, Ashland, OR). The total leucocytes counts were determined by relative expression of leucocytes subpopulation stained with specific antibody obtained in 300,000 events acquired and proportional to the leukocytes number obtained in Neubauer chamber.
2.6. Statistical Analysis
The data are expressed as the mean ± SEM and are representative of 2–4 independent experiments. The results from the individual experiments were not combined because they were analyzed individually. The means from the groups were compared by ANOVA followed by Tukey's honest significant difference (HSD) test. Statistical significance was set at P < 0.05.
3. Results
3.1. 5-LO Activation Is Required for Experimental L. infantum Infection Control
To determine whether Leishmania infantum drives the activation of 5-LO pathway, we performed a kinetic in the release of LTB4 by bone marrow-derived dendritic cells after 12, 24, 36, or 48 hours of parasite infection. L. infantum induces significant amounts of LTB4 by BMDCs at 12 hours postinfection, peaked at 24 hours, and persisted for 36 hours. At 48 hours, the heightened levels of LTB4 production were significantly reduced and similar that produced for uninfected cells (medium stimuli) (Figure 1(a)). To characterize the LTB4 function, 5-LO−/− and littermate control mice were infected with L. infantum and the course of infection was monitored by parasite quantification into the organs by a limiting dilution. We observed that WT presented progressive parasite titers into the spleen (Figure 1(b)) and the liver (Figure 1(c)) over time. The 5-LO−/− mice were more susceptible to infection, harboring more parasites in both target organs than were the WT animals in all the analyzed periods, demonstrating that 5-LO activity, and possibly LTB4, participates in the control of L. infantum.
Figure 1.
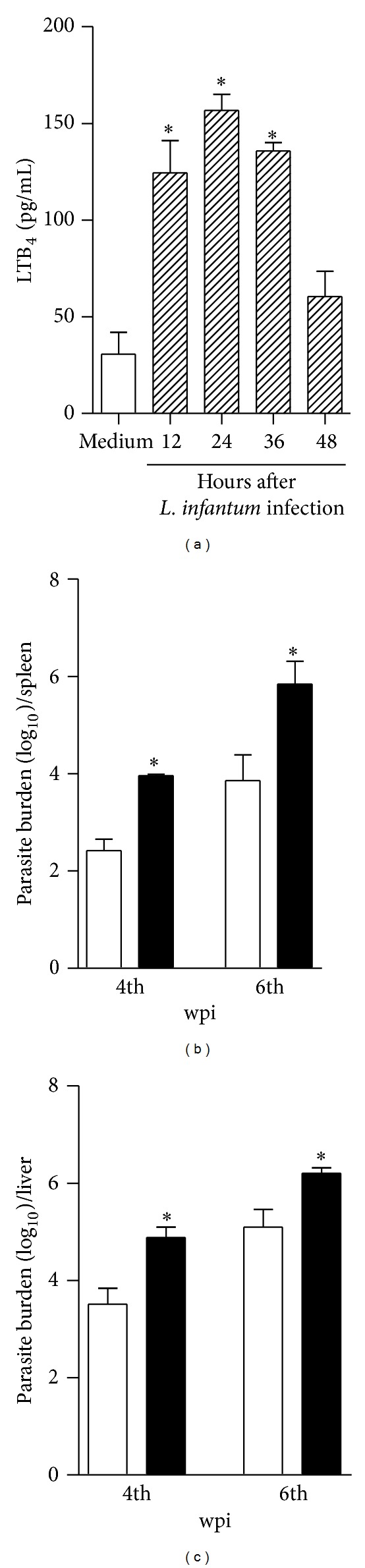
5-LO activity participates in the control of L. infantum infection. In (a), the WT BMDCs (1 × 106 cells/mL) were infected with L. infantum (1 : 5) (hatched bar) or medium (white bar) for 12, 24, 36, and 48 h and the LTB4 amount in the supernatant was determined by ELISA assay. The parasite burden in the spleen (b) and liver (c) was determined in the WT (white bars) and 5-LO−/− (black bars) mice at the 4th and 6th wpi with L. infantum promastigote forms (1 × 107 parasites/mice-i.v. route). The data are expressed as the mean ± SEM, N = 5-6. *P < 0.05 compared with the WT group.
3.2. L. infantum-Infected 5-LO−/− Mice Fail to Recruit Neutrophils to Infectious Foci
Because LTB4 presented potent neutrophil chemotactic activity and we and others reported the role of neutrophils in the control of Leishmania spp. [11], we characterized the neutrophils present in the spleens of the 5-LO−/− or WT infected mice at 6th wpi.
Based on their characteristic size (FSC) and granularity (SSC), we observed a significant reduction of cells when analyzed in the granulocytes gate from infected 5-LO−/− mice. Phenotyping the cells, we found that the frequencies of Ly6G+CD11bhigh were present in the spleen samples from the WT infected mice. The percentage of the influx of neutrophils was affected in the 5-LO−/− infected mice, which showed an approximately 30% reduction compared with that of the WT infected mice (Figures 2(b) and 2(c)). In terms of total numbers, the neutrophil reduction was ~50% in the 5-LO−/− mice (Figure 2(c)). We also observed another neutrophils population, LY6G+CD11binterm (Figure 2(b)), features of inactive neutrophils since CD11b is upregulated under proinflammatory stimuli [31, 32]. However, their frequency and total cells (Figure 2(d)) in the spleens were similar in both groups. These findings suggest that 5-LO activity participates in neutrophil recruitment to inflammatory foci and, under appropriated activation, might be required for parasite control during L. infantum infection.
Figure 2.
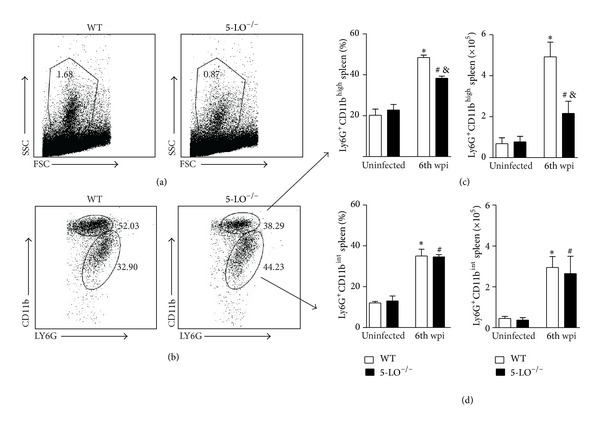
Lack of 5-LO interferes on neutrophil migration. The neutrophils were gated based on their characteristic size (FSC) and granularity (SSC) (a). The dot plots represent the frequency of neutrophils population characterized by LY6GhighCD11bhigh (upper gate) and LY6GintCD11bint (lower gate) by flow cytometry (b). The bar graphs display the percentage and the absolute number characterized as LY6GhighCD11bhigh population (c) or LY6GintCD11bint (d) in the spleen from the WT and 5-LO−/− mice at the 6th wpi or from uninfected mice (naïve mice). The data are expressed as the mean ± SEM, N = 5-6. *P < 0.05 compared with the WT naïve group, & P < 0.05 compared with the WT infected group, and # P < 0.05 compared with 5-LO−/− naïve group.
3.3. 5-LO Activity Is Associated with the Development of Host Protective Th17 Responses
Because the development of IFN-γ and IL-17-producing CD4+ T helper cells is crucial for the control of parasite replication in the target organs of LV, we investigated whether these responses were generated in a 5-LO dependent manner. Spleen cells from WT and 5-LO−/− mice at 6th wpi or naïve were in vitro restimulated with polyclonal PMA plus ionomycin and the intracellular cytokine production was analyzed. There was no difference in the frequency and absolute number of the IFN-γ-producing CD4+ T cells in the WT and 5-LO−/− mice (Figure 3(a)). The IL-17-producing CD4+ T cells were significantly impaired in the spleens of the 5-LO−/− mice (Figure 3(b)), where the Th17 cells reduction was approximately 50% of that in the WT mice.
Figure 3.
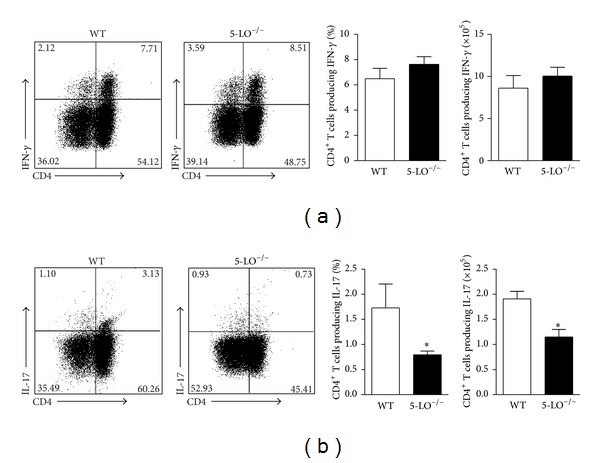
5-LO ablation decreased the Th17, but not the Th1, pattern of immune response. The spleen cells from the WT (white bars) or 5-LO−/− (black bars) mice at the 6th wpi were in vitro restimulated with PMA and ionomycin for 4 h and analyzed for intracellular cytokine production by flow cytometry. The dot plots represent the frequency of the CD4+ T cell-producing IFN-γ (a) and the CD4+ T cell-producing IL-17 (b), and the graph bars represent the percentage and the total number of these cells. The data are expressed as the mean ± SEM, N = 5-6. *P < 0.05 compared with the WT group.
Having determined that 5-LO activity participates in the development of the Th17 response, we measured the production of cytokines in the culture supernatant of the total splenic cells from the WT, 5-LO−/− naïve, or infected mice at 6th wpi and restimulated them in vitro with soluble Leishmania Ag (SLA). The stimulation with SLA did not induce significant amounts of IFN-γ (Figure 4(a)), IL-17 (Figure 4(b)), TNF-α (Figure 4(c)), IL-23 (Figure 4(d)), IL-6 (Figure 4(e)), IL-1β (Figure 4(f)), and TGF-β (Figure 4(g)) in the culture supernatants of spleens cells from the naïve WT mice compared with those induced in the control (medium). A similar effect was observed in cells from the 5-LO−/− naïve mice when stimulated with the antigen. The infection promoted pronounced levels of all the analyzed cytokines after the SLA stimulation in the WT group, compared to those in the medium (Figures 4(a)–4(g)). Infection in the 5-LO−/− mice resulted in a reduction of cytokine release related to the Th17 axis such as IL-17, TNF-α, IL-23, and IL-6 (Figures 4(b)–4(e)); however, neither IFN-γ (Figure 4(a)), IL-1β (Figure 4(f)), nor TGF-β (Figure 4(g)) productions were affected by a specific stimulus, compared to that obtained in the infected WT mice when stimulated with SLA. Additionally, proinflammatory cytokines in the liver involved in the Th17 axis such as IL-17 (Figure 4(i)), TNF (Figure 4(j)), and IL-12p40 (IL-23) (Figure 4(k)) were reduced in the absence of 5-LO. Corroborating to Figures 3(a) and 4(a), IFN-γ amounts were not altered in the deficient mice (Figure 4(h)). These data suggest that 5-LO activity is associated with Th17 response development, and this pathway might be involved in the neutrophils recruitment to inflammatory foci.
Figure 4.
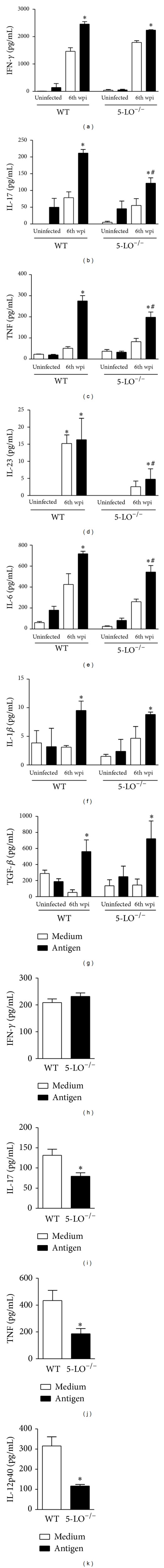
The absence of 5-LO affected the cytokine release related to Th17 pattern. The spleen cells from the WT and 5-LO−/− mice at the 6th week pi or uninfected were in vitro stimulated with the L. infantum antigen (50 μg/mL) or medium for 72 hours, and the levels of IFN-γ (a), IL-17 (b), TNF (c), IL-23 (d), IL-6 (e), IL-1β (f), and TGF-β (g) were measured in the culture supernatants by ELISA assay. The data are expressed as the mean ± SEM and one representative of two independent experiments. *P < 0.05 compared to the medium; # P < 0.05 compared with the WT stimulation. The liver fragments from the WT (white bar) or 5-LO−/− (black bar) at the 6th wpi with the L. infantum promastigote forms were collected and weighed for the determination of IFN-γ (h), IL-17 (i), TNF (j), and IL-12p40 (k) by ELISA in the homogenate supernatants. The data are expressed as the mean ± SEM, N = 5-6. *P < 0.05 compared with the WT group.
3.4. DCs Activation May Be Related to 5-LO Activity during Parasite Infection
Because dendritic cells (DCs) are the main cells involved in orchestrating immune responses during Leishmania sp. infection through the release of cytokines which might be involved in the differentiation of Th17 cells [33], we first evaluated, using flow cytometry analyses, the maturation profile of dendritic splenic cells of the WT and 5-LO−/− mice infected at 6th wpi by evaluating the costimulatory molecules in the CD11chigh cells. In terms of percentage, the DC expressing CD86 (Figure 5(b)) or MHC-II (Figure 5(c)) is slightly reduced in the absence of 5-LO that was ~20% less compared with WT. However, in terms of total cells, we observed a markedly reduction of DCs expressing CD86 or MHC-II that was ~50% into spleens of 5-LO−/− mice (Figures 5(b)-5(c), resp.). No difference was observed in the CD40 expression (Figure 5(a)). Consistent with the in vivo data, the bone marrow-derived DC (BMDC) from the WT infected in vitro with parasites enhanced the expression levels of surface markers such as MHC-II, CD40, and CD86 (Figures 6(a)–6(c)), when compared to those of the medium. In contrast, infection of BMDC from 5-LO−/− with L. infantum inhibits their activation, presenting reduced expression of CD86 surface markers (Figure 6(c)). The absence of 5-LO did not alter the LPS-induced dendritic cell maturation (Figures 6(a)–6(c)).
Figure 5.
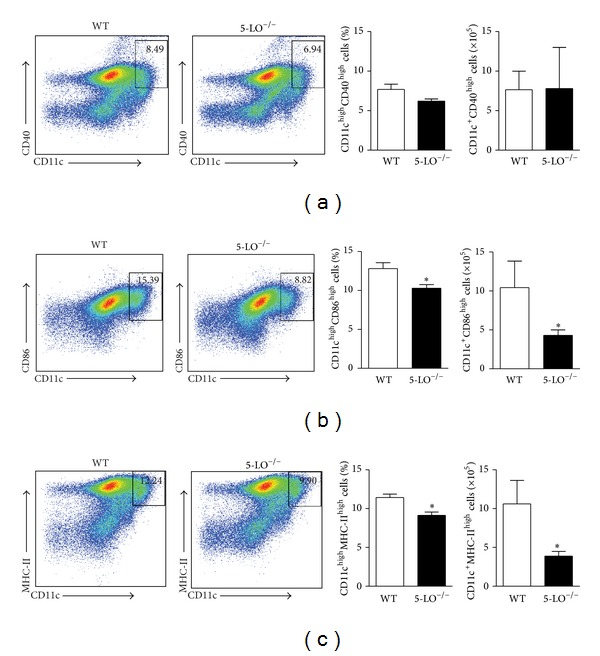
5-LO activity is required for dendritic cell activation into the inflammation site. The in vivo surface markers of DCs from the WT (white bars) or 5-LO−/− (black bars) infected mice at the 6th wpi were determined by flow cytometry. The dot plots represent the frequency of CD40 (a), CD86 (b), and MHC-II (c) in the CD11chigh population. All analyses were performed on CD11b+CD11chigh gated cells. The data are expressed as the mean ± SEM, N = 5-6. *P < 0.05 compared to the WT group.
Figure 6.

The absence of 5-LO interferes with BMDC maturation and the release of innate cytokines induced by L. infantum through BLT1 receptor. The WT or 5-LO−/− BMDC was stimulated with L. infantum (5 : 1) (black bars), LPS (200 ng/mL) (hatched bars), or medium (white bars) for 24 h. The BMDCs were harvested and the costimulatory molecules expression such CD86 (a), MHC-II (b), and CD40 (c) was evaluated by flow cytometry. All analyses were performed for the CD11chigh population. The TNF (d), IL-23 (e), IL-1β (f), and IL-6 (g) levels were measured in the supernatant of the BMDC culture by ELISA assay. The data are expressed as the mean ± SEM and are representative of three independent experiments. *P < 0.05 compared with the medium; # P < 0.05 compared with the infected WT. & P < 0.05 compared with the infected WT. In some experiment, selective BLT1 leukotriene B4 receptor antagonist (10 μM) was or not added 12 h before L. infantum infection or LPS stimuli. The levels of TNF (h), IL-23 (i), and IL-6 (j) were determinate into supernatant 24 thereafter. *P < 0.05 compared with the medium; # P < 0.05 compared with L. infantum infection. & P < 0.05 compared with LPS stimuli.
Next, we evaluated the release of innate cytokines involved in Th17 axis differentiation by DCs. Thus, we determined the levels of TNF, IL-23, IL-1β, and IL-6 in the supernatants from the WT or 5-LO−/− BMDCs cultured with L. infantum parasites or medium. As the positive control, the cells were activated with LPS. The parasites induced significant production of TNF (Figure 6(d)), IL-23 (Figure 6(e)), IL-1β (Figure 6(f)), and IL-6 (Figure 6(g)) by the DC from WT when compared with that of the respective control group. Additionally, the parasites promoted significant amounts of cytokines in the DC from 5-LO−/−, compared to the 5-LO−/− DC stimulated with the medium; however TNF (Figure 6(d)), IL-23 (Figure 6(e)), and IL-6 (Figure 6(g)) levels were significantly decreased comparing those released by infected WT DC. The levels of IL-1β (Figure 6(f)) were unaltered in the absence of 5-LO. These data suggest that 5-LO participates in DC activation, interfering with the cytokine release involved in the Th17 subset polarization during an experimental L. infantum infection.
The ablation of 5-LO lacks not only LTB4, but also cysteinyl leukotrienes including LTC4, LTD4, and LTE4 activity [34]. In other to clarify, in part, the effect of LTB4 during L. infantum infection, we use in vitro a selective BLT1 leukotriene B4 receptor antagonist (U-75302). The BLT1 antagonist was added to BMDC culture 12 h before L. infantum infection and the release of cytokines related to Th17 pattern was measured into culture supernatant by ELISA assay. As expected, TNF (Figure 6(h)), IL-23 (Figure 6(i)), and IL-6 (Figure 6(j)) were produced during infection. The ability of BMDCs infected with parasites to produce cytokines such as TNF (Figure 6(h)) and IL-23 (Figure 6(i)), but not IL-6 (Figure 6(j)), was inhibited by BLT1 blockage, confirming that LTB4 is associated with the release of cytokines involved in the Th17 axis. We do not rule out the possibility of others leukotrienes that may contribute to cytokine release, herein, that is, IL-6 release, but we undoubtedly evidenced LTB4 participation in the control of VL.
4. Discussion
In this study, we report 5-LO activity, and presumably LTB4, as an important mediator in controlling infection induced by Leishmania infantum. This eicosanoid that is released during infection may promote the activation of dendritic cells, which influence the release of mediators involved in the drive of naive CD4+ T lymphocytes to the Th17 profile. In the last instance, the Th17 subtype recruits neutrophils to the infection foci that might retrain the parasite restriction. Understanding the role of LTB4 in the inflammatory process mediated by L. infantum might elucidate some of the effector mechanisms that control the replication of the parasites.
We demonstrated that infection with L. infantum results in the production of LTB4 by dendritic cells during in vitro infection. The absence of endogenous LTB4 promoted higher susceptibility to infection. The genetic ablation of 5-LO harbored more parasites in target organs such as the spleen and liver, demonstrating its role in the control of infection. These results are consistent with those of previous studies that demonstrate the role of LTB4 in the control of infectious processes [13, 17, 21], increasing the leishmanicidal activity of macrophages [24] and of neutrophils [25] by a nitric oxide (NO)-dependent mechanism and release of reactive oxygen species (ROS), respectively.
Several studies have demonstrated that LTB4 is a potent inducer of neutrophils. During leishmaniasis, neutrophils are rapidly mobilized to the inflammatory site [1, 35], where they eliminate the pathogen by the production of reactive oxygen species (ROS) and the release of peptides and antimicrobial proteases [36–38]. In our results, the high susceptibility observed in animal 5-LO−/− was accompanied by the failure of neutrophil migration. LTB4 has a central role in controlling the migration of neutrophils to sites of inflammation through BLT1 and BLT2 (leukotriene receptors) [39], directly by inducing the expression of the CD11b and CD18 integrins [40] or indirectly by amplifying the production of inflammatory mediators such as cytokines and chemokines by others cells [41, 42]. In fact, we observed a significant reduction of activated neutrophils expressing CD11b into target organs that were infected by parasites in the absence of 5-LO. Furthermore, LTB4 enhances effectors mechanisms of neutrophils such as phagocytic capacity [43] and granules releasing and stimulates the enzymatic generation of ROS [14, 44, 45], including in vitro infection by L. amazonensis [25]. Thus, it seems that the protector role of LTB4 during LV may be played by the recruitment and activation of neutrophils to the site of infection.
The recruitment of neutrophils might be induced by cytokines such as IL-17 because they are potent granulopoietic factors [46] that induce the release of CXC chemokines [47]. We found that the absence of LTB4 synthesis impaired the Th17 response, whereas the Th1 response was unchanged in the target organs. Consistently, the production of IL-17 by spleen cells in response to the specific stimulus (i.e., Leishmania antigen) and its detection in the liver of 5-LO−/− infected mice was inhibited, confirming the interference of LTB4 in the release of IL-17. We have not evaluated whether LTB4 participates in the control of Leishmania infantum through Th17-dependent manner; however, we believe that the administration of recombinant IL-17 may rescue the protective effect of leucotrienes in susceptible 5-LO deficient mice. In fact, administration of recombinant IL-17 or IL-23 in susceptible BALB/c mice infected with L. donovani controlled parasite replication, which was associated with increased iNOS activity [3]. Furthermore, exogenous LTB4 is able to positively modulate the differentiation of Th17 cells from naive CD4+ T cells [48]. The induction of experimental autoimmune encephalomyelitis (EAE) in animals genetically deficient in BLT1 presented clinical score signs attenuated because of impairment of the Th17 generated response. Infiltration of T cells, macrophages, and granulocytes into the spinal cord was reduced in the BLT1 −/− mice [49], demonstrating the involvement of LTB4 in the development of the Th17 response.
LTB4 is produced during inflammatory and infectious processes by several leucocytes [50], including activated neutrophils, macrophages, and T cells [51–54]. Among the cells able to synthesize LTB4, DCs play an important role in the initiation of immune responses because they are the main cells involved in pathogen recognition, triggering several proinflammatory mechanisms that bridge to adaptative immune responses [55–57]. According to our results, DCs are potential sources of LTB4 during L. infantum infection. Given the importance of the role lipid mediators play in leucocyte activation, LTB4 production by DCs is a major mechanism for the modulation of the effector function of other cell subsets during LV, for example, mediating the recruitment of neutrophils to inflammation sites. We do not rule out the possibility of others leukotrienes that may contribute to cytokine release, since that the ablation of 5-LO lacks not only LTB4 but also cysteinyl leukotrienes including LTC4, LTD4, and LTE4 activity [34]. However, the pharmacological blockage of BLT1 prevented, at least in part, the release of cytokines by DC, evidencing LTB4 association with Th17 axis, and in last instance, controlling parasite replication.
Apart from sources of LTB4, DCs are the target of the action of lipid mediators as an important mechanism for modulating the immune response [58, 59]. An impaired Th17 response might result from failed DC activation in the absence of 5-LO. This hypothesis might be supported by the following explanations. First, exploring the role of LTB4 in DC activation, our data demonstrated that the maturation phenotype of DCs from animal 5-LO−/− was reduced during in vivo and in vitro infection. Consistently, the addition of LTB4 in cultured BMDCs induces maturation of these cells to increase MHC-II expression. Blockage of 5-LO with NDGA protects cells from the effects of LTB4 on DC maturation [60]. BMDCs migrate and are activated in response to LTB4, and its effect is lost in cells that lack BLT1 [61]. LTB4 upregulates the expression of CCR7 and its ligand CCL19/ELC, which mediate the migration to lymphoid organs. Second, the impaired ability of DCs from animal 5-LO−/− to secrete cytokines is involved in the polarization of naïve CD4 T cells to the Th17 profile. Naïve CD4+ T lymphocytes are polarized to the Th17 subset through the combined pattern of the action of cytokines such as IL-1β, TGF-β and IL-6 [62], whereas activation requires sustained stimulation with IL-23, which is predominantly produced by dendritic cells and TNF release [63]. Our data demonstrated that the production of TNF, IL-23, and IL-6 in vivo, at least, was compromised in the absence of 5-LO. Supporting our hypothesis, a significant reduction of IL-23, TNF, and IL-6 by BMDC was observed in the dendritic cells derived from animal 5-LO−/−. Consistently, Lefèvre and colleagues demonstrated that cytokines such as IL-1β, TGF-β, and IL-6 are highly produced by macrophages infected in vitro with L. infantum [29]. The role of LTB4 in the induction of innate cytokines related to the Th17 profile differentiation released by DCs is unprecedented. It is known that IL-1R signaling is dependent on the BLT1 downstream pathway. The requirement for the BLT1 signaling pathway is overcome by exogenous administration of IL-1β in LTB4 −/− mice [64]. Moreover, BLT1 expression is upregulated in Th17-differentiated T cells [49] and ex vivo studies have demonstrated that the production of TNF and IL-6 was impaired in the absence of BLT1 −/− cells [65, 66], confirming the role of LTB4 in driving the Th17 response.
We do not investigate the molecular mechanisms by which 5-LO activity interferes with maturation process and subsequent activation of dendritic cells, but we believe that the initial response is dependent on TLR4 signaling. During parasite recognition through TLR4 pathway, the adapter molecule MyD88 is recruited and activates factors such as NF-κB [67, 68], leading to transcription of proinflammatory cytokines such as TNF, IL-6, and IL-23. MYD88 recruitment also activated 5-LO enzyme, promoting the synthesis of leucotriens, especially LTB4 that, through BLT1 pathway, amplifies the activation of NF-κB which may induce cellular activation [66]. Interestingly, genetic deletion of 5-LO or pharmacological blockade of BLT1 receptor interferes with the secretion of proinflammatory cytokines by DCs and their maturation phenotype. The 5-LO pathway may act in autocrine manner, increasing the activation and function of DCs, and greatly influence the magnitude response of Th17 cells as well. Thus, the amplification of the inflammatory response mediated by 5-LO activation during parasite recognition by DCs appears to play an important role in controlling parasite replication.
5. Conclusion
Our data demonstrated that 5-LO activity, and perhaps LTB4, plays a prominent role in controlling L. infantum-induced visceral leishmaniasis, which may be associated with the development of the Th17 response and the subsequent recruitment of neutrophils to the inflammatory site that is dependent on dendritic cell activation. Future studies might characterize which innate receptors on DCs are involved in the recognition of the parasite, leading to a subsequent synthesis of LTB4. The results show, for the first time, the role of LTB4 in the development of the Th17 response in the context of an infectious disease.
Acknowledgments
The authors are grateful to Giuliana Bertozi for helping with the LTB4 measurement. The research leading to these results received funding from the CAPES-PRODOC, São Paulo Research Foundation (FAPESP) under Grant Agreements nos. 2012/14524-9 (Thematic Project) and 2013/08216-2 (Center for Research in Inflammatory Disease) and from the University of São Paulo NAP-DIN under Grant Agreement no. 11.1.21625.01.0.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Thalhofer CJ, Chen Y, Sudan B, Love-Homan L, Wilson ME. Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan leishmania infantum chagasi. Infection and Immunity. 2011;79(1):108–117. doi: 10.1128/IAI.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engwerda CR, Kaye PM. Organ-specific immune responses associated with infectious disease. Immunology Today. 2000;21(2):73–78. doi: 10.1016/s0167-5699(99)01549-2. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh K, Sharma G, Saha A, Kar S, Das PK, Ukil A. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. Journal of Infectious Diseases. 2013;207(6):1016–1025. doi: 10.1093/infdis/jis771. [DOI] [PubMed] [Google Scholar]
- 4.Antoniazi S, Price HP, Kropf P, et al. Chemokine gene expression in Toll-like receptor-competent and -deficient mice infected with Leishmania major . Infection and Immunity. 2004;72(9):5168–5174. doi: 10.1128/IAI.72.9.5168-5174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter U, Körner H. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunology. 2002;24(6):295–301. doi: 10.1046/j.1365-3024.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 6.Haeggström JZ, Wetterholm A. Transduction Mechanisms in Cellular Signaling: Cell Signaling Collection. 2011. Leukotriene Mediators; p. p. 349. [Google Scholar]
- 7.Rinaldo-Matthis A, Haeggström JZ. Structures and mechanisms of enzymes in the leukotriene cascade. Biochimie. 2010;92(6):676–681. doi: 10.1016/j.biochi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. Journal of Allergy and Clinical Immunology. 2006;117(3):577–582. doi: 10.1016/j.jaci.2005.12.1340. [DOI] [PubMed] [Google Scholar]
- 9.Kumar NS, Schaefer PA, Lark G, Frieri M. Late phase response during nasal challenge: effect of astemizole on leukotriene B4 levels. Allergy & Asthma Proceedings. 1996;17(2):93–99. doi: 10.2500/108854196778645047. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto A, Endo H, Hayashi I, et al. Differential expression of leukotriene B4 receptor subtypes (BLT1 and BLT2) in human synovial tissues and synovial fluid leukocytes of patients with rheumatoid arthritis. Journal of Rheumatology. 2003;30(8):1712–1718. [PubMed] [Google Scholar]
- 11.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJH. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 12.Smith MJH, Ford-Hutchinson AW, Bray MA. Leukotriene B: a potential mediator of inflammation. Journal of Pharmacy and Pharmacology. 1980;32(7):517–518. doi: 10.1111/j.2042-7158.1980.tb12985.x. [DOI] [PubMed] [Google Scholar]
- 13.Flamand L, Borgeat P, Lalonde R, Gosselin J. Release of anti-HIV mediators after administration of leukotriene B4 to humans. Journal of Infectious Diseases. 2004;189(11):2001–2009. doi: 10.1086/386374. [DOI] [PubMed] [Google Scholar]
- 14.Gaudreault E, Gosselin J. Leukotriene B4-mediated release of antimicrobial peptides against cytomegalovirus is BLT1 dependent. Viral Immunology. 2007;20(3):407–420. doi: 10.1089/vim.2006.0099. [DOI] [PubMed] [Google Scholar]
- 15.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infection and Immunity. 2011;79(7):2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infection and Immunity. 2010;78(5):2264–2271. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peres CM, de Paula L, Medeiros AI, et al. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis . Microbes and Infection. 2007;9(4):483–489. doi: 10.1016/j.micinf.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Secatto A, Rodrigues LC, Serezani CH, et al. 5-lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0031701.e31701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tristão FSM, Rocha FA, Moreira AP, Cunha FQ, Rossi MA, Silvaa JS. 5-Lipoxygenase activity increases susceptibility to experimental Paracoccidioides brasiliensis infection. Infection and Immunity. 2013;81(4):1256–1266. doi: 10.1128/IAI.01209-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos PC, Santos DA, Ribeiro LS, et al. The pivotal role of 5-lipoxygenase-derived LTB4 in controlling pulmonary paracoccidioidomycosis. PLoS Neglected Tropical Diseases. 2013;7(8) doi: 10.1371/journal.pntd.0002390.e2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong EC, Chi EY, Henderson WR., Jr. Toxoplasma gondii alters eicosanoid release by human mononuclear phagocytes: role of leukotrienes in interferon γ-induced antitoxoplasma activity. The Journal of Experimental Medicine. 1994;180(5):1637–1648. doi: 10.1084/jem.180.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavanelli WR, Gutierrez FRS, Mariano FS, et al. 5-lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microbes and Infection. 2010;12(8-9):587–597. doi: 10.1016/j.micinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Patnode ML, Bando JK, Krummel MF, Locksley RM, Rosen SD. Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. The Journal of Experimental Medicine. 2014;211(7):1281–1288. doi: 10.1084/jem.20132336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaves MM, Marques-Da-Silva C, Monteiro APT, Canetti C, Coutinho-Silva R. Leukotriene B4 modulates P2X7 receptor-mediated Leishmania amazonensis elimination in murine macrophages. Journal of Immunology. 2014;192(10):4765–4773. doi: 10.4049/jimmunol.1301058. [DOI] [PubMed] [Google Scholar]
- 25.Tavares NM, Araújo-Santos T, Afonso L, et al. Understanding the mechanisms controlling Leishmania amazonensis infection in vitro: the role of LTB4 derived from human neutrophils. Journal of Infectious Diseases. 2014;210(4):656–666. doi: 10.1093/infdis/jiu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. Journal of Immunology. 2006;177(5):3201–3208. doi: 10.4049/jimmunol.177.5.3201. [DOI] [PubMed] [Google Scholar]
- 27.Reiner NE, Malemud CJ. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: in vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. Journal of Immunology. 1985;134(1):556–563. [PubMed] [Google Scholar]
- 28.Reiner NE, Malemud CJ. Arachidonic acid metabolism in murine leishmaniasis (Donovani): Ex-vivo evidence for increased cyclooxygenase and 5-lipoxygenase activity in spleen cells. Cellular Immunology. 1984;88(2):501–510. doi: 10.1016/0008-8749(84)90181-3. [DOI] [PubMed] [Google Scholar]
- 29.Lefèvre L, Lugo-Villarino G, Meunier E, et al. The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum . Immunity. 2013;38(5):1038–1049. doi: 10.1016/j.immuni.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Carregaro V, Valenzuela JG, Cunha TM, et al. Phlebotomine salivas inhibit immune inflammation-induced neutrophil migration via an autocrine DC-derived PGE2/IL-10 sequential pathway. Journal of Leukocyte Biology. 2008;84(1):104–114. doi: 10.1189/jlb.1107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nature Medicine. 2003;9(3):315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 32.Sabroe I, Read RC, Whyte MKB, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. Journal of Immunology. 2003;171(4):1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- 33.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Current Opinion in Immunology. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Letters. 2001;487(3):323–326. doi: 10.1016/s0014-5793(00)02374-7. [DOI] [PubMed] [Google Scholar]
- 35.Beil WJ, Meinardus-Hager G, Neugebauer D-C, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. Journal of Leukocyte Biology. 1992;52(2):135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 36.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunological Reviews. 2007;219(1):88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 37.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes and Infection. 2003;5(14):1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Faurschou M, Sørensen OE, Johnsen AH, Askaa J, Borregaard N. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochimica et Biophysica Acta—Molecular Cell Research. 2002;1591(1–3):29–35. doi: 10.1016/s0167-4889(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 39.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 40.Van Pelt JPA, De Jong EMGJ, Van Erp PEJ, et al. The regulation of CD11b integrin levels on human blood leukocytes and leukotriene B4-stimulated skin by a specific leukotriene B4 receptor antagonist (LY293111) Biochemical Pharmacology. 1997;53(7):1005–1012. doi: 10.1016/s0006-2952(96)00884-2. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just TH2 cells. Nature Reviews Immunology. 2010;10(12):838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medoff BD, Seung E, Hong S, et al. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. The Journal of Immunology. 2009;182(1):623–635. doi: 10.4049/jimmunol.182.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancuso P, Nana-Sinkam P, Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae . Infection and Immunity. 2001;69(4):2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rae SA, Smith MJH. The stimulation of lysosomal enzyme secretion from human polymorphonuclear leucocytes by leukotriene B4. Journal of Pharmacy and Pharmacology. 1981;33(9):616–617. doi: 10.1111/j.2042-7158.1981.tb13884.x. [DOI] [PubMed] [Google Scholar]
- 45.Sumimoto H, Takeshige K, Minakami S. Superoxide production of human polymorphonuclear leukocytes stimulated by leukotriene B4 . Molecular Cell Research. 1984;803(4):271–277. doi: 10.1016/0167-4889(84)90117-4. [DOI] [PubMed] [Google Scholar]
- 46.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. The Journal of Experimental Medicine. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laan M, Cui Z-H, Hoshino H, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. The Journal of Immunology. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 48.Chen H, Qin J, Wei P, et al. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(4):195–200. doi: 10.1016/j.plefa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Kihara Y, Yokomizo T, Kunita A, et al. The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis. Biochemical and Biophysical Research Communications. 2010;394(3):673–678. doi: 10.1016/j.bbrc.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 50.Rouzer CA, Shimizu T, Samuelsson B. On the nature of the 5-lipoxygenase reaction in human leukocytes: characterization of a membrane-associated stimulatory factor. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(22):7505–7509. doi: 10.1073/pnas.82.22.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goetzl EJ. Selective feedback inhibition of the 5-lipoxygenation of arachidonic acid in human T-lymphocytes. Biochemical and Biophysical Research Communications. 1981;101(2):344–350. doi: 10.1016/0006-291x(81)91266-3. [DOI] [PubMed] [Google Scholar]
- 52.Goodwin JS, Atluru D, Sierakowski S, Lianos EA. Mechanism of action of glucocorticosteroids. Inhibition of T cell proliferation and interleukin 2 production by hydrocortisone is reversed by leukotriene B4. Journal of Clinical Investigation. 1986;77(4):1244–1250. doi: 10.1172/JCI112427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balderramas HA. Human neutrophils produce IL-12, IL-10, PGE2 and LTB4 in response to Paracoccidioides brasiliensis. Involvement of TLR2, mannose receptor and dectin-1. Cytokine. 2014;67(1):36–43. doi: 10.1016/j.cyto.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Conti P, Reale M, Barbacane RC, et al. Leukocyte inhibitory factor activates human neutrophils and macrophages to release leukotriene B4 and thromboxanes. Cytokine. 1990;2(2):142–148. doi: 10.1016/1043-4666(90)90008-h. [DOI] [PubMed] [Google Scholar]
- 55.Gorak P, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. European Journal of Immunology. 1998;28(2):687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 56.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. The Journal of Experimental Medicine. 1998;188(8):1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.León B, López-Bravo M, Ardavín C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania . Immunity. 2007;26(4):519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Spanbroek R, Stark H-J, Timmen UJ, et al. 5-Lipoxygenase expression in Langerhans cells of normal human epidermis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):663–668. doi: 10.1073/pnas.95.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spanbroek R, Hildner M, Steinhilber D, et al. 5-Lipoxygenase expression in dendritic cells generated from CD34+ hematopoietic progenitors and in lymphoid organs. Blood. 2000;96(12):3857–3865. [PubMed] [Google Scholar]
- 60.Harizi H, Gualde N. Dendritic cells produce eicosanoids, which modulate generation and functions of antigen-presenting cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2002;66(5-6):459–466. doi: 10.1054/plef.2002.0383. [DOI] [PubMed] [Google Scholar]
- 61.Del Prete A, Shao W-H, Mitola S, Santoro G, Sozzani S, Haribabu B. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109(2):626–631. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 63.Aggarwal S, Ghilardi N, Xie M-H, De Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. Journal of Biological Chemistry. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 64.Klickstein LB, Shapleigh C, Goetzl EJ. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. Journal of Clinical Investigation. 1980;66(5):1166–1170. doi: 10.1172/JCI109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito S, Ito Y, Katagiri H, et al. Leukotriene b4/leukotriene b4 receptor pathway is involved in hepatic microcirculatory dysfunction elicited by endotoxin. Shock. 2008;30(1):87–91. doi: 10.1097/shk.0b013e31815d06a1. [DOI] [PubMed] [Google Scholar]
- 66.Serezani CH, Lewis C, Jancar S, Peters-Golden M. Leukotriene B4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. The Journal of Clinical Investigation. 2011;121(2):671–682. doi: 10.1172/JCI43302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muraille E, de Trez C, Brait M, de Baetselier P, Leo O, Carlier Y. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. Journal of Immunology. 2003;170(8):4237–4241. doi: 10.4049/jimmunol.170.8.4237. [DOI] [PubMed] [Google Scholar]
- 68.de Trez C, Brait M, Leo O, et al. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infection and Immunity. 2004;72(2):824–832. doi: 10.1128/IAI.72.2.824-832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


