Abstract
To investigate the role of E23K polymorphism of the KCNJ11 gene on early onset of type 2 diabetes in school-aged children/adolescents in Taiwan, we recruited 38 subjects with type 2 diabetes (ages 18.6 ± 6.6 years; body mass index percentiles 83.3 ± 15.4) and 69 normal controls (ages 17.3 ± 3.8 years; body mass index percentiles 56.7 ± 29.0) from a national surveillance for childhood/adolescent diabetes in Taiwan. We searched for the E23K polymorphism of the KCNJ11 gene. We found that type 2 diabetic subjects had higher carrier rate of E23K polymorphism of KCNJ11 gene than control subjects (P = 0.044). After adjusting for age, gender, body mass index percentiles, and fasting plasma insulin, the E23K polymorphism contributed to an increased risk for type 2 diabetes (P = 0.047). K23-allele-containing genotypes conferring increased plasma insulin level during OGTT in normal subjects. However, the diabetic subjects with the K23-allele-containing genotypes had lower fasting plasma insulin levels after adjustment of age and BMI percentiles. In conclusion, the E23K variant of the KCNJ11 gene conferred higher susceptibility to type 2 diabetes in children/adolescents. Furthermore, in normal glucose-tolerant children/adolescents, K23 allele carriers had a higher insulin response to oral glucose loading.
1. Introduction
Diabetes mellitus in children and adolescents has long been considered primarily type 1 diabetes. Although type 2 diabetes (T2D) is generally considered to be a disease of adults, the past 15–20 years have seen a dramatic increase in the prevalence of T2D in children and adolescents [1–9]. This increased prevalence of pediatric T2D suggests impending future morbidity from diabetic complications in a large number of relatively young adults.
In a nationwide surveillance program with mass urine screening in Taiwan [10, 11], the incidence of T2D is 6 times that of type 1 diabetes in recent years. The identified risk factors for T2D in youth are similar to those for adult type 2 diabetes with the most prominent risk of childhood obesity for T2D [11]. T2D is generally believed to be a polygenic disorder, with disease development being influenced by both hereditary and environmental factors [12]. Genetic factors are important in determining the children who become obese and also the obese children who develop T2D [13]. Support for the role of genetic factors comes from epidemiological evidence that T2D in youth is most common in individuals from racial groups with a high prevalence of diabetes and in individuals with a strong family history [14]. A search for the contribution of certain candidate genes in the early onset T2D is mandatory for further understanding of pathogenesis of T2D in childhood.
The pancreatic islet ATP-sensitive potassium channel complex (KATP) plays a major role in glucose-stimulated insulin secretion, thus serving as a strong candidate for T2D. This channel is a heterooctameric complex composed of four sulfonylurea receptor (SUR1) subunits and four Kir6.2 subunits [15, 16]. Mutations in the SUR1 (ABCC8) and the Kir6.2 (KCNJ11) cause familial hyperinsulinemia in infancy [17], while some polymorphisms in these genes (exon 16-3t/c and exon 18 C/T of ABCC8 and E23K of KCNJ11) have been reported to be associated with T2D in several populations at different degrees [18–23]. The K23 allele is associated with higher risk of T2D, providing an overall odds ratio (OR) of 1.23 [23] and 1.26 [21] in Caucasians and Asians, respectively. According to a recent systemic meta-analysis, the E23K polymorphism was significantly associated with increased T2D risk with per-allele odds ratio (OR) of 1.12. When stratified by ethnicity, significantly increased risks were found for the polymorphism in Caucasians and East Asians. However, no such associations were detected among Indian and other ethnic populations [24]. Normoglycemic lysine carriers are shown to consistently display a defect in insulin secretion [23, 25, 26]. Furthermore, the codon 23 KCNJ11 polymorphism is shown to be related to glucose intolerance in Caucasians and progression from glucose intolerance to T2D [27, 28].
Recent studies have provided evidence that the E23K variant alters channel function by inducing spontaneous overactivity of pancreatic β-cells, thus increasing the threshold of ATP concentration for insulin release [29, 30]. Therefore, in this study, we analyzed the E23K polymorphism of KCNJ11 gene in a group of subjects with T2D and a group of controls identified in a nationwide surveillance program for diabetes in schoolchildren aged 6~18 in Taiwan. We demonstrated that the E23K polymorphism of KCNJ11 gene increased susceptibility to T2D in childhood and adolescence.
2. Materials and Methods
2.1. Subjects
With a nationwide surveillance program for diabetes in Taiwanese school-aged children, 137 subjects were newly diagnosed with T2D [10, 11]. Only 38 newly diagnosed T2D subjects and 69 nondiabetic subjects from the northern part of Taiwan were recruited for genetic analysis. Body mass index (BMI) is a measure of body fat based on body height (BH) and body weight (BW) (BMI = BW (kg)/BH2 (m2)). After BMI is calculated for children and teens, the BMI number is plotted on the Centers for Disease Control and Prevention (CDC) BMI-for-age growth charts (for either girls or boys) to obtain a percentile ranking (http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html, searched on 8.14.2014). A standard oral glucose tolerance test with 1.75 g glucose/kg of body weight or maximally with 75 g glucose was performed to classify the state of glucose tolerance, except for subjects diagnosed with a fasting plasma glucose level equal to or over 126 mg/dL. Informed consent was obtained from each participant and their parents of those under 18. This study was approved by the Institutional Review Boards.
2.2. Measurements of Metabolic Parameters
The fasting plasma glucose, serum insulin, cholesterol, and TG were measured according to the previous reports [31]. Insulin resistance index was calculated with homeostasis model assessment (HOMA-IR) as described previously [32]. The estimated β-cell function based on the HOMA-B was calculated based on the following formula: %B = 20 × fasting plasma insulin (FPI, μU/mL)/(fasting plasma glucose (FPG, mM) − 3.5) [33]. Another set of estimates of β-cell function proposed by Stumvoll et al. was also calculated using the two formulae: 1st PHs = 1283 + (1.829 × plasma insulin concentration at 30 min) − (138.7 × plasma glucose concentration at 30 min) + (3.772 × FPI) and 2nd PHs = 287 + (0.4164 × plasma insulin concentration at 30 min) − (26.07 × plasma glucose concentration at 30 min) + (0.9226 × FPI). These estimations were based on plasma glucose concentrations in mmol/L and plasma insulin concentrations in pmol/L [34]. Insulinogenic index (30 minutes) was estimated as follows: (Ins 30 − Ins 0)/(Glu 30 − Glu 0) [35]. Area under curve (AUC) of glucose and insulin during OGTT was also calculated.
2.3. Genotyping for the Polymorphism of KCNJ11
The E23K polymorphism of KCNJ11 was genotyped by PCR-restriction fragment length polymorphism (PCR-RFLP). PCR was performed with forward primer 5′-GACTCTGCAGTGAGGCCCTA-3′ and reverse primer 5′-ACGTTGCAGTTG CCTTTCTT-3′ starting with a denaturing step at 95°C for 3 min followed by 35 cycles of 95°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s with a final elongation step at 72°C for 9 min. The PCR product was 209 bps, and it was digested with BanII (New England Biolabs, Beverly, MA) and separated on 3% agarose gels. The substitution of G with A eliminated the BanII site.
2.4. Statistical Analysis
Data were represented as mean ± SD. Due to relatively small sample size, EK/KK were grouped together for regression analyses. Fisher's exact test was used to detect the distribution difference between diabetic and nondiabetic groups. Logistic regression model was further performed to adjust demographic difference. Student's t-test was applied to compare the difference of various parameters between different genotypes or between normal control and diabetic subjects. MANOVA was applied to compare the difference of glucose and insulin levels during OGTT test between different genotypes. SAS program version 8.1 (SAS institute Inc., Cary, NC) was applied for statistical analyses. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Demographic and Metabolic Characteristic
The demographic and metabolic data of the study subjects are shown in Table 1. Obesity, dyslipidemia, higher fasting plasma insulin, higher insulin resistance, and worsened β-cell function were found in subjects with T2D in childhood and adolescence (Table 1).
Table 1.
Clinical and metabolic features between normal controls and type 2 diabetic subjects in the present study.
| Non-DM (n = 69) | DM (n = 38) | P value∗ | |
|---|---|---|---|
| Age (years) | 17.3 ± 3.8 | 18.6 ± 6.6 | 0.289 |
| BMI percentiles | 56.7 ± 29.0 | 83.8 ± 15.4 | <0.001 |
| Glucose (mmol/L) | 5.19 ± 0.40 | 10.65 ± 4.05 | <0.001 |
| TCH (mmol/L) | 4.05 ± 0.77 | 4.22 ± 1.02 | 0.4159 |
| TG (mmol/L) | 0.74 ± 0.26 | 1.17 ± 0.45 | 0.0005 |
| HDL (mmol/L) | 1.43 ± 0.39 | 1.20 ± 0.50 | 0.0320 |
| Fasting insulin (pmol/L) | 7.66 ± 4.47 | 17.99 ± 20.43 | 0.0043 |
| HOMA-IR | 1.78 ± 1.06 | 7.48 ± 8.09 | <0.001 |
| Log (HOMA-IR) | 0.402 ± 0.624 | 1.449 ± 1.143 | <0.001 |
| HOMA-B | 94.62 ± 58.17 | 94.13 ± 168.52 | 0.987 |
| Log (HOMA-B) | 4.372 ± 0.625 | 3.490 ± 1.458 | 0.001 |
| Sex (M : F) | 26 : 43 | 20 : 18 | 0.157$ |
*P value with Student's t-test.
$By chi-squared test.
BMI percentiles: body mass index age- and sex-specific percentiles; TCH: total cholesterol; TG: triglyceride; HDL: high density lipoprotein-cholesterol; HOMA-IR: homeostasis model assessment-insulin resistance; HOMA-B: homeostasis model assessment-β cell.
3.2. Genotypes of KCNJ11 Genes
As shown in Table 2, K-allele-containing genotypes were significantly higher in subjects with T2D as compared to those of control (84.2% versus 65.2%, P = 0.044) (Table 2). To further adjust for potential confounding variables, logistic regression analysis was performed (Table 3). After adjustment of age, sex, and BMI age- and sex-specific percentiles (model 1), we found that higher BMI percentiles is an independent risk factor of type 2 diabetes (odds ratio = 1.060, 95% CI: 1.027–1.094, and P < 0.001). If we adjust for age, sex, BMI age- and sex-specific percentiles, and fasting plasma insulin levels (model 2), the K-allele-containing genotype is an independent risk factor of type 2 diabetes (odds ratio = 4.105, 95% CI: 1.0008–16.831, and P = 0.047). The fasting plasma insulin levels and BMI age- and sex-specific percentiles are also independent risk factors for T2D (odds ratio = 1.066, 95% CI: 1.001–1.135, and P = 0.045 for fasting insulin; odds ratio = 1.047, 95% CI: 1.014–1.080 for BMI age- and sex-specific percentiles, and P = 0.004, resp.).
Table 2.
Genotypic distribution of E23K polymorphism of the Kir6.2 between normal control and type 2 diabetes subjects.
| Non-DM (n = 69) | DM (n = 38) | P value∗ | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|---|
| Genotype∗ | |||||
| EE, n (%) | 24 (34.8%) | 6 (15.8%) | |||
| EK/KK, n (%) | 45 (65.2%) | 32 (84.2%) | 0.044 | 2.84 | 1.04–7.75 |
| Allele | |||||
| E-allele, n (%) | 81 (58.7%) | 38 (50.0%) | |||
| K-allele, n (%) | 57 (41.3%) | 38 (50.0%) | 0.251 | 1.42 | 0.81–2.50 |
*Chi-squared test.
Table 3.
Logistic regression analysis with type 2 diabetic status as dependent variables, age, sex, BMI age- and sex-specific percentiles, and genotype of E23K polymorphism in Kir6.2 as independent variables.
| Independent variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Model 1 | |||
| EK/KK versus EE | 2.941 | 0.764–11.323 | 0.117 |
| Age (every 1 year increment) | 1.156 | 0.920–1.454 | 0.214 |
| Sex (male = 1, female = 2) | 0.672 | 0.224–2.019 | 0.479 |
| BMI age- and sex-specific percentiles (every 1 percentile increment) | 1.060 | 1.027–1.094 | <0.001 |
| Model 2 | |||
| EK/KK versus EE | 4.105 | 1.0008–16.831 | 0.047 |
| Age (every 1 year increment) | 1.240 | 0.960–1.601 | 0.099 |
| Sex (male = 1, female = 2) | 0.826 | 0.252–2.711 | 0.752 |
| BMI age- and sex-specific percentiles (every 1 percentile increment) | 1.047 | 1.014–1.080 | 0.004 |
| Fasting plasma insulin levels (every 1 pmol/L increment) | 1.066 | 1.001–1.135 | 0.045 |
3.3. Effect of E23K Polymorphism of the KCNJ11 in Normal Glucose-Tolerant Subjects
To study the effect of genetic polymorphism of the E23K on insulin and glucose homeostasis during oral glucose tolerance test, we firstly compared those with EE genotype and the K23-allele-containing genotypes (EK or KK) in the normal glucose-tolerant subjects. There was no difference in the glucose levels during OGTT between subjects with different genotypes (Figure 1(a)). In contrast, subjects with EK/KK genotypes did have a significantly higher level of plasma insulin level at 60 min after glucose loading and higher AUC of insulin during OGTT (Figure 1(b)). However, this association of genotype of KCNJ11 with plasma insulin levels at 60 min after glucose loading became insignificant after adjustment of age, sex, and BMI percentiles.
Figure 1.
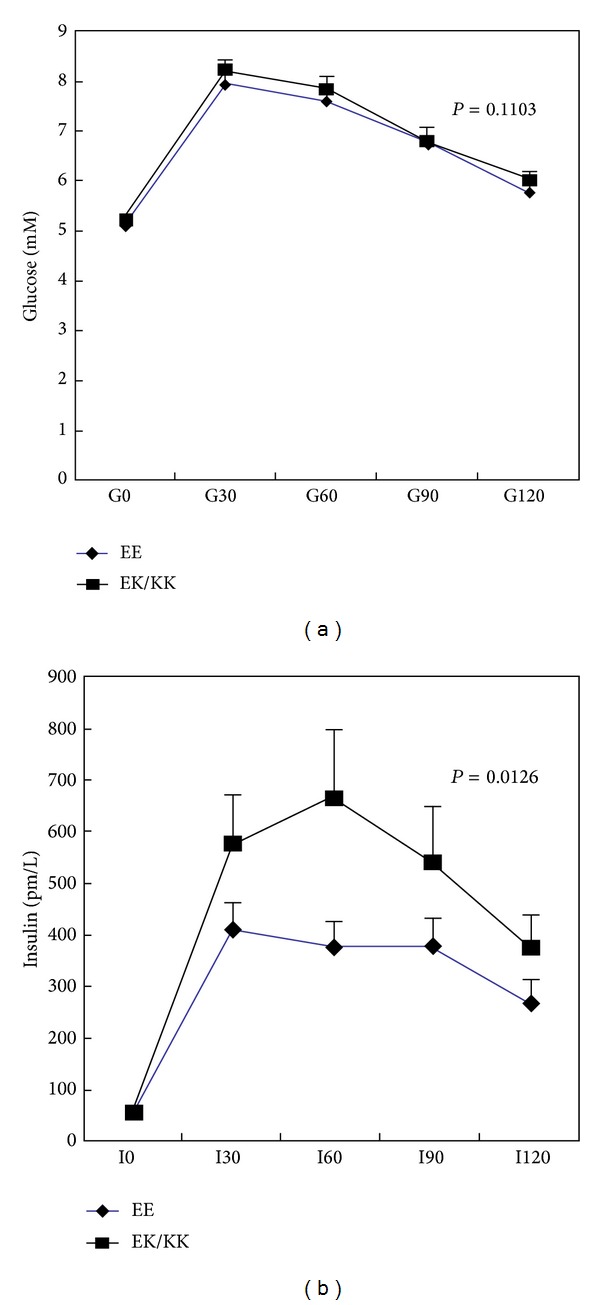
Glucose and insulin homeostasis during OGTT in the normal glucose-tolerant subjects according to genotypes of the KCNJ11 gene. There was no significant difference in plasma glucose levels in subjects with EE genotype compared with those with K23-allele-containing genotypes (EK or KK) (a). Subjects with EK/KK genotypes tended to have a higher level of plasma insulin level during OGTT (b). ∗ indicates significant difference between the two groups.
3.4. Effect of E23K Polymorphism of the KCNJ11 on Clinical Phenotypes in Diabetic and Nondiabetic Subjects
To further search for the features of E23K variants on development of type 2 diabetes in children and adolescents, we then compared various metabolic parameters between those with EE genotype and the K23-allele-containing genotypes (EK or KK) in both diabetic and nondiabetic subjects (Table 4). In general, the fasting insulin levels were higher in diabetes group. However, the diabetic subjects with the K23-allele-containing genotypes had a borderline significantly lower level of fasting plasma insulin than the diabetic subjects without K23 allele. With adjustment for age and BMI age- and sex-specific percentiles, the diabetic subjects with K-allele-containing genotypes had significantly lower fasting plasma insulin levels (fasting plasma insulin as dependent variable: β for age: −2.958 ± 1.385, P = 0.043; β for BMI age- and sex-specific percentiles: 0.548 ± 0.238, P = 0.030; β for E/E or E/K + K/K genotype: −21.451 ± 10.312, P = 0.046). In contrast, there were no significant differences of these metabolic parameters between the K23-allele-containing and the EE genotype subjects in the nondiabetic group.
Table 4.
Clinical and metabolic features between those with E/E genotype and K-containing allele among type 2 diabetic and nondiabetic subjects, respectively.
| Type 2 diabetic subjects | Nondiabetic subjects | |||||
|---|---|---|---|---|---|---|
| Genotypes | E/E (n = 6) | E/K + K/K (n = 32) | P value∗ | E/E (n = 24) | E/K + K/K (n = 45) | P value∗ |
| Sex (M : F) | 4 : 2 | 16 : 16 | 0.663$ | 11 : 13 | 15 : 30 | 0.434$ |
| Age (years) | 21.5 ± 9.7 | 18.0 ± 5.8 | 0.242 | 17.2 ± 3.3 | 17.4 ± 4.1 | 0.861 |
| BMI age- sex-specific percentiles | 84.02 ± 22.07 | 83.73 ± 14.31 | 0.970 | 56.65 ± 25.63 | 56.79 ± 30.87 | 0.987 |
| Glucose (mmol/L) | 10.0 ± 3.8 | 10.8 ± 4.1 | 0.715 | 5.1 ± 0.4 | 5.2 ± 0.4 | 0.133 |
| Fasting plasma insulin levels (pmol/L) | 237.21 ± 230.48 | 112.18 ± 126.24 | 0.076 | 52.2 ± 31.1 | 56.5 ± 32.8 | 0.602 |
| Ins-30′ | — | — | 414.67 ± 53.23 | 582.09 ± 94.71 | 0.222 | |
| Ins-60′ | — | — | 381.81 ± 51.51 | 671.57 ± 132.39 | 0.046 | |
| Ins-90′ | — | — | 379.09 ± 55.41 | 545.79 ± 107.51 | 0.280 | |
| Ins-120′ | — | — | 268.32 ± 48.95 | 375.18 ± 67.10 | 0.283 | |
| AUC-glucose | — | — | 832.92 ± 21.83 | 855.72 ± 17.02 | 0.422 | |
| AUC-insulin | 40074.46 ± 4153.74 | 60457.87 ± 10721.20 | 0.180 | |||
| HOMA-B | 127.0 ± 194.8 | 89.89 ± 168 | 0.685 | 97.69 ± 70.03 | 92.98 ± 51.55 | 0.751 |
*P value with Student's t-test.
$ P value with Fisher's exact test.
4. Discussion
In our present study, we found that a common polymorphism of E23K of the KCNJ11 confers higher susceptibility to T2D in childhood and adolescence of the Han-Chinese in Taiwan.
Childhood obesity is the single most important risk factor for type 2 diabetes in our schoolchildren [10, 11] and the present study. In the present study, we found that even with adjustment for age, sex, BMI age- and sex-specific percentiles, and fasting plasma insulin level, the K-allele-containing genotypes (EK and KK) confer an independent risk, with a relative high odds ratio of 4.105, for T2D in youth. Until recently, the Pro12Ala polymorphism in the peroxisome proliferator-activated receptor gamma (PPARG) was one of the other few polymorphisms that demonstrated an alteration in type 2 diabetes susceptibility across different populations [36]. More recently, several genome-wide association studies (GWAS) independently confirmed the strong associations of SNP rs7903146 in the TCF7L2 locus with type 2 diabetes [37–40]. Evidence accumulated so far suggests that the E23K polymorphism of the KCNJ11 gene, which encodes the Kir6.2 subunit of the KATP channel, is a candidate gene for type 2 diabetes reported mostly from adults [24, 41]. In children, one recent study indicated that six single nucleotide polymorphisms, including an activating R201H mutation on KCNJ11 gene, contribute to permanent neonatal diabetes [42]. Besides, several mutations on KCNJ11 gene have been reported to cause permanent hyperinsulinemic hypoglycemia of infancy [43–45]. A recent study reported that E23K variant did not affect metabolic disorders in prepubertal children who is small for gestational age at birth [46]. On the other hand, the association of the E23K polymorphism with type 1 diabetes was not statistically significant in the evaluated Korean population [47]. To our knowledge, no study has been reported for the impact of E23K polymorphism of the KCNJ11 gene on the early onset type 2 diabetes in children/adolescents. In consistence with previous studies in adult populations, we showed that school-aged children/adolescents with T2D in this study had higher E23K carrier rate of KCNJ11 gene than normal subjects. According to Genetic Power Calculator (S. Purcell et al., 2003; http://pngu.mgh.harvard.edu/~purcell/gpc/), the estimated number of diabetic cases for 80% power will be 181 with P value less than 0.05. Though only 38 diabetic subjects were recruited in our study group, the E23K polymorphism still contributed to a significantly increased risk for type 2 diabetes independent of age, gender, BMI age- and sex-specific percentiles, and fasting plasma insulin level. Furthermore, from the meta-analysis of candidate-gene studies and GWAS for T2D in adults, the average odds ratio of each genetic variant to increased T2D risk is in the range from 1.10 to 1.37 [48]. According to a recent systemic meta-analysis, the E23K polymorphism was significantly associated with increased T2D risk with per-allele odds ratio (OR) of 1.12. However, in this study, the odds ratio of K-allele of KCNJ11 to increased T2D risk in childhood and adolescence reached 4.105 after adjusting age, gender, BMI age- and sex-specific percentiles, and fasting plasma insulin levels. It inferred that the E23K polymorphism of KCNJ11 contributed a much higher risk to T2D in children and adolescence than in adults.
How E23K variation leads to diabetes is not completely understood. In previous studies, it has been shown that insulin secretion is significantly reduced in both heterozygous (E/K) and homozygous (K/K) variants among the normal glucose-tolerant adults [49, 50]. In contrast, we found that those carrying K-allele exhibited a higher insulin response after oral glucose loading in the normal glucose-tolerant children (Figure 1(b)). In support of our findings, studies in the glucose-tolerant offspring of T2D patients carrying the E23K variants demonstrated significantly higher 2-hour insulin concentrations compared with those with control subjects [51]. Moreover, the E23K variant has been linked to an increase in BMI in the Danish population [50]. Taken together, these data including ours suggest that the higher response in insulin secretion to oral glucose loading might be due to the compensatory hypersecretion of insulin to maintain normal glucose homeostasis in the presence of insulin resistance. In spite of the small case number in this study, we found that there is a decline in fasting plasma insulin levels in diabetes subjects carrying K-alleles compared to those with homozygous EE genotype when adjusted for age, sex, and BMI age- and sex-specific percentiles. Whether the reduced fasting insulin levels observed in the diabetic children/adolescents with K23-allele-containing genotypes are due to inadequate compensation of β-cell failure is not known. Future longitudinal study will be required to establish the effect of E23K polymorphism in the KCNJ11 gene on changes of body build, insulin resistance, and β-cell dysfunction during disease progression.
5. Conclusions
In conclusion, a common E23K variant of the KCNJ11 gene conferred higher susceptibility to T2D in children/adolescents in Taiwan. Furthermore, in the normal glucose-tolerant children and adolescents, K23 allele carriers had a significantly higher insulin response to oral glucose loading, suggesting a compensatory insulin secretion in the presence of insulin resistance. However, the functional impact of the E23K polymorphism on progression of glucose intolerance and diabetes needs further investigation.
Acknowledgments
The authors thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital, for technical support during the study. This work was supported by the Grant NSC 91-3112-B-002-019 from the National Science Council of Taiwan.
Conflict of Interests
The authors report no conflict of interests.
Authors' Contribution
Yi-Der Jiang, Lee-Ming Chuang, Tien-Jyun Chang, Jun-Nan Wei, and Fung-Chang Sung participated in concept/design. Lee-Ming Chuang, Dee Pei, and Yann-Jinn Lee participated in the collection of clinical and laboratory data. Yi-Der Jiang, Lee-Ming Chuang, and Tien-Jyun Chang participated in data analysis/interpretation and drafting of the paper. Tien-Jyun Chang participated in critical revision of the paper and approval of the paper. Yi-Der Jiang and Lee-Ming Chuang contributed equally to this work.
References
- 1.Kahn R. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23(3):381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D'Agostino RB, Jr., et al. Incidence of diabetes in youth in the United States. Journal of the American Medical Association. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.Mayer-Davis EJ, Bell RA, Dabelea D, et al. The many faces of diabetes in American youth: Type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for diabetes in youth study. Diabetes Care. 2009;32(supplement 2):S99–S101. doi: 10.2337/dc09-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu LL, Yi JP, Beyer J, et al. Type 1 and type 2 diabetes in Asian and Pacific Islander U.S. youth. The SEARCH for diabetes in youth study. Diabetes Care. 2009;32(supplement 2):S133–S140. doi: 10.2337/dc09-S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabelea D, DeGroat J, Sorrelman C, et al. Diabetes in Navajo youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(supplement 2):S141–S147. doi: 10.2337/dc09-S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(supplement 2):S123–S132. doi: 10.2337/dc09-S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell RA, Mayer-Davis EJ, Beyer JW, et al. Diabetes in non-hispanic white youth. Diabetes Care. 2009;32, supplement 2:S102–S111. doi: 10.2337/dc09-S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean H. NIDDM-Y in first nation children in Canada. Clinical Pediatrics. 1998;37(2):89–96. doi: 10.1177/000992289803700205. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa T, Owada M, Urakami T, Tajima N. Epidemiology of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Research and Clinical Practice. 1994;24:S7–S13. doi: 10.1016/0168-8227(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 10.Wei J-N, Chuang L-M, Lin C-C, Chiang C-C, Lin R-S, Sung F-C. Childhood diabetes identified in mass urine screening program in Taiwan, 1993–1999. Diabetes Research and Clinical Practice. 2003;59(3):201–206. doi: 10.1016/s0168-8227(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 11.Wei J-N, Sung F-C, Lin C-C, Lin R-S, Chiang C-C, Chuang L-M. National surveillance for type 2 diabetes mellitus in Taiwanese children. Journal of the American Medical Association. 2003;290(10):1345–1350. doi: 10.1001/jama.290.10.1345. [DOI] [PubMed] [Google Scholar]
- 12.Polonsky KS, Sturis J, Bell GI. Non-insulin-dependent diabetes mellitus—a genetically programmed failure of the beta cell to compensate for insulin resistance. The New England Journal of Medicine. 1996;334(12):777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 13.Gill-Carey O, Hattersley AT. Genetics and type 2 diabetes in youth. Pediatric Diabetes. 2007;8(9):42–47. doi: 10.1111/j.1399-5448.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 14.Rubio-Cabezas Ó, Argente J. Current insights into the genetic basis of diabetes mellitus in children and adolescents. Journal of Pediatric Endocrinology and Metabolism. 2008;21(10):917–940. doi: 10.1515/jpem.2008.21.10.917. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar-Bryan L, Clement JP, IV, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of K(ATP) channels. Physiological Reviews. 1998;78(1):227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 16.Miki T, Nagashima K, Seino S. The structure and function of the ATP-sensitive K+ channel in insulin-secreting pancreatic β-cells. Journal of Molecular Endocrinology. 1999;22(2):113–123. doi: 10.1677/jme.0.0220113. [DOI] [PubMed] [Google Scholar]
- 17.Sharima N, Crane A, Gonzalez G, Bryan J, Aguilar-Bryan L. Familial hyperinsulinism and pancreatic β-cell ATP-sensitive potassium channels. Kidney International. 2000;57(3):803–808. doi: 10.1046/j.1523-1755.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- 18.Gloyn AL, Hashim Y, Ashcroft SJ, et al. Association stud ies of variants in promoter and coding regions of beta-cell ATP -sensitive K-channel genes SUR1 and Kir6.2 with Type 2 diabetes mellitus (UKPDS 53) Diabetes Medicines. 2001;18(3):206–212. doi: 10.1046/j.1464-5491.2001.00449.x. [DOI] [PubMed] [Google Scholar]
- 19.Gloyn AL, Weedon MN, Owen KR, et al. Large-scale association studies of variants in genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52(2):568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 20.Florez JC, Jablonski KA, Kahn SE, et al. Type 2 diabetes-associated missense polymorphisms KCNJ11 E23K and ABCC8 A1369S influence progression to diabetes and response to interventions in the diabetes prevention program. Diabetes. 2007;56(2):531–536. doi: 10.2337/db06-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto Y, Inoue H, Keshavarz P, et al. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. Journal of Human Genetics. 2007;52(10):781–793. doi: 10.1007/s10038-007-0190-x. [DOI] [PubMed] [Google Scholar]
- 22.Doi Y, Kubo M, Ninomiya T, et al. Impact of Kir6.2 E23K polymorphism on the development of type 2 diabetes in a general Japanese population: the Hisayama study. Diabetes. 2007;56(11):2829–2833. doi: 10.2337/db06-1709. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen EM, Hansen L, Carstensen B, et al. The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes. 2003;52(2):573–577. doi: 10.2337/diabetes.52.2.573. [DOI] [PubMed] [Google Scholar]
- 24.Qiu L, Na R, Xu R, et al. Quantitative assessment of the effect of KCNJ11 gene polymorphism on the risk of type 2 diabetes. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093961.e93961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florez JC, Burtt N, de Bakker PIW, et al. Haplotype structure and genotype-phenotype correlations of the sulfonylurea receptor and the islet ATP-sensitive potassium channel gene region. Diabetes. 2004;53(5):1360–1368. doi: 10.2337/diabetes.53.5.1360. [DOI] [PubMed] [Google Scholar]
- 26.Lyssenko V, Almgren P, Anevski D, et al. Genetic prediction of future type 2 diabetes. PLoS Medicine. 2005;2(12, article e345) doi: 10.1371/journal.pmed.0020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dam RM, Hoebee B, Seidell JC, Schaap MM, De Bruin TWA, Feskens EJM. Common variants in the ATP-sensitive K+ channel genes KCNJ11 (Kir6.2) and ABCC8 (SUR1) in relation to glucose intolerance: population-based studies and meta-analyses. Diabetic Medicine. 2005;22(5):590–598. doi: 10.1111/j.1464-5491.2005.01465.x. [DOI] [PubMed] [Google Scholar]
- 28.Laukkanen O, Pihlajamäki J, Lindström J, et al. Polymorphisms of the SUR1 (ABCC8) and Kir6.2 (KCNJ11) genes predict the conversion from impaired glucose tolerance to type 2 diabetes. The Finnish Diabetes Prevention Study. Journal of Clinical Endocrinology and Metabolism. 2004;89(12):6286–6290. doi: 10.1210/jc.2004-1204. [DOI] [PubMed] [Google Scholar]
- 29.Schwanstecher C, Meyer U, Schwanstecher M. KIR6.2 Polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic β-cell ATP-sensitive K+ channels. Diabetes. 2002;51(3):875–879. doi: 10.2337/diabetes.51.3.875. [DOI] [PubMed] [Google Scholar]
- 30.Riedel MJ, Light PE. Saturated and cis/trans unsaturated acyl CoA esters differentially regulate wild-type and polymorphic β-cell ATP-sensitive K+ channels. Diabetes. 2005;54(7):2070–2079. doi: 10.2337/diabetes.54.7.2070. [DOI] [PubMed] [Google Scholar]
- 31.Yang W-S, Lee W-J, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. The Journal of Clinical Endocrinology & Metabolism. 2001;86(8):3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 34.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 35.Seino Y, Ikeda M, Yawata M, Imura H. The insulinogenic index in secondary diabetes. Hormone and Metabolic Research. 1975;7(2):107–115. [Google Scholar]
- 36.Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nature Genetics. 2000;26(1):76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 37.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 38.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 39.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1339–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love-Gregory L, Wasson J, Lin J, Skolnick G, Suarez B, Permutt MA. An E23K single nucleotide polymorphism in the islet ATP-sensitive potassium channel gene (Kir6.2) contributes as much to the risk of Type II diabetes in Caucasians as the PPARγ Pro12Ala variant (3) Diabetologia. 2003;46(1):136–137. doi: 10.1007/s00125-002-0947-x. [DOI] [PubMed] [Google Scholar]
- 42.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP sensitive potassium channel subunit Kir6.2 and permanent neonatal diabetes. New England Journal Medicine. 2004;350(18):1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 43.Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Human Molecular Genetics. 1996;5(11):1809–1812. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- 44.Nestorowicz A, Inagaki N, Gonoi T, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46(11):1743–1748. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- 45.Marthinet E, Bloc A, Oka Y, et al. Severe congenital hyperinsulinism caused by a mutation in the Kir6.2 subunit of the adenosine triphosphate-sensitive potassium channel impairing trafficking and function. Journal of Clinical Endocrinology and Metabolism. 2005;90(9):5401–5406. doi: 10.1210/jc.2005-0202. [DOI] [PubMed] [Google Scholar]
- 46.Stawerska R, Szalapska M, Borowiec M, et al. Frequency of the E23K polymorphism of the KCNJ11 gene in children born small for gestational age and its influence on auxological and metabolic parameters in the prepubertal period. Journal of Pediatric Endocrinology and Metabolism. 2013;26(5-6):457–462. doi: 10.1515/jpem-2012-0196. [DOI] [PubMed] [Google Scholar]
- 47.Ko JM, Yang S, Kim SY, Lee HS, Hwang JS, Hwang IT. E23K polymorphism of the KCNJ11 gene in Korean children with type 1 diabetes. World Journal of Pediatrics. 2012;8(2):169–172. doi: 10.1007/s12519-012-0355-3. [DOI] [PubMed] [Google Scholar]
- 48.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nature Reviews Genetics. 2007;8(9):657–662. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 49.Florez JC, Burtt N, de Bakker PIW, et al. Haplotype structure and genotype phenotype correlations of the sulfonylurea receptor and the islet ATP sensitive potassium channel gene region. Diabetes. 2004;53(5):1360–1368. doi: 10.2337/diabetes.53.5.1360. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen E-MD, Hansen L, Carstensen B, et al. The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes. 2003;52(2):573–577. doi: 10.2337/diabetes.52.2.573. [DOI] [PubMed] [Google Scholar]
- 51.Ezenwaka CE, Kalloo R, Uhlig M, Schwenk R, Eckel J. The E23K variant in the Kir6.2 subunit of the ATP-sensitive K+ channel does not augment impaired glucose tolerance in Caribbean subjects with a family history of type 2 diabetes. Journal of Endocrinology. 2005;185(3):439–444. doi: 10.1677/joe.1.06117. [DOI] [PubMed] [Google Scholar]


