Abstract
Lymphoseek™ is a molecular imaging agent specifically designed for sentinel lymph node (SLN) mapping. We conducted a Phase I trial which measured the injection site clearance and sentinel lymph node accumulation after a single intra-dermal injection of Lymphoseek or [99mTc]sulfur colloid protocol. Ten patients with breast cancer participated in this study. Five patients received an intradermal administration of 1.0 nmole of 99mTc-labeled Lymphoseek and five patients received an intra-dermal administration of filtered [99mTc]sulfur colloid (fTcSC). Lymphoseek, exhibited a significantly (P < .001) faster injection site clearance than fTcSC. The mean Lymphoseek clearance half-time was 2.61 ± 0.72 hr compared to 24.1 ± 17.7 hr for fTcSC. The mean sentinel lymph node uptake of Lymphoseek (1.1 ± .5%), and TcSC (2.5 ± 4.9%) were statistically equivalent (P = 0.28). When an intra-dermal injection was employed, Lymphoseek demonstrated faster injection site clearance than filtered [99mTc]sulfur colloid.
Keywords: sentinel lymph node biopsy, radiopharmaceutical - [99mTc]DTPA-mannosyl-dextran, Lymphoseek, molecular imaging, breast cancer
1. Introduction
Lymphoseek™ is a molecular imaging agent specifically designed for sentinel lymph node mapping. The technetium-99m-labeled radiopharmaceutical attains rapid clearance from the injection site by virtue of it’s small molecular diameter [10]. In rabbits Lymphoseek exhibited significantly faster injection site clearance technetium-99m sulfur colloid [10]. Submucosal administration into pig colon and stomach resulted in lymph node accumulation within 10 minutes [2, 6, 11]. Rapid sentinel lymph node uptake was also observed after direct injection into the porcine prostate gland [7]. The molecular structure of Lymphoseek contains the carbohydrate, mannose, with provides the radiopharmaceutical with a high affinity [10] for a receptor, mannose binding protein [9], which is specific to lymphoid tissue. This molecular feature provides Lymphoseek with sustained sentinel lymph node uptake without distal lymph node accumulation, a property demonstrated in rabbit [10] and pig [2] studies.
Clinical trials of Lymphoseek also demonstrated rapid injection site clearance compared with filtered technetium-99m-labeled sulfur colloid and sustained sentinel lymph node uptake. In women with breast cancer, Lymphoseek demonstrated significantly faster clearance from the injection site and equivalent sentinel lymph node accumulation [13]. No adverse events or clinically significant changes in clinical and laboratory values were observed. These findings were also demonstrated at the 5-nmole dose level [1] and by patients with melanoma [12].
We present the injection site clearance and sentinel lymph node accumulation after a single intradermal injection of Lymphoseek or filtered [99mTc]sulfur colloid which employed a protocol for sentinel lymph node (SLN) mapping of breast cancer. Our previous studies employed paratumoral/intradermal technique where the injection of the radiopharmaceutical was initiated paratumorally and finished with the needle in the intradermal position.
2. Materials and Methods
2.1. Patient Enrollment
Ten female patients with breast cancer who would normally be offered sentinel lymph node biopsy as per University of California, San Diego guidelines participated in this study. We entered women that presented a challenge to successful SLN mapping and lymphoscintigraphy; at least one of the following criteria was required: 1) over 60 years old, 2) having a nonpalable lesion, or 3) having an upper-outer quadrant lesion. The need to have follow-through node dissection was determined by pathologic outcome of the sentinel node and did not affect this study. Consenting subjects were randomly into one of two groups: Lymphoseek, or filtered [99mTc]sulfur colloid. Pregnant and lactating females, patients with known metastatic disease, and patients currently enrolled in another protocol were excluded from this study. The subjects ranged in age from 46 to 83 years (Table 1). Lesion size ranged from 0.8 to 2.1 cm. Two sentinel lymph nodes from 2 subjects were positive on frozen section. A third sentinel lymph node from a third subject was positive on immunohistochemistry.
TABLE 1.
Subject and Radiopharmaceutical Summary
| Subject and Pathology
|
SN Status+ (#) | Radiopharmaceutical
|
||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Age (yrs) | Diagnosis | Size (cm) | Stage | Site | RP | Dose (mCi) | |
| 1 | 59 | Inv ductal & Lob | 0.8 | T1bN0MX | RUOQ | 0/2 | fTcSC | 0.55 |
| 2 | 60 | Inv spindle-cell | 2.1 | T2N0M0 | LUOQ | 0/2 | Lymphoseek | 0.48 |
| 3 | 55 | Inv ductal | 1.0 | T1N0MX | RUOQ | 0/4 | Lymphoseek | 0.46 |
| 4 | 65 | Multifocal DCIS | 1.1 | TisN0MX | LUOQ | 0/7 | Lymphoseek | 0.56 |
| 5 | 57 | Inv mixed duc & lob | 1.5 | T1cN1M0 | LUOQ | 1/2/12 | fTcSC | 0.49 |
| 6 | 64 | Inv mixed duc & lob | 2.0 | T1cN0MX | LLIQ | 0/5 | fTcSC | 0.47 |
| 7 | 46 | Inv ductal | 1.2 | T1cN0 (I+)MX | RUIQ | 1†/3 | Lymphoseek | 0.48 |
| 8 | 51 | Inv mixed duc & lob | 2.0 | T1cN2aMX | RUOQ | 4/8/35 | Lymphoseek | 0.50 |
| 9 | 83 | Inv duc & DCIS | 1.8 | T1bN0MX | LUOQ | 0/10 | fTcSC | 0.43 |
| 10 | 55 | Inv duc & DCIS | 1.1 | T1cN0MX | RUIQ | 0/4 | fTcSC | 0.52 |
Inv, invasive; DCIS, ductal carcinoma in situ; fTcSC, filtered [99mTc]sulfur colloid;
positive/SLN/axillary dissection,
Immunopositive
The protocol received the consent of the Division of Medical Imaging and Radiopharmaceutical Drug Products of the U. S. Food and Drug Administration as a Physician-Sponsored Investigational New Drug. The protocol and the informed consent form were approved by the University of California, San Diego, Office for Human Research Protection, the Moores UCSD Cancer Center Protocol Review Monitoring Committee, and the UCSD Human Exposure Review Committee.
2.2. Agent Preparation
Lymphoseek was synthesized [10] and radiolabeled [3] as previously described. This study used the same Lymphoseek preparation as our Phase I Breast cancer [1, 13] and melanoma trials [12]. The mean molecular diameter was 7.0 nanometers and the average molecular weight was 28,200 grams per mole. The average DTPA and mannose densities were 2.1 and 42 moles per mole of dextran, respectively. The mean Lymphoseek radiochemical purity was 97% (range: 96 – 98). Technetium-99m-labeled filtered sulfur colloid (CIS-US, Bedford, MA) and was prepared by a commercial radiopharmacy. All radiopharmaceutical preparations were administered within two hours of preparation.
2.3. Nuclear Imaging
Each patient received a single injection (0.1 ml) of either Lymphoseek (1 nmole) or filtered [99mTc]sulfur colloid above the lesion using an intradermal technique [4, 5]. The administration site was massaged for several minutes. Each subject was monitored for any sign of an allergic reaction such as the occurrence of a rash, hives, edema, or other cutaneous manifestations.
Nuclear imaging of the Lymphoseek or filtered [99mTc]sulfur colloid employed the same imaging protocol. Images of the injection site were acquired immediately after the injection and at 15-minute intervals for two hours. An imaging standard of a known dilution and volume of injectate was placed within the field-of-view. All images were acquired (256x256x16) for 3 minutes and stored on an image processing computer. The injection site clearance rate constant kc and half-life Tc of Lymphoseek and filtered [99mTc]sulfur colloid were calculated using decay-corrected counts obtained from the nuclear images of the injection site.[13]
2.4. Sentinel Lymph Node Detection and Measurement
Sentinel lymph node biopsy was performed utilizing standard technique. At the start of the surgical procedure, isosulfan blue (Lymphazurin 1%, U. S. Surgical Corp., Norwalk, CT) was injected at the 3-, 6-, 9-, and 12-O’clock positions (1 ml per position) surrounding the lesion. The administration site was massaged for several minutes. Also during this time a hand-held gamma probe (Neoprobe 2000, Neoprobe Corp., Dublin, OH) was used to localize the sentinel lymph node. Scinitigrams were used to guide the search. After marking the skin at the highest count rate, the patient was prepped and an incision was made at the marked location. With the aid of the gamma probe the dissection was carried out to the hot lymph node and/or to the lymphatic with blue dye accumulation. The lymph node was isolated, removed, and placed on the tip of the gamma probe for radioactive counting. A background measurement was made by placing the tip of the gamma probe perpendicular to the skin surface at lease 20 cm from the administration site. Finally, the gamma probe was placed back within the nodal basin to ensure no significant residual radioactivity remained. Verification that the node contained radioactivity at least ten times background was performed before sending it to pathology. Frozen section analysis was then performed to identify metastases. Sentinel lymph nodes were defined by having a node-to-background ratio of at least 10-to-1. If the sentinel node was histologically positive, the lymph node dissection was completed.
All lymph nodes and injection standards were assayed for radioactivity using a dose calibrator located adjacent to the operating room. The percent-of-injected dose %ID in each lymph node was calculated as previously described [13].
2.5. Subject Monitoring
Vital signs and EKGs were obtained before Lymphoseek and filtered TcSC administration and at 15, 30, 45, 60, and 120 minutes post-administration. Samples for urinalysis, CBC with differential and platelet counts, and a blood chemistry panel were acquired at the pre-op anesthesia appointment (baseline) and again just prior to surgery.
2.6. Statistical Methods
For the measures kc, Tc, %IDIS, and %IDSN, statistical significance was evaluated by comparisons of the Lymphoseek group to the filtered [99mTc]sulfur colloid group using the nonpaired Students–t -test (JMP software, SAS Institute, Cary NC).
3. Results
Table 1 lists the subject number and age. The table also lists the tumor diagnosis, size, stage and location. Also, listed are the agent and the amount of radioactivity administered. Table 2 lists the subject number, radiopharmaceutical, the injection site clearance rate constant and half-life, the time at which the sentinel lymph node was excised relative to the time injected. Also, listed is the probe count rate after excision of each sentinel lymph node as defined by the radiotracer or blue dye. Entries for subjects 1 and 5 contain zeros, indicating a sentinel lymph node detected only via blue dye. Subjects 3 and 10 exhibited blue sentinel lymph nodes that did not attain probe counts that exceeded 10-times background.
TABLE 2.
Injection Site Clearance and Sentinel Lymph Node Uptake
| Subject | Radiopharmaceutical | Injection Site Clearance+
|
Sentinel Lymph Node Uptake‡
|
|||||
|---|---|---|---|---|---|---|---|---|
| Rate Constant kc (hr−1) | Half-Life Tc (hr) | Excision Time (hr) | Probe Count Rate
|
|||||
| primary (cps) | 2nd (cps) | 3rd (cps) | 4th (cps) | |||||
| 1 | fTcSC | 0.036 ± 0.044 | 19.5 ± 24.4 | 5.1 | 0† | |||
| 2 | Lymphoseek | 0.284 ± 0.026 | 2.44 ± 0.22 | 3.4 | 7,000 | 1,300 | ||
| 3 | Lymphoseek | 0.249 ± 0.022 | 2.79 ± 0.25 | 4.3 | 1,100 | 650 | 90† | |
| 4 | Lymphoseek | 0.176 ± 0.034 | 3.93 ± 0.76 | 4.4 | 3,200* | 450 | 2,200 | 1,208* |
| 5 | fTcSC | 0.000 | 4.4 | 0† | 141* | |||
| 6 | fTcSC | 0.019 ± 0.003 | 36.7 ± 6.5 | 7.3 | 45 | 225 | 109* | |
| 7 | Lymphoseek | 0.294 ± 0.023 | 2.36 ± 0.19 | 6.1 | 2,600 | 2,136 | ||
| 8 | Lymphoseek | 0.322 ± 0.023 | 2.15 ± 0.16 | 4.9 | 5,081 | |||
| 9 | fTcSC | 0.032 ± 0.019 | 21.4 ± 12.7 | 3.5 | 6,869 | 2,169* | 5,070* | |
| 10 | fTcSC | 0.057 ± 0.018 | 12.2 ± 3.9 | 6.1 | 38,942 | 2,700 | 75† | |
fTcSC, filtered [99mTc]sulfur colloid;
mean ± SD;
hot or blue;
not blue,
not hot
Lymphoseek, exhibited a significantly (P < 0.001) faster injection site clearance than filtered or unfiltered TcSC. The mean Lymphoseek clearance half-time (Table 3) was 2.62 ± 0.55 hr (n = 5) compared to 24.1 ± 17.7 hr for filtered TcSC (n = 5). The mean Lymphoseek sentinel lymph node uptake of 1.1 ± 0.5 % and percent-of-injected dose of 2.5 ± 4.9 % for filtered TcSC, which were statistically equivalent (P = 0.28). Figure 1 demonstrates the clearance of radioactivity from the injection site of two sentinel node cases: Lymphoseek (diamonds, Subject #8) exhibited a half-life of 2.15 ± 0.16 hours, filtered [99mTc]sulfur colloid (triangles, Subject #8) exhibited a half-life of 36.7 ± 6.5 hours.
TABLE 3.
Injection Site Clearance and Sentinel Lymph Node Uptake of Lymphoseek and Filtered [99mTc]Sulfur Colloid
| Group | Radiopharmaceutical | Injection Site Clearance
|
Sentinel Lymph Node
|
|||
|---|---|---|---|---|---|---|
| Studies (#) | Rate Constant kc+ (hr−1) | Half-Life Tc* (hr) | Studies (#) | %IDSN+ (%) | ||
| 1 | Lymphoseek | 5 | 0.265 ± 0.055 | 2.62 ± 0.55 | 5 | 1.1 ± 0.5 |
| 2 | Filtered [99mTc]sulfur colloid | 5 | 0.029 ± 0.021 | 24.1 ± 17.7 | 5 | 2.5 ± 4.9 |
| P-value | < 0.001 | < 0.001 | 0.275 | |||
Mean ± SD;
Based on the Rate Constant kc
Figure 1.
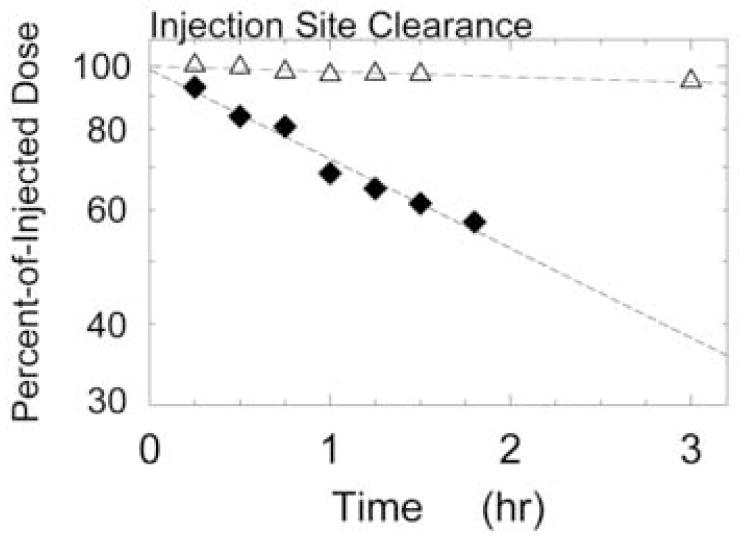
Clearance of radioactivity from the injection site of two sentinel node cases: Lymphoseek (diamonds, Subject #8) exhibited a half-life of 2.15 ± 0.16 hours, filtered [99mTc]sulfur colloid (triangles, Subject #6) exhibited a half-life of 36.7 ± 6.5 hours.
None of the adverse events that occurred during this study were attributed to either radiopharmaceutical. No grade 3 or 4 hematologic adverse events occurred on this study, and one non-hematologic adverse event occurred. This subject (#8), who was a member of the filtered TcSC group, experienced a mild rash on her left breast from the time of surgery. Approximately one month after the operation, some fullness and erythema was noted toward the incision. This was aspirated and found to be a hematoma.
Both radiopharmaceuticals exhibited the same mean number of sentinel lymph nodes per study. The five subjects in the Lymphoseek group yielded a total of eleven sentinel lymph node for an average of 2.2 nodes per study. The five filtered TcSC subjects yielded nine sentinel lymph nodes; one study failed to produce a hot lymph node. To calculate the average we used four as the denominator to calculate 2.3 nodes per study.
4. Discussion
This study demonstrated significantly faster injection site clearance by Lymphoseek than filtered [99mTc]sulfur colloid. Contrary to our expectation, the intradermal administration did not exhibit a faster clearance half-life (2.62 +/− 0.55 hr) than Phase I study group that utilized a periturmoral/intradermal injection [13], which exhibited a clearance half-life of 2.72 +/− 1.57 hr. This was also true for our melanoma study [12], where the measured clearance half-life was 2.05 +/− 0.89 hr. One noticeable difference between the three studies was the wide dispersion of clearance half-lives exhibited by the periturmoral/intradermal injections; this was denoted by relative standard deviation of 0.58, which is almost three-fold greater than the relative standard deviation of the intradermal injection (0.21).
The sentinel lymph node accumulation of the Lymphoseek group was higher compared to the accumulation of Lymphoseek after intradermal and intradermal/peritumoral administration to melanoma or breast cancer patients. The mean sentinel lymph node accumulation after a 1-nmole dose of Lymphoseek using a standard mapping protocol for SLN of melanoma [12] was 0.49 ± 0.59 percent of injected dose. A breast cancer mapping protocol using an intradermal/periturmoral administration of Lymphoseek [13] yielded mean SLN uptakes of 0.55 ± 0.43 %. These values are lower than the means 1.1 ± 0.5 % exhibited by the Lymphoseek group of this study; the differences are not, however, significant.
The study was designed to measure the rates at which Lymphoseek and filtered [99mTc]sulfur colloid clear the injection site after an intradermal administration to the breast. The study was powered to provide a statistical comparison of the injection site clearance rates exhibited by the Lymphoseek and fTcSC groups. We also measured each subject’s sentinel lymph node accumulation. This study was not powered to test for a significant difference in SLN accumulation between Lymphoseek and fTcSC. This is due to the extremely high variability in SLN uptake between subjects [8].
The entrance criteria for this study, over 60 years old, a nonpalable, or upper-outer quadrant lesion, was designed to enroll subjects who’s lymphosintigraphic or gamma probe detection is more difficult. The intradermal method [4, 5, 14] is extremely convenient, is less stressful for the patient, generates higher SLN uptake, and produces less scatter from the injection site. Given the propensity for many surgeons to use an intradermal approach, we conducted this study to ensure that Lymphoseek was compatible with this technique. Todate, we have not administered Lymphoseek using a sub-areolar injection.
5. Conclusion
When a single intradermal injection is employed, Lymphoseek demonstrated significantly faster injection site clearance than filtered [99mTc]sulfur colloid. The injection site clearance rate and sentinel lymph node accumulation were comparable to clearance and accumulation of Lymphoseek after intradermal or intradermal/peritumoral administration to melanoma or breast cancer patients.
Acknowledgments
This work was supported by grant R21-CA09764 of the Quicktrials Program of the National Cancer Institute. We thank Dr. Ernest V Belezzuoli, MD and the Neoprobe Corporation (Dublin OH) for the use of a Model 2100 intraoperative radioisotope detector. Lymphoseek is a registered trademark of the Neoprobe Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellner SJ, Hoh CK, Vera DR, Darrah DD, Schulteis G, Wallace AM. Dose-dependent biodistribution of [99mTc]DTPA-mannosyl-dextran for breast cancer sentinel node mapping. Nucl Med Biol. 2003;30:805–10. doi: 10.1016/j.nucmedbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ellner SJ, Mendez J, Vera DR, Hoh CK, Ashburn WL, Wallace AM. Sentinel lymph node mapping of the colon and stomach using lymphoseek in a pig model. Ann Surg Oncol. 2004;11:674–81. doi: 10.1245/ASO.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Hoh CK, Wallace AM, Vera DR. Preclinical studies of [99mTc]DPTA-mannosyl-dextran. Nucl Med Biol. 2003;30:457–64. doi: 10.1016/s0969-8051(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 4.Linehan DC, Hill ADK, Akhurst T, Yeung H, Yeh S, Tran KN, Borgen PI, Cody HS. Intradermal radiocolloid and intraparenchymal blue dye injection optimize sentinel node identification in breast cancer patients. Ann Surg Oncol. 1999;6:450–4. doi: 10.1007/s10434-999-0450-4. [DOI] [PubMed] [Google Scholar]
- 5.McMasters K, Wong S, Martin RI, Chao C, Tuttle T, Noyes R, Carlson D, Laidley A, McGlothin T, Ley P, Brown C, Glaser R, Pennington R, Turk P, Simpson D, Cerrito P, Edwards M. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001;233:676–87. doi: 10.1097/00000658-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez J, Wallace AM, Hoh CK, Vera DR. Detection of gastric and colonic sentinel nodes via endoscopic administration of Lymphoseek in pigs. J Nucl Med. 2003;44:1677–81. [PubMed] [Google Scholar]
- 7.Salem CE, Wallace AM, Hoh CK, Vera DR. A preclinical study of prostate sentinel node mapping with Lymphoseek. J Urol. 2006;175:744–8. doi: 10.1016/S0022-5347(05)00139-4. [DOI] [PubMed] [Google Scholar]
- 8.Schoder H, Glass EC, Pecking AP, Harness JK, Wallace AM, Hirnle P, Alberini JL, Vilain D, Larson SM, Hoh CK, Vera DR. Molecular targeting of the lymphovascular system for imaging and therapy. Cancer metastasis reviews. 2006;25:185–201. doi: 10.1007/s10555-006-8498-0. [DOI] [PubMed] [Google Scholar]
- 9.Steer CJ. In: Hepatology A Textbook of Liver Disease. Zakim D, Boyer TD, editors. W. B. Saunders; Philadelphia: 1996. pp. 149–214. [Google Scholar]
- 10.Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: [99mTc]DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–9. [PubMed] [Google Scholar]
- 11.Wallace AM, Ellner SJ, Mendez J, Hoh CK, Salem CE, Bosch CM, Orahood RC, Vera DR. Minimally invasive sentinel lymph node mapping of the pig colon with lymphoseek. Surgery. 2006;139:217–23. doi: 10.1016/j.surg.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: A molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14:913– 21. doi: 10.1245/s10434-006-9099-4. [DOI] [PubMed] [Google Scholar]
- 13.Wallace AM, Hoh CK, Vera DR, Darrah D, Schulteis G. Lymphoseek: A molecular radiopharmceutical for sentinel node detection. Ann Surg Oncol. 2003;10:531–8. doi: 10.1245/aso.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Yeung H, Cody HS, III, Turlakow A, Riedel ER, Fey J, Gonen M, Nunez R, Yeh SD, Larson SM. Lymphoscintigraphy and sentinal node localization in breast cancer patients: a comparison between 1-day and 2-day protocols. J Nucl Med. 2001;42:420–3. [PubMed] [Google Scholar]


