SUMMARY
Toxoplasma gondii is an apicomplexan intracellular protozoan parasite responsible for toxoplasmosis, a disease with considerable medical and economic impact worldwide. Toxoplasma gondii cells never lose the nuclear envelope and their chromosomes do not condense. Here, we tested the murine monoclonal antibody PL2-6, which labels epichromatin (a conformational chromatin epitope based on histones H2A and H2B complexed with DNA), in T. gondii cultured in human fibroblasts. This epitope is present at the exterior chromatin surface of interphase nuclei and on the periphery of mitotic chromosomes in higher eukaryotes. PL2-6 reacted with T. gondii H2A and H2B histones in Western blot (WB) assays. In addition, the antibody reacted with the nuclear fraction of tachyzoites, as a single band coincident with H2B histone. In the T. gondii tachyzoite stage, PL2-6 also had peripheral nuclear localization, as observed by epifluorescence/confocal microscopy and immunoelectron microscopy. Confocal analysis showed that epichromatin is slightly polarized to one face of the parasite exterior chromatin surface. In replicating tachyzoites, PL2-6 also labels the exterior chromatin surface, covering the face of both segregating nuclei, facing the plasma membrane of the mother cell. The possible role of epichromatin in T. gondii is discussed.
Keywords: Toxoplasma gondii, epichromatin, nuclear envelope, cell cycle, replication
INTRODUCTION
Epigenetics is among the most conserved mechanisms of chromatin modulation and gene regulation present in all eukaryotes. This mechanism can be observed in all living organisms from animals to protozoan cells. In fact, histones H2A, H2B, H3 and H4, chromatin remodellers and histone code, can be found in the genera Giardia and Trichomonas, the most primitive eukaryote cells (Dalmasso et al. 2012). Noteworthy, the conserved epigenetic mechanism coexists with low conservation of gene organization and cell cycle progress. As an example, apicomplexan and trypanosomatid protozoan cells conserve similar features of replication behaviour, which are highly different from those occurring in higher eukaryotes: they never lose the nuclear envelope during mitosis and their chromosomes do not present the higher order level of condensation as observed in mitotic chromosomes of higher eukaryotes.
Toxoplasma gondii is a protozoan parasite belonging to the phylum Apicomplexa together with the genera Plasmodium, Cryptosporidium and Eimeria. These protozoa are responsible for a range of human and animal diseases with considerable medical and economic impact worldwide (Black and Boothroyd, 2000). Toxoplasma is an intracellular obligate parasite whose cell cycle occurs inside the host cell, confined to a vacuolar space named the parasitophorous vacuole (PV) (Ferguson and Dubremetz, 2007). The parasite replication process includes classical events: DNA replication, chromosome segregation, nuclear division and cytokinesis or budding. Nevertheless, the T. gondii tachyzoite, a highly replicative stage, divides in cells of intermediate hosts through a type of internal budding known as endodyogeny, in which two daughters develop internally within the parental cell. As such, two, four, eight, etc. tachyzoites per PV are found after several rounds of replication (Striepen et al. 2007). In contrast to other apicomplexans, T. gondii is easily maintained in cell culture and there are several tools available to analyse its replication. Taking into account all these data, T. gondii could be considered a good model for studying Apicomplexa replication.
An epichromatin epitope has been recently found while using an autoimmune murine monoclonal antibody named PL2-6 (Olins et al. 2011). Based on a co-localization assay with this antibody, it could be observed that epichromatin is present in the interphase and mitotic chromosomes of mammalian, Drosophila melanogaster, Caenorhabditis elegans and Arabidopsis thaliana cells, suggesting a high degree of conservation during evolution, at least in higher eukaryotes. Interestingly, PL2-6 labels the exterior chromatin surface of interphase nuclei, whereas during mitosis, when the nuclear envelope disappears, epichromatin is located at the periphery of the condensed chromosomes adjacent to the dissociating nuclear envelope (Olins et al. 2011). Recently, Prudovsky et al. (2012) showed that the anionic glycerophospholipid phosphatidylserine co-localizes with epichromatin, staining also the surfaces of chromatin in the interphase nucleus and of the periphery of condensed chromosomes during mitosis. Therefore, it is possible that epichromatin is a conserved mechanism of eukaryotic cells to break down and restructure the nuclear envelope during mitosis (Prudovsky et al. 2012).
Apicomplexan parasites neither break down nor restructure the nuclear envelope during mitosis (Striepen et al. 2007). Nevertheless, it is possible that the interaction between the nuclear envelope and chromatin remains to organize the chromosomes at interphase and during mitosis. Consequently, these parasites may represent an interesting model to analyse the epichromatin epitope. Here, we show the presence of epichromatin in T. gondii by indirect immunofluorescence (IFA), confocal analysis and immunoelectron microscopy (immunoEM) and determine its location by 3D reconstruction analysis in interphase and replicating tachyzoites. The possible role of epichromatin in T. gondii is discussed.
MATERIALS AND METHODS
Toxoplasma gondii culture
Parasites of the RHΔhxgprt strain were grown in standard tachyzoite conditions in vitro: human foreskin fibroblast (HFF) monolayers grown on cover slips were infected with tachyzoites and incubated with Dulbecco’s modified Eagle medium (DMEM, Gibco BRL) supplemented with 1% fetal bovine serum at 37 °C and 5% CO2.
Nuclear extraction of T. gondii tachyzoites
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) was used according to the kit instructions. Protease inhibitor Cocktail kit (Thermo Scientific) was added to avoid protein degradation. 1×108 fresh, filtered tachyzoites were processed with the kit. Cytoplasmic and nuclear fractions were resolved in 15% SDS-PAGE and either assayed by Western blot (WB) or silver stained as detailed in Blum et al. (1987).
Western blot analysis
Recombinant histones (Dalmasso et al. 2009) (~1·5 μg of recombinant protein per well) or the nuclear fraction of tachyzoites were loaded and resolved by 15% SDS-PAGE. Proteins were transferred to cellulose acetate membrane for 1 h at 100 V. WB was then performed as described before (Echeverria et al. 2005), taking care to use Bovine SeroAlbumin (BSA) 5% in Tris Buffered Saline-Tween 20 (TBS-T) instead of defatted milk for blocking and diluting the primary and secondary antibodies. The primary antibody α-PL2-6 was used at 1/2000 for 1 h at room temperature. Rabbit anti-H2Bv and anti-H2AX antibodies were previously described in Dalmasso et al. (2009). Rabbit anti-H4 trimethylated K20 (Abcam ab9053) and –rabbit anti-histone H3 (Millipore 05928) polyclonal antibodies were also used at dilution 1/1000 and 1/500, respectively. Phosphatase alkaline-conjugated goat anti-mouse (Sigma-Aldrich Argentina S. A.) along with the NBT and BCIP (Promega) detection system or horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit along with the ECL detection system (Amersham-GE) were used.
Immunofluorescence assay (IFA)
Extracellular tachyzoites of the RHΔhxgprt strain were fixed in cover slips using alternatively cold methanol 100% for 8 min at −20 °C, or 4% paraformaldehyde (PAF) for 20 min. For intracellular conditions, RHΔhxgprt strain parasites were cultured for 24 h in Vero cells and then were fixed with 4% PAF and blocked with 1% BSA. The primary antibodies α-H2AX, α-H2AZ, and α-IMC1 diluted 1/500 and α-PL2-6 diluted 1/100 with 0·5% BSA were incubated in the cover slips infected with tachyzoites for 1 h at room temperature. After several washes with Phosphate Buffered Saline (PBS) cover slips were incubated with secondary antibodies Alexa Fluor 594 goat anti-mouse/rabbit or Alexa Fluor 488 goat anti-rabbit/mouse (Invitrogen Life Technologies). DAPI was used to stain nuclei of tachyzoites and host cells.
Confocal microscopy
Fluorescence was detected with an Olympus confocal microscope (Olympus FV1000) with an emission filter of 490–540 nm for Alexa Fluor 488 detection (laser 473 nm); of 575–675 nm for Alexa Fluor 594 detection (laser 559 nm) and of 430–455 nm for DAPI detection (laser 405 nm). Phase-contrast images were obtained with the 405 nm laser. An objective of 40× (UPlanSAPO) with a NA of 0·95 was used. 3D reconstruction of stack images was performed using image analyser software (cellSens Dimension®, Olympus, Tokyo).
Immunoelectron microscopy
Tachyzoite-infected cells were fixed with 4% formaldehyde and 0·05% glutaraldehyde in PBS for 15 min, dehydrated in ethanol and embedded in LR White (London Resin Co.). Thin sections were collected on nickel grids and incubated in blocking buffer with 3% BSA in TBS 0·05 M pH 7·8 for 30 min. Sections were immunolabelled with mouse monoclonal PL2-6 antibody diluted 1: 10, in 0·05 M TBS containing BSA 3%, pH 7·6, overnight at 4 °C, followed by anti-mouse IgG antibody gold conjugated (Sigma). A negative control, without primary antibody, was included. After immunolabelling, sections were post-fixed with 2% of glutaraldehyde in deionized water and stained with 0·25% uranyl acetate in water and observed with a Zeiss EM 109T, equipped with a Zeiss EM109T Digital Camera 11 Mpx for electron images GATAN ES1000W.
RESULTS
PL2-6 antibody reacts with T. gondii H2A and H2B histones
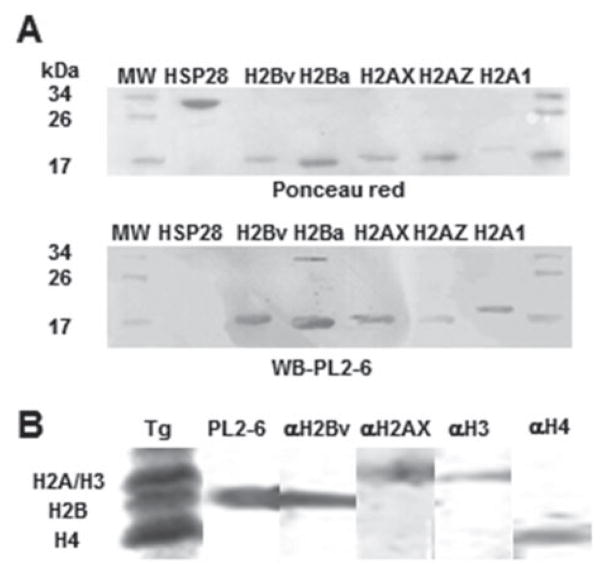
It has been previously observed that PL2-6 antibody shows significant reactivity against recombinant H2A and H2B Xenopus histones (Olins et al. 2011). Among histones, H2A and H2B families are the least conserved in nature, showing significant differences between T. gondii H2A and H2B histones in comparison with its human or yeast counterparts (Dalmasso et al. 2006, 2009). In addition, canonical H2A and H2A variants (H2AX and H2AZ) show significant differences mainly at the N-terminal tail (Dalmasso et al. 2009). Similarly, canonical H2B and its variant, H2Bv, also show most of their differences at the N-terminal tail (Dalmasso et al. 2009). For this reason, in the present work, the T. gondii recombinant histones H2A.1 (canonical), H2AX, H2AZ, H2Ba (canonical) and H2Bv were assayed by WB with PL2-6 antibody. Recombinant HSP28 was included as non-histone control. The antibody reacted with similar intensity with all the recombinant histones, but did not react with HSP28 (Fig. 1A).
Fig. 1.
PL2-6 antibody reactivity with Toxoplasma gondii histones. (A) T. gondii recombinant histones H2A.1 (canonical), H2AX, H2AZ, H2Ba (canonical) and H2Bv were assayed by Western blot (WB) with PL2-6 antibody. Recombinant HSP28 was included as non-histone control. The upper panel shows the Ponceau red staining as loading control. The lower panel shows the WB using mAb PL2·6 antibody. Molecular weight markers are shown in both marginal lanes; (B) The nuclear fraction of T. gondii extracellular tachyzoites was resolved in 15% SDS-PAGE. Lane one was Coomassie-blue stained to detect H2A, H2B, H3 and H4 histones. The other lane were cut into pieces and assayed by WB with PL2-6, anti-H2Bv, anti-H2AX, anti-H3 and anti-H4 trimethylated K20 (H4) antibodies.
In order to know whether PL2-6 is able to react with T. gondii H3 and/or H4 histones, the nuclear fraction of extracellular tachyzoites was analysed by WB (Fig. 1B). It can be observed that PL2-6 reacted as one band at H2B region but not in H4, H3 and H2A regions. These data suggest that T. gondii histones H2B and H2A conserve the epitope recognized by PL2-6. The fact that PL2-6 only reacted as one band at H2B region of T. gondii histone preparation, may be due to a weaker interaction with H2A, or the relative quantities of H2A in tachyzoites, as discussed later.
Epichromatin is present in T. gondii chromosomes
It has been previously observed that PL2-6 antibody reacts weakly with isolated Xenopus histones in comparison with nucleosomes, indicating that the epitope is conformational and that these families of histones expose sequences present in such epitopes (Olins et al. 2011). In addition, epichromatin is located at the exterior chromatin surface of interphase nuclei (Olins et al. 2011). In order to analyse whether T. gondii presents similar features in its PL2-6 associated epitope, extracellular tachyzoites were labelled with PL2-6 and DAPI and analysed by epifluorescence microscopy. Extracellular tachyzoites correspond to a non-replicative stage of the parasite. Parasites were fixed by two methods (methanol and PAF) in order to avoid technical artefacts due to fixation. PL2-6 stained the periphery of the interphase nuclei of the parasite regardless of the method used to fix (Fig. 2).
Fig. 2.
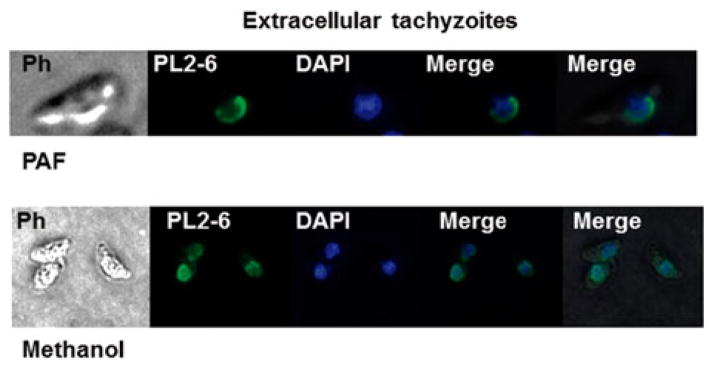
Identification of epichromatin in Toxoplasma gondii tachyzoites. Extracellular T. gondii tachyzoites were fixed either with paraformaldehyde (PAF) or methanol (see Materials and Methods) and assayed by immunofluorescence assay (IFA) using PL2-6 antibody and DAPI to stain the nuclear DNA. Epichromatin epitope identified with murine mAb antibody (PL2-6) was stained with Alexa Fluor 488 (green). PH, phase contrast.
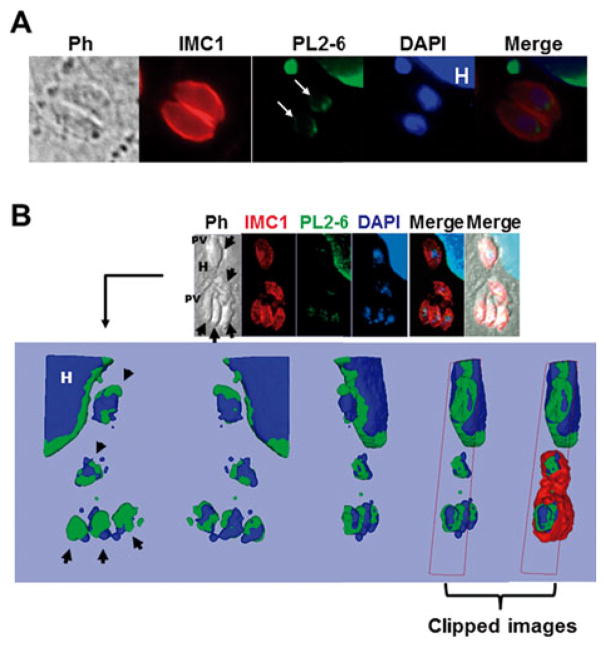
Once the parasite enters the host cell, tachyzoites become a highly replicative form. In order to determine whether PL2-6 label is maintained along the cell cycle, intracellular tachyzoites were labelled with PL2-6 and anti-IMC1 antibody and analysed by epifluorescence/confocal microscopy. IMC1 is a cytoskeletal protein located at the inner membrane complex (IMC), a continuous patchwork of flattened vesicles located below the plasma membrane that extends along the entire parasite from the apical to the posterior region (Hu et al. 2002). PL2-6 stained the periphery of the interphase nuclei of the parasite (Fig. 3A). The host cell nucleus acts as control of immunostaining since PL2-6 also presented peripheral localization (Fig. 3A).
Fig. 3.
Immunolocalization of the epichromatin epitope in intracellular Toxoplasma gondii tachyzoites. (A) The image shows host cells infected with one parasitophorous vacuole (PV) containing two tachyzoites, sagittal plane. The epichromatin was identified by murine mAb antibody (PL2-6) stained with Alexa Fluor 488 (green). The panel (white arrows) shows an example representative of the typical PL2-6 labelling. IMC1, inner membrane complex 1 protein stained with Alexa Fluor 594 (red). DAPI was used to stain the nuclear DNA. PH, phase contrast; H, host cell; (B) Confocal section of an infected cell with three PV immunostained with mAb PL2-6, anti-IMC1 and DAPI staining. The arrows show single tachyzoites. The upper panel shows one slice of the Z projection, while the lower panel shows a 3D reconstruction (isosurface projection of the Z direction) of the entire stack in frontal, caudal and lateral view of the parasite. Colour coding: blue, the host (H) and parasite nuclei; green, the epichromatin. The last two representations on the right represent clipped images to demonstrate that epichromatin surrounds the parasite and host nuclei and that IMC surrounds epichromatin.
On the other hand, to confirm that PL2-6 is only confined to the periphery of parasite nuclei, confocal analysis was performed. For this purpose, stacks of 0·2 μm optical cuts in Z direction were obtained. The 3D analysis showed that the PL2-6-associated epitope is located at the nuclei periphery, although slightly polarized to one face (Fig. 3B).
By immunoEM (Fig. 4A and B), PL2-6 immunogold labelling is clearly concentrated in the periphery of the non-replicating nucleus, associated with heterochromatin. Although in some cases it seems to label the whole nucleus periphery, there are some parasites where it is only partial (Fig. 4A, black arrow), as was observed by confocal microscopy. A negative control without primary antibody did not show any significant gold label (not shown).
Fig. 4.
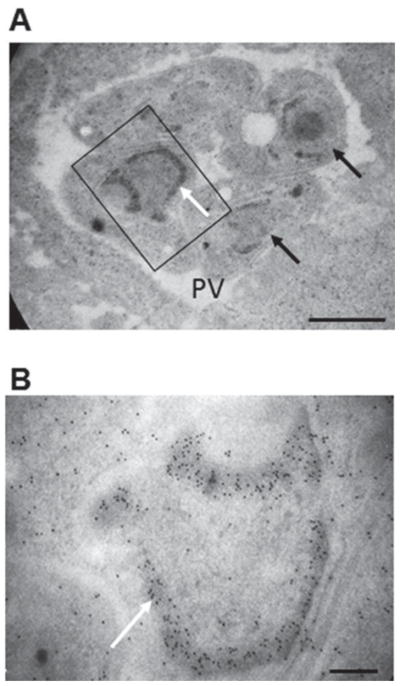
Immunoelectron microscopy of the epichromatin epitope in intracellular Toxoplasma gondii tachyzoites. (A) Immunoelectron microscopic labelling of the epichromatin epitope at the nuclear periphery of intracellular tachyzoites; (B) Enlarged region taken from (A) (indicated by a rectangle) White arrows show immunogold labelling visibly concentrated at the nucleus periphery, probably associated to heterochromatin. Black arrows show nucleus where the label is not homogeneous in the nucleus periphery. PV, parasitophorous vacuole. Magnification bar values: (A) 1 μm; (B) 0·2 μm.
Taken together, these data support that epichromatin is present in T. gondii with a localization similar to that observed in other organisms where PL2-6 was studied. Based on that, we consider the epitope labelled by PL2-6 to be Toxoplasma epichromatin.
Peripheral nuclei localization of epichromatin persists in replicative tachyzoites
One of the most interesting aspects of epichromatin is that, during mitosis, its peripheral nuclear localization disappears along with the nuclear envelope breakdown and it is relocated at the periphery of the condensed chromosomes adjacent to the dissociating nuclear envelope (Olins et al. 2011). Since T. gondii does not lose its nuclear envelope during mitosis and chromosomes do not show such high order level of condensation, the localization of PL2-6 antibody was analysed by immunostaining of replicative intracellular tachyzoites. Anti-IMC1 and anti-H2AX antibodies were also used in the analysis. The parasite IMC appears surrounding the new tachyzoites during daughter cell segregation, whereas mother cell structure is conserved (Hu et al. 2002). The upper panel in Fig. 5(A) shows one PV with two different replicative stages inside a host cell. In the most advanced stage of replication, where both nuclei are split and almost completely inside the daughter cell, PL2-6 showed a localization similar to that in the mature tachyzoite (Fig. 5A, yellow arrows). Noteworthy, at an earlier stage of replication, PL2-6 staining showed a nuclear polarity, oriented posteriorly (Fig. 5A, red arrow), whereas the segregating nuclei that are entering the daughter cells showed a weaker signal (Fig. 5A, white arrows). Confocal 3D analysis confirmed that epichromatin presents nuclear polarization, covering the posterior face of the two segregating nuclei that are outside the budding cells, whereas the anterior part of these nuclei that are entering the daughter cells were not labelled with PL2-6 (Fig. 5B, red arrows).
Fig. 5.
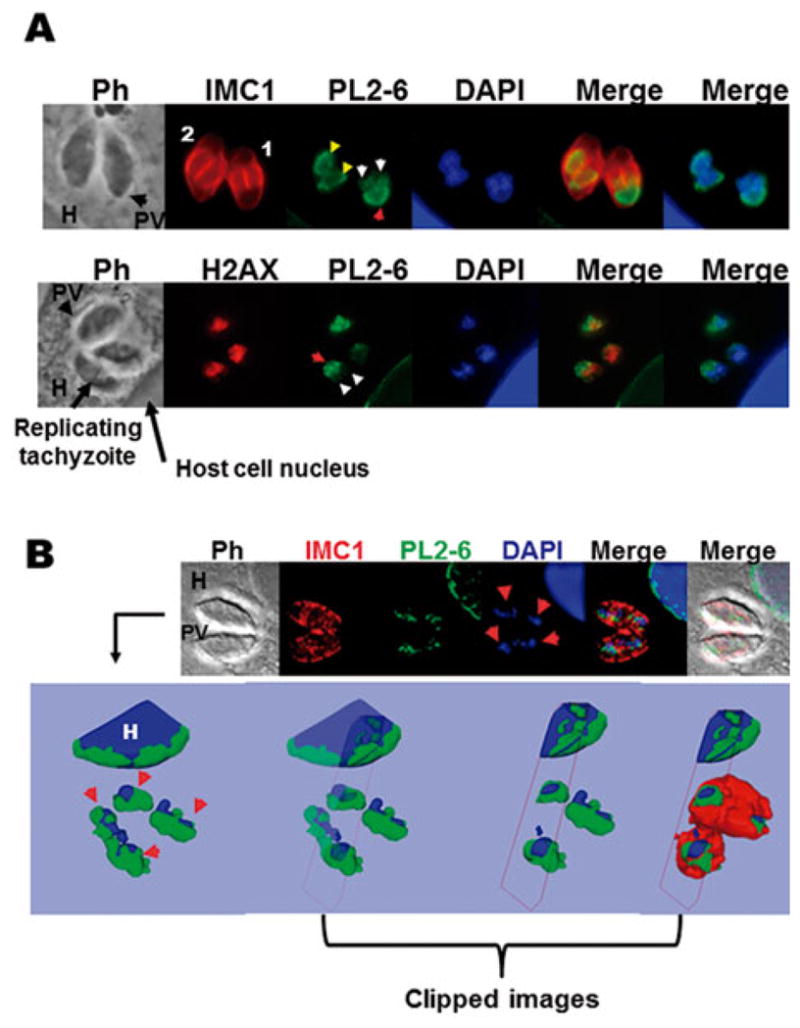
Immunolocalization of the epichromatin epitope in intracellular replicative tachyzoites. (A) The upper panel shows host cells infected with one parasitophorous vacuole (PV) containing two replicating tachyzoites in different degrees; numbers 1 and 2 indicate the lower and higher stage of replication, respectively. IMC1, inner membrane complex 1 protein stained with Alexa Fluor 594; PL2-6 epichromatin stained with Alexa Fluor 488 and DAPI used to dye the nuclear DNA. PH, phase contrast; H, host cell. The lower panel shows host cells infected with one PV containing three tachyzoites, one of them replicating. H2AX, variant T. gondii histone H2AX stained with Alexa Fluor 594; PL2-6, epichromatin stained with Alexa Fluor 488 and DAPI used to dye the nuclear DNA. Red arrow: the nuclear face facing the plasma membrane. Yellow head arrows: PL2-6 showing localization similar to that of mature tachyzoites in higher stage of replication. White head arrows: tops of the two nuclei entering the daughter cell; (B) One optical confocal section of an infected cell with one PV immunostained with mAb PL2-6 (Alexa Fluor 488); anti-IMC1 (Alexa Fluor 594) and DAPI staining. The arrows show the replicating nuclei of tachyzoites. The upper panel shows a 3D reconstruction (isosurface projection of the Z direction) of the entire stack of T. gondii culture. Colour coding: blue, the host (H) and parasite nuclei; green, the epichromatin. The last three representations on the right represent clipped images to demonstrate that epichromatin surrounds the parasite and host nuclei and that IMC surrounds epichromatin.
Although PL2-6 reacted with recombinant H2AX by WB (Fig. 1), it did not co-localize, at least completely, with native H2AX by immunostaining (Fig. 5A, lower panel). This result agrees with the idea that histone nucleosomes within interphase and mitotic chromatin possess the histone amino acid residues involved in the epichromatin epitope, but that these residues are not exposed (Olins et al. 2011).
These data indicate that PL2-6 is present along the parasite cell cycle, but with a behaviour different from that in higher eukaryotes, probably due to the conservation of the nuclear envelope of this parasite during replication, the uncondensed chromosomes, or both. Figure S1 (in Online version only) shows an immunofluorescence, where PL2-6 is co-localized with Morn1, an antibody that is a marker of the T. gondii cell cycle (Heaslip et al. 2010; Lorestani et al. 2010). The rings which appear early as well as in a more advanced stage of replication (Fig S1 A and B, respectively) were clearly located far from the PL2-6 label (white arrows), confirming that PL2-6 is mainly oriented posteriorly, facing the parasite posterior end.
DISCUSSION
Knowledge about the evolution of the nuclear envelope during apicomplexan parasite replication and its interaction with chromatin and role in spatial organization of parasite chromosomes is still very modest. Epichromatin has been recently discovered as a conformational epitope of histones H2A and H2B complexed with DNA, exposed at the periphery of the nucleus, which is juxtaposed to the nuclear envelope (Olins et al. 2011). This epitope is present in chromosomes of mammalian, D. melanogaster, C. elegans and A. thaliana cells, indicating a high degree of conservation through evolution as well as an early appearance in the evolution of higher eukaryotes. Our finding that epichromatin is also present in T. gondii suggests that this conformational chromosome epitope arose very early in evolution.
PL2-6 antibody reacted with parasite recombinant H2A and H2B, as well as native H2B. The fact that native H2A seems not to be recognized by this epitope in the WB (Fig. 1B) may be due to the relative amount of each histone in T. gondii, and/or to the fact that PL2-6 can recognize a homologous epitope within both histones H2A and H2B. Indeed, H2A exhibits ~85% reaction intensity compared with 100% intensity with H2B, as shown by immunoblot experiments in Olins et al. (2011). The H2A and H2B families are the least conserved histones in nature, especially at N-terminal tails, whereas the globular regions maintain a higher degree of conservation (Henikoff and Ahmad, 2005). In fact, the H3 and H4 histones present only a few amino acid variations among organisms, except for trypanosomatids (Dalmasso et al. 2012). In addition, T. gondii H2AX and H2AZ showed significant differences at the N-terminal sequences, similar to the case of H2Ba and H2Bv (Dalmasso et al. 2006, 2009). Perhaps, the regions of H2A and H2B involved in epichromatin are present in the highly conserved globular domains.
One of the most remarkable findings regarding epichromatin is that it is present throughout the cell cycle, but confined to the exterior surface of high-order condensed mitotic chromosomes (Olins et al. 2011). Here we observed that in T. gondii, the epichromatin is located at the periphery of the interphase nuclei of the non-replicative and replicative parasite. An antibody against phosphatidylserine, an anionic glycerophospholipid present in the nuclear envelope, co-localizes with epichromatin throughout the cell cycle, suggesting that epichromatin could have a conserved role as a nucleation site for the binding of the endoplasmic reticulum and re-establishment of the nuclear envelope (Prudovsky et al. 2012). Toxoplasma gondii provides an interesting model for our understanding of the role of epichromatin. This parasite, like other apicomplexan protozoa, does not break down or reform the nuclear envelope during mitosis, but probably conserves the nuclear envelope–chromatin interaction to organize the chromosomes at interphase and during mitosis. Based on the subcellular localization of the H3K9me3 histone, a marker of heterochromatin, and FISH analysis, it has been observed that the subtelomeric region, as well as other heterochromatic regions of Plasmodium falciparum are anchored at the nuclear periphery (Lopez-Rubio et al. 2009). A similar spatial organization of T. gondii chromosomes has been previously modelled based on the parasite chromodomain 1, a protein that co-immunoprecipitates with the H3K9me3 histone and is positioned in peri-centromeric and other heterochromatic regions (e.g. subtelomeres) along the parasite chromosomes (Gissot et al. 2012). Moreover, Toxoplasma chromodomain 1 is associated with the nuclear membrane, maintained near the centrocone and centrosome during the parasite cell cycle, suggesting a role of this protein in the spatial organization of the nucleus. Unfortunately, the chromosomes do not achieve high-order condensation during mitosis so as to become visible by microscopy. For this reason, the interaction of parasite chromosomes and the nuclear envelope cannot be visualized with precision during replication and daughter cell segregation. However, the PL2-6 label seems to be stronger at one face of the replicating nuclei, out of the nucleus region that is entering the daughter cell. Since here we used a model different from that used by Olins et al. (2011), we could not associate epichromatin as a nucleation site in the chromosome. However, we can hypothesize that, anciently, the main role of epichromatin was to order the chromosomes into the nucleus during DNA replication, while they were contacting the nuclear envelope. Later in evolution, when the nuclear envelope disappeared during mitosis, a new function as a nucleation site could have arisen.
Interestingly, P. falciparum presents a polarization of the nuclear pore complex: in the early multi-nucleated schizont, it clusters in the nucleus region facing the mother plasma membrane, whereas in late stages, when prepared for budding, it clusters towards the cytoplasm of the incipient merozoite (Weiner et al. 2011). These data, along with our results, suggest that the nuclear envelope and chromosomes of apicomplexan parasites show a dynamic re-localization and/or rotation during parasite budding. Moreover, epichromatin polarization seems to persist in mature tachyzoites. Maybe, it could be a consequence of chromosome polarization within the nucleus to generate different chromatin domains. Further analysis should be performed to shed light on this question.
In conclusion, we suggest that epichromatin is conserved in the evolution from simple to high eukaryotes, and that it has a particular location through the T. gondii cell cycle, suggesting a role in chromosome arrangement during parasite replication.
Supplementary Material
Acknowledgments
We would like to thank Agustina Ganuza (Consejo de Investigaciones Científicas, Prov. Buenos Aires) for her valuable help in the nuclear extraction experiment, and Andrés Mariano Alonso for his help in the Morn-1 assay and images processing. The electron microscopy was performed by Lic. Margarita Lopez leading professional. CONICET, Institute of Cell Biology and Neuroscience, “E. De Robertis” LANAIS MIE. Directed by Professor Dr Alicia Brusco. EL Portiansky and SO Angel (Researchers), MC Dalmasso and L. Vanagas (Postdoctoral Fellows) are members of the National Research Council of Argentina (CONICET). The authors thank M. Monestier (Temple University) for the gift of PL2-6.
FINANCIAL SUPPORT
This work was supported by National Institute of Health–National Institute of Allergy and Infectious Diseases (USA) 1R01AI083162-01 (to SOA) and by ANPCyT PICT 0623 (to SOA).
References
- Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiology and Molecular Biology Reviews. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B, Beter H, Gross H. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- Dalmasso MC, Echeverria PC, Zappia MP, Hellman U, Dubremetz JF, Angel SO. Toxoplasma gondii has two lineages of histones 2b (H2B) with different expression profiles. Molecular and Biochemical Parasitology. 2006;148:103–107. doi: 10.1016/j.molbiopara.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dalmasso MC, Onyango DO, Naguleswaran A, Sullivan WJ, Jr, Angel SO. Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family. Journal of Molecular Biology. 2009;392:33–47. doi: 10.1016/j.jmb.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso MC, Sullivan WJ, Jr, Angel SO. Canonical and variant histones of protozoan parasites. Frontiers in Bioscience. 2012;17:2086–2105. doi: 10.2741/3841. [DOI] [PubMed] [Google Scholar]
- Echeverria PC, Matrajt M, Harb OS, Zappia MP, Costas MA, Roos DS, Dubremetz JF, Angel SO. Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. Journal of Molecular Biology. 2005;350:723–734. doi: 10.1016/j.jmb.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Ferguson DJP, Dubremetz JF. Toxoplasma gondii The Model Apicomplexan: Perspectives and Methods. 1. Elsevier; Oxford, UK: 2007. The ultrastructure ofToxoplasma gondii . [Google Scholar]
- Gissot M, Walker R, Delhaye S, Huot L, Hot D, Tomavo S. Toxoplasma gondii chromodomain protein 1 binds to heterochromatin and colocalises with centromeres and telomeres at the nuclear periphery. PLoS ONE. 2012;7:e32671. doi: 10.1371/journal.pone.0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K. Assembly of variant histones into chromatin. Annual Review of Cell and Developmental Biology. 2005;21:133–153. doi: 10.1146/annurev.cellbio.21.012704.133518. [DOI] [PubMed] [Google Scholar]
- Heaslip AT, Dzierszinski F, Stein B, Hu K. TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii. PLoS Pathogens. 2010;6:e1000754. doi: 10.1371/journal.ppat.1000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Mann T, Striepen B, Beckers CJ, Roos DS, Murray JM. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Molecular Biology of the cell. 2002;13:593–606. doi: 10.1091/mbc.01-06-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubio JJ, Mancio-Silva L, Scherf A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host and Microbe. 2009;5:179–190. doi: 10.1016/j.chom.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, Brooks CF, Ferguson DJ, Striepen B, Gubbels MJ. A Toxoplasma MORN1 null mutant undergoes repeated divisions but is defective in basal assembly, apicoplast division and cytokinesis. PLoS ONE. 2010;5:e12302. doi: 10.1371/journal.pone.0012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins AL, Langhans M, Monestier M, Schlotterer A, Robinson DG, Viotti C, Zentgraf H, Zwerger M, Olins DE. An epichromatin epitope: persistence in the cell cycle and conservation in evolution. Nucleus. 2011;2:47–60. doi: 10.4161/nucl.2.1.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Vary C, Markaki Y, Olins AL, Olins D. Phosphatidylserine colocalizes with epichromatin in interphase nuclei and mitotic chromosomes. Nucleus. 2012;3:200–210. doi: 10.4161/nucl.19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepen B, Jordan CN, Reiff S, van Dooren GG. Building the perfect parasite: cell division in apicomplexa. PLoS Pathogens. 2007;3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Dahan-Pasternak N, Shimoni E, Shinder V, von Huth P, Elbaum M, Dzikowski R. 3D nuclear architecture reveals coupled cell cycle dynamics of chromatin and nuclear pores in the malaria parasite Plasmodium falciparum. Cellular Microbiology. 2011;13:967–977. doi: 10.1111/j.1462-5822.2011.01592.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.