Abstract
Purpose of review
This review focuses on the latest data that elucidates the role of the NLRP3 inflammasome in kidney diseases.
Recent findings
The NLRP3 inflammasome is not limited by traditional microbial stimuli of innate immunity and its connection with autophagy, apoptosis, fibrosis, and pro-inflammatory cytokines has broader implications for a variety of kidney diseases. In a wide spectrum of glomerular and tubulointerstitial diseases, the NLRP3 inflammasome is upregulated in both classical immune cells such as infiltrating macrophages and resident dendritic cells as well as in renal tubular epithelial cells, and even podocytes. Inhibition of the NLRP3 inflammasome ameliorates renal injury in a variety of animal models. Interestingly, this extends to models of proteinuria, which suggests that the deleterious effect of albuminuria on the proximal tubular epithelium and podocytes is, in part, mediated by inflammasome activation.
Summary
Recent studies in animal models, and still limited studies in humans, suggest a broad role for inflammasome activation in renal disease. Surprisingly, individual components of the inflammasome, independent of inflammasome activation, may also contribute to progressive renal injury. Additional, studies are needed to define the relative importance of the inflammasome in specific diseases and the therapeutic opportunities afforded by targeting the inflammasome.
Keywords: NLRP3, tubulointerstitial inflammation, glomerulonephritis, proteinuria
INTRODUCTION
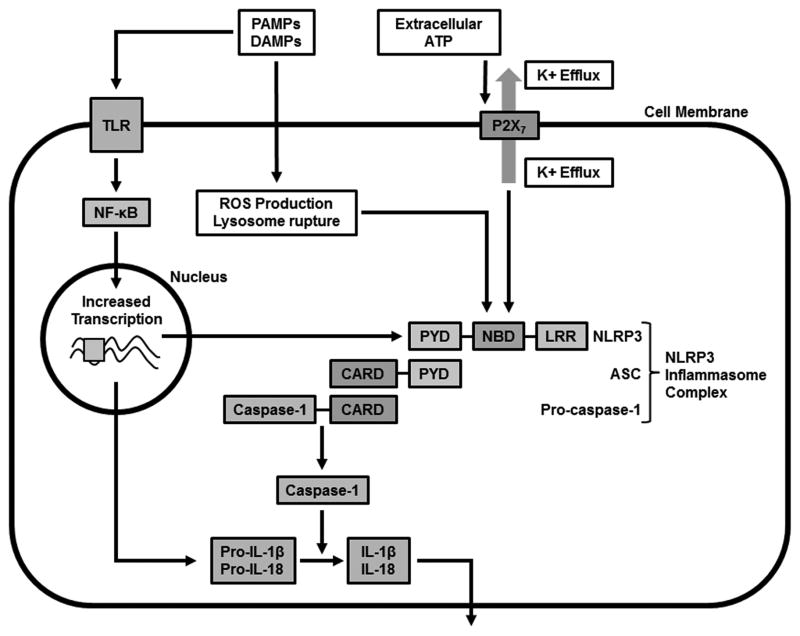
The inflammasome was initially discovered in 2002 as an important component of innate immunity that could be induced by lipopolysaccharide (LPS), which influenced the creation of its name [1]. Additional stimuli of the inflammasome include other microbial-derived molecules or pathogen-associated molecular patterns (PAMPs), such as bacterial toxins and viral nucleic acids. Other non-infectious, host-derived stimuli of the inflammasome or damage-associated molecular patterns (DAMPs) include potassium efflux, excess ATP or reactive oxygen species (ROS), mitochondrial stress, endoplasmic reticulum stress [2*], or cell swelling [3]. Urate crystals, calcium phosphate, amyloid fibrils, silica, or asbestos are also additional triggers of the inflammasome pathway (Figure 1). The inflammasome has been increasingly implicated in autoimmunity, other chronic inflammatory diseases, and even cancer, which broadens the importance of this homeostatic mechanism.
Figure 1. Activation of NLRP3 Inflammasome.
Various microbial PAMPs and endogenous/exogenous DAMPs can activate NLRP3 inflammasome indirectly through different intermediate mechanisms including K+ efflux through the purinergic 2X7 receptor, lysozyme rupture, and ROS production. Assembly of the NLRP3 inflammasome complex leads to activation of caspase-1 which in turn cleaves pro-IL-1β and pro-IL-18 resulting in their extracellular release. PAMPs and DAMPs also activate the NLRP3 inflammasome through TLRs leading to increased transcription via NF-κB. Abbreviations: PAMPs = pattern-associated molecular patterns, DAMPs = damage-associated molecular patterns, NBD = nucleotide-binding domain, LRR = leucine-rich repeat, PYD = pyrin domain, CARD = caspase activation and recruitment domain, NLRP3 = NOD-, LRR- and PYD-containing 3, ROS = reactive oxygen species, TLR = toll-like receptor
The inflammasome is a complex of cytosolic proteins that typically consists of three components: 1) a sensor (NOD-like receptor (NLR) or non-NLR); 2) adapter protein; and 3) caspase 1. The NLR family of sensor molecules consists of NOD-, LRR- and PYD-containing proteins, which includes NLRP1, NLRP3, NLRP6, NLRP7, and NLRP12. They consist of leucine-rich repeat (LRR) domains involved in autoinhibition prior to direct or indirect sensing of signals, nucleotide-binding domain (NBD) involved in inflammasome assembly and either a pyrin domain (PYD) or caspase activation and recruitment domain (CARD) for downstream signaling. The adaptor protein is usually ASC (apoptosis-associated speck-like protein containing a CARD). Some sensor molecules can activate caspase 1 without an adaptor protein. Another newly discovered adaptor, mitochondrial antiviral signaling (MAVS), can interact with NLRP3 [4], but a pathologic role for this protein has not been identified. Upon stimulation by different PAMPs or DAMPs or potassium efflux through the purinergic 2X7 receptor (P2X7R), the inflammasomes activate caspase-1 from its inactive zymogen (pro-caspase-1) by binding to its CARD, either directly or indirectly through ASC. Activated caspase-1 in turn cleaves pro-IL-1β and pro-IL-18 to produce interleukin-1β (IL-1β) and interleukin-18 (IL-18), which are both proinflammatory cytokines (Figure 1) [5**–7]. The complex activating mechanisms of the various inflammasomes has been well reviewed [5**,8].
PAMPs and DAMPs not only activate caspase-1 through the NLRP3 inflammasome, but may also stimulate membrane-bound pattern recognition receptors, such as the Toll-like receptors (TLRs). Signaling of this pathway leads to activation of transcription factor, nuclear factor-κB (NF-κB), resulting in increased transcription of pro-IL-1β, pro-IL-18 and NLRP3 [5**].
The NLRP3 (also known as NALP3 or cryopyrin) inflammasome is implicated in many diseases, including microbial infection, rheumatic diseases, diabetes, and atherosclerosis [6,7]. This review will focus on the role of the NLRP3 inflammasome in kidney diseases, because there is a paucity of literature about the role of the other inflammasomes.
NLRP3 and Tubulointerstitial Disease
Some roles of the inflammasome in kidney disease have been well reviewed in the context of acute kidney injury/chronic kidney disease [9] and kidney inflammation and fibrosis [10]. NLRP3 and ASC can be found in the kidneys of both humans and mice [11], and the renal dendritic cells, infiltrating macrophages and tubules are all known contributors. The contribution of the inflammasome by macrophages is of particular interest given the pathogenic role of macrophages in a wide spectrum of renal diseases [12]. Furthermore, mechanisms operative in macrophages might be applicable to the dendritic cells of the renal mononuclear phagocytic system as these cells appear to be phenotypically and functionally related [13].
The NLRP3 inflammasome would be expected to be activated and amplify renal inflammation during an infection, such as acute pyelonephritis, but there is a surprising absence of studies on the subject. For non-infectious causes of renal inflammation, NLRP3 can also be a critical mediator. Using unilateral ureteral obstruction, Vilaysane et al. observed that NLRP3 deficiency abrogated the magnitude of kidney injury in mice [14]. Furthermore, the upregulation of NLRP3 mRNA was demonstrated in human kidney biopsies from patients with a variety of kidney diseases, including IgA nephropathy, acute tubular necrosis, focal segmental glomerulosclerosis, lupus nephritis, minimal change disease, and hypertensive nephrosclerosis. In another experimental model of tubulointerstitial nephritis, Correa-Costa et al. confirmed important roles for both TLRs and the NLRP3 inflammasome, and the use of allopurinol downregulated the individual components of the inflammasome pathway and diminished this injury [15]. Chen et al. analyzed the renal interstitial inflammation of human diabetic patients and found that increased levels of the P2X4 receptor (another member of the P2X receptor family), NLRP3, IL-1β and IL-18 correlated with the presence of diabetic nephropathy compared to normal controls and these components co-localized in the renal tubules [16*].
In other conditions, such as oxalate nephropathy, direct toxicity of calcium oxalate kills tubular epithelial cells [17,18], which leads to IL-1β production through NLRP3 activation. NLRP-null mice were protected from the harmful effects of oxalate, even though the intrarenal calcium oxalate deposits were present [17,18]. Also, in chronic obstructive conditions, uromodulin leakage into the renal interstitium can stimulate renal dendritic cells to utilize the NLRP3 inflammasome [19].
In the setting of ischemia-reperfusion injury (IRI), Shigeoka et al. [11] demonstrated a protective effect for NLRP3 deficiency, but this finding was surprisingly not observed in the setting of either ASC or caspase 1 deficiency. Therefore, the absence of NLRP3 did not mediate its protective effect by blocking the inflammasome, but decreased apoptosis was observed. The similarities between the inflammasome and apoptosome are striking [5] and some inhibitors of apoptosis protein (IAP) can both positively and negatively influence NLRP3 inflammasome activation [20,21]. Although the precise mechanisms that link the NLRP3 inflammasome to apoptosis remain to be elucidated, the close interconnection of these pathways is a theme that will re-emerge in studies that are discussed later in this review. Kim et al. recently confirmed the protective effect from IRI in NLRP3-knockout mice, but found in contrast that caspase 1 activity was increased in their study [22*]. Given the use of similar experimental methods, the reason for these discrepant findings with the prior study is not clear. This same group also found that NLRP3 was not increased in cisplatin-induced acute kidney injury and NLRP3 deficiency did not protect against this injury, but the investigators suggested that NLRP1 could be more important.
In addition to both infectious and immune-mediated etiologies of tubulointerstitial nephritis, interstitial inflammation is likely important in the pathogenesis of severe acute tubular necrosis (ATN), which is the most common cause of acute kidney injury [23]. If the above disease examples are any guide, then it is likely that the NLRP3 inflammasome could provide a therapeutic target in even ATN.
NLRP3 and Glomerular Disease
Data from murine models of kidney disease implicate the NLRP3 inflammasome in a wide variety of glomerular diseases. In a murine model of anti-glomerular basement membrane disease, Lichtnekert et al. observed that mice that were deficient for IL-1R had decreased levels of IL-1 and were mildly protected from the necrotizing and crescentic glomerular injury when compared with wild-type mice [24]. IL-18 deficiency did not result in the same glomerular protection, but there was a reduction in tubular atrophy. Further investigation of the inflammasome pathway in this model using mice with targeted deletions for NLRP3, ASC or caspase-1 demonstrated no difference in glomerular pathology. Isolated murine glomeruli did not produce pro-IL-1β after LPS stimulation or caspase-1 activation after exposure to ATP, an NLRP3 agonist. Therefore, the harmful effects of IL-1β and IL-18 were produced independently of the inflammasome pathway and the contribution of these cytokines were most likely from the renal dendritic cell network that normally reside throughout the tubulointerstitium along the peritubular capillaries. In contrast, using a rat model of nephrotoxic nephritis with crescentic glomerulonephritis, Deplano et al. found that an antagonist of purinergic 2X7 receptor (P2X7R) could reduce both NLRP3 activation and crescentic glomerular injury [25*]. Although they noted upregulation of NLRP3 in the isolated glomeruli compared to controls, this was determined to be primarily due to infiltrating macrophages.
In murine models of lupus nephritis, several groups have confirmed that the inflammasome plays a key role. Zhao et al. also blocked P2X7R, which reduced the glomerular injury in MRL/lpr mice, decreased anti-dsDNA antibody levels and serum IL-1β and IL-17 by inhibiting NLRP3 activation and subsequent caspase 1 activity [26*]. However, only whole kidney lysates were analyzed, which does not specifically localize the site of inflammasome activity within the kidney or exclude the contribution of circulating macrophages. This group using similar experimental methods previously determined that administration of Bay11-7082, an inhibitor of the phosphorylation of the inhibitor of NF-κB (IκB), attenuated murine lupus nephritis and resulted in reduced macrophage infiltration, NLRP3 inhibition, and improved survival [27]. In the NZB/W F1 murine model of lupus nephritis, an antagonist of Toll-like receptors 7, 8, and 9 reduced glomerular injury and interstitial inflammation with decreased transcription of both IL-1β and NLRP3 in the kidney [28]. In this same murine model, a different group found that epigallocatechin-3-gallate, a major antioxidant in green tea, significantly reduced the kidney injury, which was mediated through decreased ROS production and both marked decreased transcription and protein levels of NLRP3 compared with control mice [29].
Several compounds, such as antroquinonol and osthole, which are found in Chinese herbal medications have demonstrated reduced activation of the NLRP3 inflammasome pathway in a murine model of IgA nephropathy. Yang et al. determined that antroquinonol markedly reduced T cell activation and renal inflammation, which was due in part to decreased ROS generation resulting in less NLRP3 activation [30]. In a separate study using the same murine model, the same research group also observed ROS production in a murine macrophage cell line and cultured murine mesangial cells after LPS treatment, which was inhibited and significantly reduced, respectively, by osthole administration [31].
Surprisingly, within glomeruli, activation of the inflammasome within podocytes might significantly contribute to inflammation and glomerular damage. As demonstrated by Zhang et al., murine podocytes can express all the key components (NLRP3, ASC, and caspase 1) of the inflammasome and its activation contributes to glomerulosclerosis in a model of hyperhomocystinenemia [32**]. The introduction of small interfering RNA for ASC or use of a caspase 1 inhibitor could markedly reduce IL-1β production. This research group subsequently identified both NADPH oxidase [33] and ROS [34] as potential triggers of the NLRP3 inflammasome in podocytes using this same model system. As further confirmation, another research group using a murine model of metabolic syndrome that results in renal inflammation after a high-fat diet demonstrated that deletion of the P2X7 receptor attenuated kidney injury [35*]. Increased NLRP3 was detected in murine cultured podocytes, and human kidneys with mild diabetic nephropathy revealed immunohistochemical staining for NLRP3 and P2X7R in proximal and distal tubules and occasional cells that appeared to be in the location of podocytes [35]. This represents only the second study of the NLRP3 inflammasome in human kidney tissue sections by immunohistochemistry [35]. In light of recent evidence demonstrating the antigen presenting capacity of murine podocytes [36], podocytes appear to be functionally similar to renal dendritic cells and kidney-infiltrating macrophages in their abilities to contribute to both adaptive immunity and inflammation.
Interstitial inflammation is an important complication of most glomerular diseases and specifically an independent predictor of disease progression in IgA nephropathy [37] and lupus nephritis [38]. Glomerular cells (mesangial or podocytes) have the capacity to activate the NLRP3 inflammasome and could possibly initiate inflammatory pathways that secondarily involve the tubulointerstitium. Alternatively, tubulointerstitial injury, either secondary to glomerular injury and downstream ischemia, or as a primary pathogenic process could initiate local NLRP3 inflammasome activation. This could occur either in cells resident in the kidney (e.g., tubulointerstitial and dendritic cells) and/or after inflammatory cells have infiltrated. Future studies will be needed to elucidate the interrelationships between glomerular and tubulointerstitial inflammation and inflammasome activation.
NLRP3 and Proteinuria
Proteinuria is an independent risk factor of disease progression in numerous kidney diseases [39], but the underlying mechanism for this harmful effect on the kidney remains poorly understood. Several recent studies implicate the inflammasome in this injury process.
Erkan et al. previously established that albumin exposure alone caused apoptosis in cultured human proximal tubular epithelial cells [40]. Building upon this prior study, Nishi and colleagues studied the role of the inflammasome in the setting of proteinuria with an interest in the potential protective impact of estrogen [41**]. There was moderate tubulointerstitial inflammation and fibrosis in their murine model of nephrotic syndrome which was also mediated through inflammasome activation, as indicated by the presence of elevated mRNA and protein levels for NLRP3, ASC and pro-caspase 1/caspase 1. Interestingly, the inflammasome pathway was dampened by administration of raloxifene in ovariectomized female mice, which is a selective estrogen receptor modulator that is used for hormone replacement therapy. This protective effect may account in part for the epidemiologic data that the male gender is at greater risk for developing chronic kidney disease [42]. However, the beneficial effect of raloxifene was only observed in the tubulointerstitium and the glomerular injury did not improve, and only partial inhibition of the inflammasome was achieved, so these data cannot resolve whether albumin-induced injury of the proximal tubular cells is only mediated through the NLRP3 inflammasome. A second recent study also confirmed the activation of the inflammasome in renal tubules in the setting of proteinuria. Fang et al demonstrated that the severity of proteinuria correlated with protein expression of caspase 1, IL-1β and IL-18 in both proximal and distal tubules of human kidney biopsies regardless of the underlying disease (IgA nephropathy, focal segmental glomerulosclerosis, minimal change disease or membranous nephropathy), [2*]. These investigators also found that GRP78, a heat shock protein localized in the endoplasmic reticulum, was upregulated after bovine serum albumin exposure, and administration of ursodeoxycholic acid blunted this effect in both cultured tubular epithelial cells and a streptozotocin-induced model of diabetic nephropathy. Therefore, the authors suggest that endocytosis of albumin by tubules might activate the inflammasome by inducing endoplasmic reticulum stress.
In addition to tubules, albuminuria might induce the inflammasome in podocytes. Okamura et al. demonstrated that endocytosis of albumin into human podocytes derived from urine samples can result in increased IL-1β and TNF RNA expression [43**]. The other components of the inflammasome pathway, such as NLRP3, ASC, and caspase 1, were not analyzed. Therefore, the possibility of caspase 1-mediated pyroptosis, which has been postulated by others [10], was not specifically studied, but IL-1β is cleaved by caspase 1 from its proenzyme form and represents a surrogate marker of this pathway. Of note, an increase of caspase 3 and 7 activity and TUNEL-positive podocytes was noted, which suggested that apoptosis resulted from albumin endocytosis. These data were confirmed in a murine albumin overload model and provide an intriguing mechanism for the harmful effects of proteinuria. However, future studies are needed to determine the broader applicability of these findings to other causes of severe proteinuria or nephrotic syndrome.
The harmful effects of proteinuria on the kidney are not exclusively mediated via the canonical inflammasome pathway. Wang et al. recently discovered that NLRP3 augments TGF-β in kidney epithelium [44**], which occurs independently of the inflammasome. The precise mechanism remains unknown, but TGF-β has a central role in renal fibrosis [45] and can also induce apoptosis of tubular epithelial cells and podocytes [46]. This additional line of evidence further broadens the influence of NLRP3 with a pro-fibrotic effect in addition to its well-established pro-inflammatory role.
Sequential human kidney biopsies suggest that minimal change disease can transform to focal segmental glomerulosclerosis (FSGS). Current knowledge of the inflammasome suggests a possible mechanism. The presence of proteinuria by itself is predicted to activate the inflammasome and result in podocyte apoptosis. If the podocyte loss outpaces its replenishment rate and exceeds 20%, then FSGS can ensue [47]. In addition, if sufficient apoptosis, inflammation, and fibrosis resulted from inflammasome activation as previously delineated within the tubulointerstitial compartment, the formation of aglomerular tubules and atubular glomeruli might also contribute to a segmental sclerotic glomerular injury [48].
In contrast to FSGS, glomerulonephritis that is primarily mediated by immune complexes and/or inflammation may have a greater contribution of the inflammasome by infiltrating macrophages or local renal dendritic cells with minimal or no podocyte contribution of the inflammasome. Further studies will be needed to determine whether the degree of inflammasome activation by glomerular cells and specifically podocytes may be the distinguishing feature between nephrotic and nephritic glomerular diseases.
Conclusion
The inflammasome is gaining recognition for its role in both tubulointerstitial and glomerular diseases. Understanding the different ways in which the NLRP3 inflammasome contributes to glomerular diseases characterized by proteinuria, glomerulonephritis and tubulointerstitial injury will provide additional insights to kidney disease mechanisms and identify potential therapeutic targets.
Key Points.
Animal and human data demonstrate activation of the NLRP3 inflammasome in a variety of glomerular and tubulointerstitial diseases.
The deleterious effect of proteinuria on the proximal tubular epithelium and podocytes may be primarily mediated through the NLRP3 inflammasome.
Inhibition of the inflammasome may be a promising therapeutic target in kidney diseases.
Acknowledgments
Anthony Chang currently has grant funding from the Roche Organ Transplantation Research Foundation. Kichul Ko is funded by an Arthritis Foundation Clinical to Research Transition award. Marcus Clark currently has grant funding from the National Institutes of Health (AI082724 and AR055646), Abbvie, and MedImmune, LLC.
Footnotes
No conflicts of interest.
Reference section
- 1.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- *2.Fang L, Xie D, Wu X, et al. Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PloS One. 2013;8:e72344. doi: 10.1371/journal.pone.0072344. This study further confirms the notion that proteinuria can negatively affect renal tubules by activating the inflammasome and induction of pro-inflammatory cytokines which correlates with the severity of proteinuria in human kidney biopsies. Reduction of endoplasmic reticulum stress and inflammasome activation in a murine model of diabetic nephropathy attenuated the kidney injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compan V, Baroja-Mazo A, López-Castejón G, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian N, Natarajan K, Clatworthy MR, et al. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **5.Wen H, Miao EA, Ting JP-Y. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. This is a very detailed and comprehensive review of the myriad mechanisms and molecular interactions that are involved with the activation of the inflammasome. There are also excellent figures that illustrate the complex canonical and non-canonical inflammasome activation pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 7.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigue-Gervais IG, Saleh M. Caspases and immunity in a deadly grip. Trends Immunol. 2013;34:41–49. doi: 10.1016/j.it.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Anders H-J, Muruve DA. The Inflammasomes in Kidney Disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz G, Darisipudi MN, Anders H-J. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 11.Shigeoka AA, Mueller JL, Kambo A, et al. An Inflammasome-Independent Role for Epithelial-Expressed Nlrp3 in Renal Ischemia-Reperfusion Injury. J Immunol. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Q, Wang Y, Harris DCH. Pathogenic and protective role of macrophages in kidney disease. Am J Physiol Renal Physiol. 2013;305:F3–11. doi: 10.1152/ajprenal.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson PJ, Rees AJ, Griffin MD, et al. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilaysane A, Chun J, Seamone ME, et al. The NLRP3 Inflammasome Promotes Renal Inflammation and Contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa-Costa M, Braga TT, Semedo P, et al. Pivotal role of Toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PloS One. 2011;6:e29004. doi: 10.1371/journal.pone.0029004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Chen K, Zhang J, Zhang W, et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45:932–943. doi: 10.1016/j.biocel.2013.02.009. Renal interstitial inflammation and increased levels of the P2X4 receptor, NLRP3, IL-1β and IL-18 correlated with the presence of diabetic nephropathy in human patients. This is the first study to localize the inflammasome in human kidney tissue sections using immunofluorescence and immunohistochemistry. [DOI] [PubMed] [Google Scholar]
- 17.Mulay SR, Kulkarni OP, Rupanagudi KV, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauf F, Asplin JR, Granja I, et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darisipudi MN, Thomasova D, Mulay SR, et al. Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol. 2012;23:1783–1789. doi: 10.1681/ASN.2012040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labbé K, McIntire CR, Doiron K, et al. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Vince JE, Wong WW-L, Gentle I, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- *22.Kim H-J, Lee DW, Ravichandran K, et al. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther. 2013;346:465–472. doi: 10.1124/jpet.113.205732. NLRP3-knockout mice were protected from ischemia-reperfusion injury, and caspase 1 activity was increased. However, NLRP3 was not increased in cisplatin-induced acute kidney injury and NLRP3 deficiency did not protect against this injury, but NLRP1 may be important. This is the first study to suggest a role for NLRP1 in any kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lameire N. The pathophysiology of acute renal failure. Crit Care Clin. 2005;21:197–210. doi: 10.1016/j.ccc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lichtnekert J, Kulkarni OP, Mulay SR, et al. Anti-GBM glomerulonephritis involves IL-1 but is independent of NLRP3/ASC inflammasome-mediated activation of caspase-1. PloS One. 2011;6:e26778. doi: 10.1371/journal.pone.0026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Deplano S, Cook HT, Russell R, et al. P2X7 receptor-mediated Nlrp3-inflammasome activation is a genetic determinant of macrophage-dependent crescentic glomerulonephritis. J Leukoc Biol. 2013;93:127–134. doi: 10.1189/jlb.0612284. Inhibition of P2X7R in this rat model of crescentic glomerulonephritis reduced the NLRP3 inflammasome activation and crescentic glomerular injury, and this contribution was primarily attributed to infiltrating macrophages. [DOI] [PubMed] [Google Scholar]
- *26.Zhao J, Wang H, Dai C, et al. P2X7 Blockade Attenuates Murine Lupus Nephritis by Inhibiting Activation of the NLRP3/ASC/Caspase 1 Pathway. Arthritis Rheum. 2013;65:3176–3185. doi: 10.1002/art.38174. Inhibition of P2X7R in murine lupus nephritis reduces glomerular injury, anti-dsDNA antibody levels, serum IL-1β and IL-17 levels through the inhibition of NLRP3 activation and subsequent caspase 1 activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Zhang H, Huang Y, et al. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Int Immunopharmacol. 2013;17:116–122. doi: 10.1016/j.intimp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Zhu F-G, Jiang W, Bhagat L, et al. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity. 2013;46:419–428. doi: 10.3109/08916934.2013.798651. [DOI] [PubMed] [Google Scholar]
- 29.Tsai P-Y, Ka S-M, Chang J-M, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51:744–754. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Yang S-M, Ka S-M, Hua K-F, et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic Biol Med. 2013;61C:285–297. doi: 10.1016/j.freeradbiomed.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Hua K-F, Yang S-M, Kao T-Y, et al. Osthole Mitigates Progressive IgA Nephropathy by Inhibiting Reactive Oxygen Species Generation and NF-κB/NLRP3 Pathway. PloS One. 2013;8:e77794. doi: 10.1371/journal.pone.0077794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Zhang C, Boini KM, Xia M, et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688. This is the first study to demonstrate the ability of murine podocytes to express NLRP3, ASC and caspase 1 due to activation of the inflammasome by hyperhomocystinenemia and inhibition reduced IL-1β production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abais JM, Zhang C, Xia M, et al. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abais JM, Xia M, Li G, et al. Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Solini A, Menini S, Rossi C, et al. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol. 2013;231:342–353. doi: 10.1002/path.4237. This is the third study to detect NLRP3 in podocytes, which was found in both murine cultured and human kidney tissue sections from diabetic nephropathy patients. [DOI] [PubMed] [Google Scholar]
- 36.Goldwich A, Burkard M, Olke M, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol. 2013;24:906–916. doi: 10.1681/ASN.2012020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts ISD, Cook HT, Troyanov S, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh C, Chang A, Brandt D, et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 2011;63:865–874. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49:800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 40.Erkan E, Devarajan P, Schwartz GJ. Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J Am Soc Nephrol. 2007;18:1199–1208. doi: 10.1681/ASN.2006040407. [DOI] [PubMed] [Google Scholar]
- **41.Nishi Y, Satoh M, Nagasu H, et al. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int. 2013;83:662–673. doi: 10.1038/ki.2012.475. This is the first study to identify an important contribution of the inflammasome in a murine model of proteinuria-induced tubular injury and cultured human proximal tubular epithelial cells. This injury may have been mediated in part through pyroptosis, which provides an intriguing mechanism for the harmful effect of proteinuria on renal tubules. An interesting aspect was the beneficial effect of raloxifene, a selective estrogen receptor modulator, in attenuating the tubulointerstitial but not glomerular injury, which may have been mediated through decreased pyroptosis and mitochondrial oxidative stress (a recognized stimulus of the inflammasome) [DOI] [PubMed] [Google Scholar]
- 42.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5 (Suppl A):S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- **43.Okamura K, Dummer P, Kopp J, et al. Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PloS One. 2013;8:e54817. doi: 10.1371/journal.pone.0054817. This is the first study to demonstrate increased IL-1β and TNF RNA expression in human podocytes from urine samples after albumin endocytosis. The NLRP3 inflammasome pathway was not specifically studied and may not be involved in this injury, but increased apoptosis was observed, which provides a novel mechanism for the harmful effects of proteinuria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Wang W, Wang X, Chun J, et al. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J Immunol. 2013;190:1239–1249. doi: 10.4049/jimmunol.1201959. This is the first study to demonstrate that NLRP3 has a direct role in TGF-β signaling in kidney epithelial cells, which is independent of the actions of caspase 1, IL-1β and IL-18. The precise mechanism remains to be elucidated, but this new pro-fibrotic role of NLRP3 in conjunction with its well established pro-inflammatory actions further supports the important contribution of NLRP3 to tubulointerstitial injury. [DOI] [PubMed] [Google Scholar]
- 45.Gewin L, Zent R. How does TGF-β mediate tubulointerstitial fibrosis? Semin Nephrol. 2012;32:228–235. doi: 10.1016/j.semnephrol.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 48.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]