Abstract
Background
Patients with advanced biliary tract cancers have limited therapeutic options. Preclinical data suggest proteasome inhibition may be an effective therapeutic strategy. We thus evaluated the clinical efficacy of bortezomib in advanced biliary tract cancers.
Patients and Methods
Patients with locally advanced or metastatic cholangiocarcinoma or gallbladder adenocarcinoma who had received 0–2 prior therapies received bortezomib 1.3 mg/m2 days 1, 4, 8, and 11 of a 21-day cycle. The primary endpoint was objective response rate. A Simon two-stage design was employed (null response rate of < 5% and response rate of ≥ 20% of interest).
Results
Twenty patients enrolled: bile duct/gallbladder cancer (14/6), prior treatments 0/1/2 (10/6/3). The trial was discontinued early due to lack of confirmed partial responses. No unanticipated adverse events were noted. There was one unconfirmed partial response. Ten patients achieved stable disease as best response. Median time to progression was 5.8 months (95% CI 0.7–77.6 months). Median survival was 9 months (95% CI 4.6–18.5 months). The 6-month and 1-year survival rates were 70% and 38%. There was no difference in survival based on primary disease site.
Conclusions
Single agent bortezomib does not result in objective responses in biliary tract cancers. However, the rate of stable disease and time to progression benchmark is encouraging. Further development of bortezomib in combination with other therapies in this disease setting should be considered.
Keywords: bortezomib, biliary tract cancers, proteosome inhibition, cholangiocarcinoma
INTRODUCTION
Biliary tract cancers, including tumors of the gallbladder and bile ducts, are relatively uncommon in the United States. However, the incidence of intrahepatic cholangiocarcinomas is rising and is now among the top ten causes of cancer death in both men and women 1. Although surgical resection is the only curative therapy, most patients present with locally advanced or metastatic disease with limited systemic treatment options 2. Two year survival rates for metastatic disease in gallbladder and extrahepatic cholangiocarcinoma are 2% and 4%, respectively, while survival rates for intrahepatic cholangiocarcinoma are minimally better 2–4.
At the time this trial was conceived, no randomized trial had demonstrated a survival benefit for systemic therapy. Fluoropyrimidine-based therapies produced response rates ranging from 9% to 34% in small single arm phase II trials, with median survival times ranging from 21 weeks to 12 months 5–7. Single agent gemcitabine produced slightly better results, with response rates of 17.5% to 36% and median survival times of approximately 7.5 months 8,9. Single agent phase II trials of other cytotoxic agents were similarly disappointing, with median survival times of less than one year 10,11. Thus, development of novel agents and targets was, and remains, urgently needed in these tumors.
One such target is the ubiquitin-proteasome pathway. Inhibition of this pathway results in cell death via sensitization of cells to apoptosis. This may be accomplished through inhibition of nuclear factor kappa B (NFκB), increased activity of p53 and Bax proteins, accumulation of cyclin-dependent kinase inhibitors p27 and p21, increased c-myc and bcl-2 expression, and upregulation of heat shock proteins and caspase-mediated apoptotic pathways 12,13. Alterations in expression of many of these (p53, p27, bcl-2, NFκB) have been described in biliary tract cancers 14–20. Further, preclinical data in cholangiocarcinoma cell lines suggested proteasome inhibition was a possible therapeutic strategy via induction of endoplasmic reticulum dysfunction and caspase-independent cell death 21–23.
Bortezomib (Millenium Pharmaceuticals) is a dipeptide boronic acid and selective inhibitor of the 26S proteasome 12. Although bortezomib has broad spectrum activity against a range of tumor types in preclinical models and in breast and lung xenografts, bortezomib is currently only approved for the treatment of multiple myeloma and mantle cell lymphoma 12,24,25. Bortezomib is well-tolerated in patients with solid tumors and has a predictable side effect profile 25,26. Given the tolerability of bortezomib in solid tumors and the preclinical data suggesting a therapeutic benefit for proteasome inhibition in biliary tract cancers 21–23,26, this single arm phase II trial of bortezomib in unresectable or metastatic adenocarcinoma of the bile duct or gallbladder was undertaken.
PATIENTS AND METHODS
Study Population
Eligible patients were age 18 years or older with histologically confirmed adenocarcinoma of the intrahepatic or extrahepatic bile ducts or gallbladder that was not amenable to surgical resection for cure. All patients were required to have measurable disease and provided informed consent. The initial protocol stipulated that patients could not have received prior chemotherapy for metastatic or locally advanced disease. However, as evolving evidence suggested a benefit to chemotherapy in this setting, the protocol was amended to allow up to two prior chemotherapy regimens 27–31. Chemotherapy used as a radiosensitizer was not considered a prior treatment regimen.
Key additional inclusion criteria included an Eastern Cooperative Oncology Group performance status of 0–2 and a total bilirubin up to 1.5 times the upper limit of normal (ULN), AST/ALT up to 2.5 times the ULN, and adequate hematologic and renal function. Exclusion criteria included known brain metastases, preexisting peripheral neuropathy of grade 2 or higher, known history of allergic reaction to bortezomib, history of immunodeficiency or uncontrolled intercurrent illness, or the receipt of more than two prior chemotherapy regimens for metastatic or locally advanced disease. The study was approved by the Institutional Review Board of Fox Chase Cancer Center and all patients provided written informed consent.
Study Design
This was a single arm, two-stage, open-label study. Bortezomib was administered at a dose of 1.3 mg/m2 on days 1, 4, 8 and 11 of a 21-day cycle. Bortezomib was given as an intravenous bolus over 3–5 seconds. Treatment cycles were initiated on Mondays, Wednesdays, or Fridays to avoid treatment days falling on a weekend. Treatment was continued until disease progression, intercurrent illness precluding further dosing, unacceptable adverse events, patient withdrawal of consent, death, or changes in patient condition that rendered the patient unacceptable for further treatment in the judgment of the investigator. Toxicity was graded according to NCI Common Toxicity Criteria version 3.0 32. Dose modifications were made for ≥ grade 3 non-hematologic toxicity, excluding neuropathy; grade 1 neuropathy with pain or grade ≥ 2 neuropathy without pain; and hematologic toxicity (neutrophil count ≤ 750/mm3 or platelet count ≤ 75,000/mm3). Dose reduction levels were 1 mg/m2 and 0.7 mg/m2 per dose. Patients requiring more than two dose reductions were removed from the study.
Study Evaluations
All patients were assessed for tumor response every 2 cycles, or 6 weeks, by radiologic assessment (computed tomography or magnetic resonance imaging) according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 33. Confirmatory scans were obtained at least four weeks following initial documentation of objective response. All patients who received at least one dose of bortezomib were considered evaluable for toxicity and response assessment.
Study Endpoints and Statistical Considerations
The primary endpoint was objective response rate (ORR) based on investigator assessment. Secondary endpoints included time to progression and overall survival (OS). The study design required 35 patients for 90% power to detect a response rate of at least 20% with a type I error of at least 0.05. Response rate was defined as the percent of patients who achieved a complete or partial response confirmed ≥ 28 days after initial documentation of response. An early stopping point of 20 patients based on efficacy was employed. If one or fewer of the first 20 patients enrolled had a complete or partial response to bortezomib, the trial would be terminated. Otherwise the study would continue to a total accrual of 35 patients.
Time to progression was defined as the time from initiation of therapy to first progressive disease. Overall survival was defined as the time from initiation of therapy to death or last follow-up. In an exploratory analysis done after study completion, the clinical benefit rate was also calculated, defined as the total number of patients who achieved a complete or partial response, unconfirmed response, or stable disease. Safety was evaluated in all patients who received at least one dose of bortezomib. The clinical benefit was compared between patients with and without prior therapy via Fisher’s exact test. Survival analysis was assessed by Kaplan Meier estimation method and log rank test.
RESULTS
Between February 2004 and August 2008, twenty patients were enrolled. The median age was 62 years. Patient characteristics are listed in Table 1. The majority of patients had adenocarcinoma of the bile duct (cholangiocarcinoma) and had not received prior therapy for metastatic disease. Sixteen study participants presented with metastatic disease at baseline, while four patients were initially diagnosed with locally advanced disease and developed recurrence. These four patients had gallbladder carcinoma and underwent cholecystectomy and fluoropyrimidine-based chemoradiotherapy in the curative setting. Fourteen patients had metastatic disease to liver.
Table 1.
Patient Characteristics
| Demographic or Clinical Characteristics | Treatment Study Population (N=20) N (%) |
|---|---|
|
| |
| Age | |
| Median | 62 |
| Range | 41–79 |
|
| |
| Gender | |
| Male | 11 (55%) |
| Female | 9 (45%) |
|
| |
| Site of Primary Tumor | |
| Gallbladder | 6 (30%) |
| Cholangiocarcinoma (Intrahepatic or Extrahepatic) | 14 (70%) |
|
| |
| Site of Metastases | |
| Liver | 14 (70%) |
| Lymph Nodes | 7 (35%) |
| Lung | 5 (25%) |
| Other | 2 (10%) |
|
| |
| ECOG PS | |
| 0 | 7 (35%) |
| 1 | 13 (65%) |
|
| |
| Prior Treatment | |
| None | 10 (50%) |
| Chemotherapy Only | 4 (20%) |
| Chemotherapy/Radiation | 5 (25%) |
| Other | 1 (5%) |
|
| |
| Prior Surgery | |
| Yes | 5 (25%) |
| Cholecystectomy | 4 (20%) |
| Liver Resection | 1 (5%) |
| No | 15 (70%) |
The median number of cycles received was three, with a range of one to nine. Accrual was halted after twenty patients were enrolled and treated based on the predefined early stopping rule. Median follow up was 9.6 months (range 1.3 to 77.6 months).
Toxicity
Table 2 summarizes adverse events experienced by all patients. The most common non-hematologic toxicities observed were fatigue (18 patients), nausea (11 patients), and vomiting (7 patients). Neuropathy was reported in six patients, with one patient having grade 3 neuropathy. The most common hematologic toxicities observed were anemia (15 patients), thrombocytopenia (14 patients), and leukopenia (5 patients). Grade 3/4 hematologic and non-hematologic toxicities were rare and consistent with the adverse event profile of bortezomib 25. Eight serious adverse events were reported, four of which were felt to be at least possibly related to bortezomib and included grade 3 aseptic vasculitis in one patient, grade 4 cerebrovascular accident in one patient, grade 2 meningitis in one patient, and grade 2 renal insufficiency in one patient. Five patients discontinued treatment due to adverse events, all with at least stable disease at first disease evaluation (6 weeks). Dose reductions were required in seven patients. Fatigue (n=3) was the most common reason for dose reduction, followed by thrombocytopenia (n=2) and neuropathy (n=1).
Table 2.
Adverse events by highest grade toxicity experienced per patient
| Toxicity | All Grade Toxicity N (%) | Grade 3/4 Toxicity N (%) |
|---|---|---|
| Hematologic | ||
| Anemia | 15 (75%) | 2 (10%) |
| Leukopenia | 5 (25%) | 0 |
| Neutropenia | 5 (25%) | 1 (5%) |
| Thrombocytopenia | 14 (70%) | 3 (15%) |
| Non-Hematologic | ||
| Dehydration | 3 (15%) | 2 (10%) |
| Dizziness | 4 (20%) | 0 |
| Edema (all sites) | 5 (25%) | 0 |
| Elevated Alkaline Phosphatase | 3 (15%) | 0 |
| Elevated AST/ALT | 7 (35%) | 0 |
| Elevated creatinine | 3 (15%) | 0 |
| Fatigue | 18 (90%) | 4 (20%) |
| GI obstruction | 0 | 2 (10%) |
| Hyperbilirubinemia | 4 (20%) | 3 (15%) |
| Hypertension | 4 (20%) | 0 |
| Nausea | 11 (55%) | 2 (10%) |
| Pain (all sites) | 9 (45%) | 2 (10%) |
| Rash/Ulceration | 4 (20%) | 0 |
| Sensory Neuropathy | 6 (30%) | 1 (5%) |
| Vomiting | 7 (35%) | 2 (10%) |
Clinical Efficacy
One patient withdrew consent prior to the first disease evaluation. Of the remaining nineteen patients, one patient with intrahepatic cholangiocarcinoma achieved an unconfirmed partial response in thoracic lymph node metastases, but withdrew consent prior to confirmation of response. Ten patients (two with gallbladder carcinoma and eight with cholangiocarcinoma) achieved stable disease as best response. The median duration of stable disease was 11.3 weeks (range 3 weeks to 18 weeks). Thus, the clinical benefit rate was 53%. No confirmed partial or complete responses were observed. There was no difference in clinical benefit rate between patients who had and had not received prior therapy (21% versus 32% p=0.66). The median time to progression for the entire study population was 5.8 months (95% CI 0.7–77.6 months).
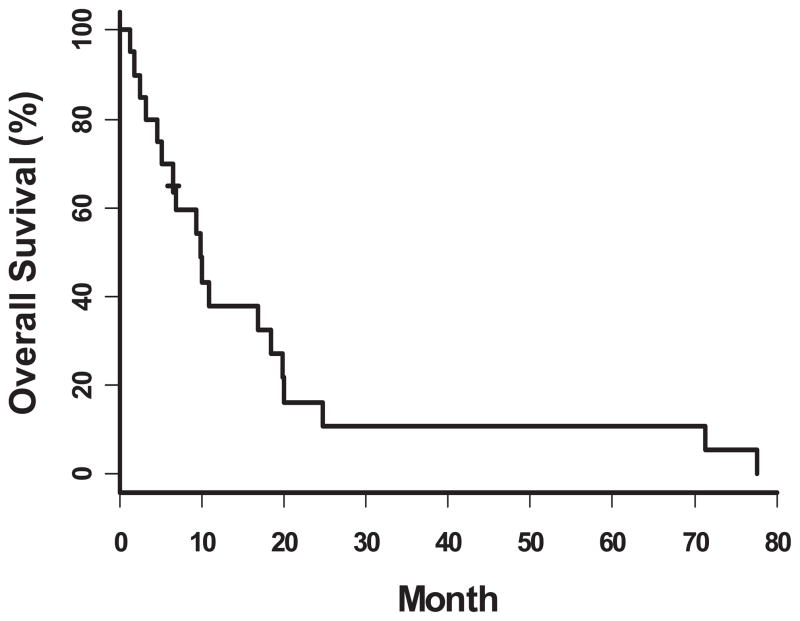
The median survival for the entire study population was 9 months (95% CI 4.6–18.5 months) (Figure 1). The 6-month and 1-year survival rates for the entire study population were 70% and 38%, respectively. Patients who had a clinical benefit had a 6 month and 1 year survival of 90% and 69%, respectively, as compared to patients whose best response was progressive disease (6 month and 1 year survival of 44% and 11%, respectively, p=0.01). There was no difference in survival at 6 months and 1 year based on primary disease site (gallbladder versus bile duct, p=0.63)
Figure 1.
Overall Survival for all patients treated with bortezomib
DISCUSSION
Our results demonstrate that objective responses with single agent bortezomib treatment are rare in biliary tract cancers. Since we did not observe a confirmed response, our pre-defined stopping rule triggered early discontinuation of this trial. However, our clinical benefit rate of 53%, time to progression of 5.8 months, and overall survival of 9 months are encouraging and compare favorably to prior studies of single agents such as 5-fluorouracil or gemcitabine in advanced biliary cancer. Previous studies of 5-fluorouracil with leucovorin in this disease have demonstrated a median survival of 6 months, while single agent gemcitabine trials have reported median survivals ranging from 6 to 11.5 months with varying doses and schedules. Time to progression was also similar between our results and those reported in other single agent studies 8,9,34–36.
While the low median number of cycles relative to prolonged time to progression may define a patient population with indolent disease, our results are intriguing given that half of the patients enrolled had received prior therapy. However, of those patients whose best response was stable disease, only three had received prior systemic therapy including gemcitabine and/or fluoropyrimidines and one patient had undergone prior chemoembolization. In addition, many of the patients with stable disease had liver-only or liver-dominant metastases with some patients’ metastases limited to regional intraabdominal lymph nodes. Intrahepatic disease is thought to be a good prognostic factor in resectable disease, and this may apply to our population as well 37. Additional prognostic factors from surgical series of resected cholangiocarcinomas include tumor differentiation, peripheral versus central location, and gross subtype (mass forming, intraductal growth, or periductal infiltrating) 37,38. We were unable to further analyze this in our study as most patients did not have surgical resection. Limited data exist in the literature regarding prognostic features in metastatic cholangiocarcinoma.
While these results may indicate that patient selection played a role in the high rate of stable disease, the clinical benefit rate suggests that proteasome inhibition may have some role in the management of this disease by delaying time to progression. The toxicity profile of bortezomib in this population was similar to other studies evaluating this agent in a variety of disease sites 25,26. Given that many of these patients had liver involvement with tumor with potential loss of functional hepatic parenchyma, it is worth noting that bortezomib was well tolerated in this population.
Since the initiation of this study, combination systemic therapy has proven effective in advanced biliary tract cancers and is associated with a survival benefit. Based on these data, combination therapy in the front-line setting is now recommended based on level one evidence for appropriate patients with unresectable or metastatic disease 39. The combination of cisplatin and gemcitabine has been established as a standard of care for advanced disease based on the results of a large randomized phase III study demonstrating an improvement in overall survival from 8.1 months to 11.7 months (p<0.001) with the combination compared to gemcitabine alone 40. Other combination chemotherapy regimens have also demonstrated promising antitumor activity. Recent phase II trials of gemcitabine and oxaliplatin have produced response rates of 35% to 50%, and overall survival times of up to 18 months in the front-line setting 28,31. A pooled analysis of 104 chemotherapy trials in advanced biliary tract cancers further suggested that gemcitabine may be the most active agent in these diseases, with the addition of platinum agents to gemcitabine resulting in higher response rates and tumor control rates 41. Combination therapy with gemcitabine and fluoropyrimidines has also demonstrated activity in the front-line setting. The addition of 5-fluorouracil to gemcitabine resulted in a response rate of 9.5% and a median overall survival of 9.7 months 27. The combination of gemcitabine and capecitabine may be more active, with response rates of slightly over 30%, a clinical benefit rate of over 60%, and a median overall survival of 14 months 29,30. Thus, combination chemotherapy has been established as a standard of care in advanced biliary cancers.
Given the increasing role of chemotherapy in biliary tract cancers, further combination studies with bortezomib should be considered. Preclinical data suggest that bortezomib enhances the activity of multiple chemotherapy agents including gemcitabine, 5-fluorouracil, and irinotecan, providing at least additive cell killing 24,42–45. This may be due to downregulation of NFkB, a known candidate mediator of chemotherapy resistance 45. This principle is evident in the multiple myeloma setting, where recent studies have consistently demonstrated improved response rates, longer progression-free survival times, and better survival with the addition of bortezomib to traditional therapeutic regimens 46–50. Further preclinical work to define the best cytotoxic combinations is clearly needed.
The limitations of our study include the nonrandomized, single arm trial design, the small sample size, and the inclusion of a heterogeneous patient population. The population was not uniform in treatment history, with some patients being treatment-naïve and others having received prior therapy for their advanced disease. However, the primary endpoint of our study was response rate. This is an endpoint which may be less affected by patient heterogeneity.
In contrast, the observation of prolonged time to progression and the high rate of stable disease despite a low number of treatment cycles received may be due to inherent biology of the disease, with some patients potentially harboring a biologically indolent disease. While a formal study of predictive and prognostic markers was planned at study inception, the majority of patients enrolled did not have surgical speciments nor biliary fluid available for analysis. In future evaluations of bortezomib for biliary tract cancers, correlative studies attempting to better characterize the clinicopathologic factors and biomarkers within tumors of those patients who experience clinical benefit from therapy and those that do not would be worthwhile.
CONCLUSION
Our data demonstrate that single agent bortezomib has minimal clinical activity as defined by tumor response in adenocarcinomas of the biliary tract. However, the rate of stable disease, TTP, and OS are comparable to other studies of single agents in this disease. Currently, combination chemotherapy with gemcitabine and cisplatin is the standard of care for biliary tract cancer. Given preclinical evidence of chemotherapy potentiation, future study of bortezomib with cytotoxic agents known to be active in these diseases is warranted.
CLINICAL PRACTICE POINTS.
Combination therapy is recommended in the front-line setting for the treatment of advanced biliary cancers. Cisplatin and gemcitabine are a standard treatment option, with a known survival benefit over single agent gemcitabine.
Therapeutic options are limited for those unable to receive platinum-based combination therapy or in patients who have failed front-line combination therapy.
Bortezomib does not result in antitumor responses as a single agent in advanced biliary tract cancers.
Investigation of bortezomib in combination with other active therapies for advanced biliary tract cancers is warranted.
Acknowledgments
Funding source: This study was supported by NCI P30 CA006927 and the Cancer Therapy Evaluation Program (NCI # 6135). Clinicaltrials.gov Identifier: NCT00085410. Registered June 10, 2004.
This study was supported by the National Cancer Institute P30 CA006927 (PI: Fisher) and the Cancer Therapy Evaluation Program NCI # 6135 (PI: Cohen). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Manufacturer: Millennium Pharmaceuticals
Footnotes
FINANCIAL DISCLOSURES/CONFLICTS OF INTEREST
Conflict of Interest statement: All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.de Groen PC, Gores GJ, LaRusso NF, et al. Biliary Tract Cancers. New England Journal of Medicine. 1999;341:1368–78. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 3.Henson DE, Albores-Saavedra J, Code D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493–97. doi: 10.1002/1097-0142(19920915)70:6<1493::aid-cncr2820700608>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1498–501. doi: 10.1002/1097-0142(19920915)70:6<1498::aid-cncr2820700609>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Falkson G, Macintyre JM, Moertel CG. Eastern cooperative oncology group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54:965–69. doi: 10.1002/1097-0142(19840915)54:6<965::aid-cncr2820540603>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Ducreux M, Rougier P, Fandi A, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Annals of Oncology. 1998 Jun 1;9:653–56. doi: 10.1023/a:1008241008379. [DOI] [PubMed] [Google Scholar]
- 7.Patt Y, Jones D, Hoque A, et al. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. Journal of Clinical Oncology. 1996 Aug 1;14:2311–15. doi: 10.1200/JCO.1996.14.8.2311. [DOI] [PubMed] [Google Scholar]
- 8.Okusaka T, Ishii H, Funakoshi A, et al. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemotherapy and Pharmacology. 2006;57:647– 53. doi: 10.1007/s00280-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 9.Gallardo JO, Rubio B, Fodor M, et al. A phase II study of gemcitabine in gallbladder carcinoma. Annals of Oncology. 2001 Oct 1;12:1403–06. doi: 10.1023/a:1012543223020. [DOI] [PubMed] [Google Scholar]
- 10.Papakostas P, Kouroussis C, Androulakis N, et al. First-line chemotherapy with docetaxel for unresectable or metastatic carcinoma of the biliary tract. A multicentre phase II study. European Journal of Cancer. 2001;37:1833–38. doi: 10.1016/s0959-8049(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 11.Sanz-Altamira PM, O'Reilly E, Stuart KE, et al. A phase II trial of irinotecan (CPT-11) for unresectable biliary tree carcinoma. Annals of Oncology. 2001 Apr 1;12:501–04. doi: 10.1023/a:1011135014895. [DOI] [PubMed] [Google Scholar]
- 12.Adams J. Development of the Proteasome Inhibitor PS-341. Oncologist. 2002 Feb 1;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Richardson PG, Hideshima T, et al. Proteasome Inhibition As a Novel Therapeutic Target in Human Cancer. Journal of Clinical Oncology. 2005 Jan 20;23:630–39. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Hui A-M, Li X, Shi Y-Z, et al. p27Kip1 expression in normal epithelia, precancerous lesions, and carcinomas of the gallbladder: Association with cancer progression and prognosis. Hepatology. 2000;31:1068–72. doi: 10.1053/he.2000.6127. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino M, Altimari A, D’Errico A, et al. Low p27 Expression Is an Independent Predictor of Survival for Patients with Either Hilar or Peripheral Intrahepatic Cholangiocarcinoma. Clinical Cancer Research. 2001 Dec 1;7:3994–99. [PubMed] [Google Scholar]
- 16.Taguchi K-I, Aishima S-I, Asayama Y, et al. The role of p27kip1 protein expression on the biological behavior of intrahepatic cholangiocarcinoma. Hepatology. 2001;33:1118–23. doi: 10.1053/jhep.2001.24028. [DOI] [PubMed] [Google Scholar]
- 17.Hui A-M, Cui X, Makuuchi M, et al. Decreased p27kip1 expression and cyclin D1 overexpression, alone or in combination, influence recurrence and survival of patients with resectable extrahepatic bile duct carcinoma. Hepatology. 1999;30:1167–73. doi: 10.1002/hep.510300506. [DOI] [PubMed] [Google Scholar]
- 18.Harnois DM, Que FG, Celli A, et al. Bcl-2 is overexpressed and alters the threshold for apoptosis in a cholangiocarcinoma cell line. Hepatology. 1997;26:884–90. doi: 10.1002/hep.510260413. [DOI] [PubMed] [Google Scholar]
- 19.Tannapfel A, Weinans L, Geißler F, et al. Mutations of p53 Tumor Suppressor Gene, Apoptosis, and Proliferation in Intrahepatic Cholangiocellular Carcinoma of the Liver. Digestive Diseases and Sciences. 2000;45:317–24. doi: 10.1023/a:1005412626515. [DOI] [PubMed] [Google Scholar]
- 20.Xoapfang L, Shengquan Z, Fazu Q. Expression of nuclear factor kappa B in hepatitis C virus core gene transfected cholangiocarcinoma cells. Chinese Medical Journal. 2002;115:998–1001. [PubMed] [Google Scholar]
- 21.Ustundag, Bronk SF, Gores GJ. Proteasome inhibition-induces endoplasmic reticulum dysfunction and cell death of human cholangiocarcinoma cells. World J Gastroenterol. 2007;13:851–57. doi: 10.3748/wjg.v13.i6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baradari V, Hopfner M, Huether A, et al. Histone deacetylase inhibitor MS-275 alone or combined with bortezomib or sorafenib exhibits strong antiproliferative action in human cholangiocarcinoma cells. World Journal of Gastroenterology. 2007;13:4458–66. doi: 10.3748/wjg.v13.i33.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaeteewoottacharn K, Kariya R, Matsuda K, et al. Perturbation of proteasome function by bortezomib leading to ER stress-induced apoptotic cell death in cholangiocarcinoma. Journal of Cancer Research and Clinical Oncology. 2013;139:1551–62. doi: 10.1007/s00432-013-1473-6. [DOI] [PubMed] [Google Scholar]
- 24.Teicher BA, Ara G, Herbst R, et al. The Proteasome Inhibitor PS-341 in Cancer Therapy. Clinical Cancer Research. 1999 Sep 1;5:2638–45. [PubMed] [Google Scholar]
- 25.Millenium Pharmaceuticals. [Accessed May 25, 2011.];Velcade Package Insert. 2011 http://www.velcade.com/
- 26.Aghajanian C, Soignet S, Dizon DS, et al. A Phase I Trial of the Novel Proteasome Inhibitor PS341 in Advanced Solid Tumor Malignancies. Clinical Cancer Research. 2002 Aug 1;8:2505–11. [PubMed] [Google Scholar]
- 27.Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma. Cancer. 2005;103:111–18. doi: 10.1002/cncr.20753. [DOI] [PubMed] [Google Scholar]
- 28.André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Annals of Oncology. 2004 Sep 1;15:1339–43. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 29.Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104:2753–58. doi: 10.1002/cncr.21591. [DOI] [PubMed] [Google Scholar]
- 30.Knox JJ, Hedley D, Oza A, et al. Combining Gemcitabine and Capecitabine in Patients With Advanced Biliary Cancer: A Phase II Trial. Journal of Clinical Oncology. 2005 Apr 1;23:2332–38. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 31.Verderame F, Russo A, Di Leo R, et al. Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Annals of Oncology. 2006 Jun;2006:17, vii68–vii72. doi: 10.1093/annonc/mdl955. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services. [Accessed February 10, 2012.];Common Terminology Criteria for Adverse Events version 3.0. 2006 http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 33.Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. Journal of the National Cancer Institute. 2000 Feb 2;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.Choi CW, Choi K, Seo JH, et al. Effects of 5-Fluorouracil and Leucovorin in the Treatment of Pancreatic-Biliary Tract Adenocarcinomas. American Journal of Clinical Oncology. 2000;23:425–28. doi: 10.1097/00000421-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 35.Gebbia V, Giuliani F, Maiello E, et al. Treatment of Inoperable and/or Metastatic Biliary Tree Carcinomas With Single-Agent Gemcitabine or in Combination With Levofolinic Acid and Infusional Fluorouracil: Results of a Multicenter Phase II Study. Journal of Clinical Oncology. 2001 Oct 15;19:4089–91. doi: 10.1200/JCO.2001.19.20.4089. [DOI] [PubMed] [Google Scholar]
- 36.Penz C, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Annals of Oncology. 2001 Feb 1;12:183–86. doi: 10.1023/a:1008352123009. [DOI] [PubMed] [Google Scholar]
- 37.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one year experience with 564 patients at a single institution. Annals of Surgery. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isa T, Kusano T, Shimoji H, et al. Predictive factors for long-term survival in patients with intrahepatic cholangiocarinoma. The American Journal of Surgery. 2001;181:507–11. doi: 10.1016/s0002-9610(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 39.NCCN Practice Guidelines in Oncology. Hepatobiliary Cancers. 2013;1 [Google Scholar]
- 40.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. New England Journal of Medicine. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 41.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo SM, Tepper JE, Baldwin AS, Jr, et al. Enhancement of radiosensitivity by proteasome inhibition: Implications for a role of NF-κB. International Journal of Radiation Oncology*Biology*Physics. 2001;50:183–93. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 43.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of Pancreatic Cancer by Inhibition of the 26S Proteasome. Journal of Surgical Research. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 44.Cusack JC, Liu R, Houston M, et al. Enhanced Chemosensitivity to CPT-11 with Proteasome Inhibitor PS-341. Cancer Research. 2001 May 1;61:3535–40. [PubMed] [Google Scholar]
- 45.Voorhees PM, Dees EC, O’Neil B, et al. The Proteasome as a Target for Cancer Therapy. Clinical Cancer Research. 2003 Dec 15;9:6316–25. [PubMed] [Google Scholar]
- 46.Harousseau J-L, Attal M, Avet-Loiseau H, et al. Bortezomib Plus Dexamethasone Is Superior to Vincristine Plus Doxorubicin Plus Dexamethasone As Induction Treatment Prior to Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: Results of the IFM 2005–01 Phase III Trial. Journal of Clinical Oncology. 2010 Oct 20;28:4621–29. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 47.Sonneveld P, Schmidt-Wolf I, van der Holt B, et al. HOVON-65/GMMG-HD4 Randomized Phase III Trial Comparing Bortezomib, Doxorubicin, Dexamethasone (PAD) Vs VAD Followed by High-Dose Melphalan (HDM) and Maintenance with Bortezomib or Thalidomide In Patients with Newly Diagnosed Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2010 Nov 19;116:40. [Google Scholar]
- 48.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. New England Journal of Medicine. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 49.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. The Lancet. 2010;376:2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 50.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III Study of Pegylated Liposomal Doxorubicin Plus Bortezomib Compared With Bortezomib Alone in Relapsed or Refractory Multiple Myeloma: Combination Therapy Improves Time to Progression. Journal of Clinical Oncology. 2007 Sep 1;25:3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]