Abstract
A hallmark of metastatic cancer cells is their invasion through the basal membrane and endothelial layer, which requires a highly elastic cytoskeleton and nucleus. Therefore, cellular deformability can serve as a universal biophysical marker for detecting a tumor’s propensity for invasion, migration, and metastasis. In this review, we define the importance of the biophysical features of cancer cells in tumor metastasis and summarize the state-of-the-art technology for the study of cell biomechanics. This review will serve as a brief introduction to the interdisciplinary character of cancer cell biophysics for cancer biologists, physicists, and engineers.
Keywords: Cancer metastasis, Biophysics, Cell biomechanics, Microfluidics, Mechanobiology
INTRODUCTION
Metastases cause 90% of human cancer deaths and continue to be difficult to study (Plodinec et al., 2012). Cancer and its metastasis require a marvelously complex system involving heterogeneous cell populations with diverse phenotypes, genotypes, and karyotypes and their surrounding microenvironment (Gupta and Massagué, 2006; Polyak and Weinberg, 2009). Recent insights into physical and mechanical processes at each step of the invasion-metastasis cascade may help unravel this complexity (Michor et al., 2011; Salaita et al., 2010). Mechanical phenotyping of cells provides an indication of flexibility and stiffness, which reflect the mechano-response changes of cells. From a biophysical perspective, the mechanical properties are defining characteristics for cancer cells to metastasize (Fritsch et al., 2010). A hallmark of metastatic tumor cells is the ability to invade locally and metastasize distantly (Fig. 1). To achieve this, cytoskeletal components, such as keratin filaments, reorganize from an ordered and rigid structure to an irregular and pliant architecture, and from a cytoplasmic to a juxtanuclear location.
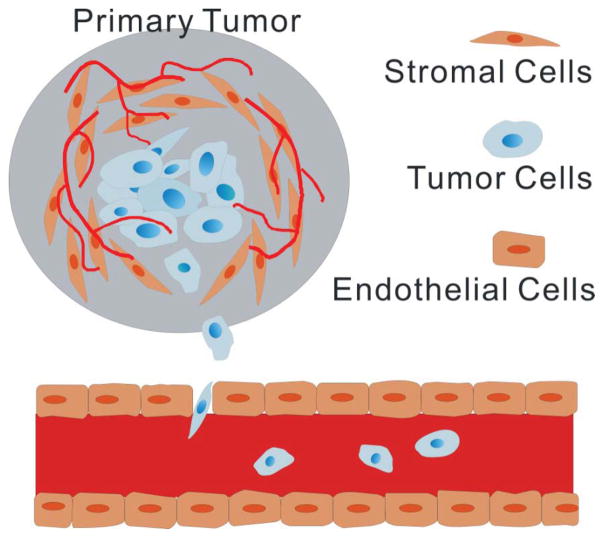
Figure 1.
Schematic showing cancer cell metastasis. Invasive tumor cells migrate from the primary tumor site into the surrounding tissue towards the circulation. These disseminated tumor cells penetrate the vessel wall and circulate via blood flow. Eventually, these cells extravasate through the vessel wall and form a secondary tumor.
In this review, recent advances are discussed to illustrate how biophysical tools can aid in the understanding of cancer biology, in particular, how microfluidics technology has contributed to better measurement of the biomechanical characteristics of metastatic cancer cells. This progress has enriched the field of cell migration, invasion, and tumor microenvironment, and may enhance the development of metastatic cancer diagnostics and anticancer targets.
DISEASES ASSOCIATED WITH THE BIOPHYSICAL PROPERTIES OF A CELL
Studies of a variety of diseases utilizing different biophysical techniques including membrane stretching, atomic force microscopy (AFM), optical traps, and micropipette aspiration have shown that abnormalities in the elastic properties of cells are associated with disease pathogenesis and progression (Huang et al., 2004). The biomechanical property of a cell is a sensitive indicator of its health due to the strong connection between cytoskeleton and disease status. With every motion of muscle, cartilage, and viscera, cells undergo a substantial amount of stretching, contraction, or other deformation. It has been demonstrated that living cells immediately fluidize with a rapid rearrangement of the cytoskeleton in response to a transient physical force; these cells then slowly re-solidify and the cytoskeleton becomes relaxed (Trepat et al., 2007). The cytoskeleton, a crowded framework of intracellular structural proteins, not only plays a significant role in multiple biological functions but also provides cells with mechanical deformability and rigidity (Bao and Suresh, 2003). Cytoskeletal remodeling and the complex sequence of biophysical events that allow these changes are properties of some diseases, e.g., mechanical changes of blood cells in diabetes mellitus (Williamson et al., 1985), anemia (Bosch et al., 1994), capillary clogs (Worthen et al., 1989), and motor neuron diseases (Fuchs and Cleveland, 1998; Xu et al., 1993). More specifically, dividing cells express less adherent protein and several-fold increased cortical stiffness relative to interphase cells (Kunda et al., 2008; Matzke et al., 2001).
Another well-known example of biomechanical changes caused by the cytoskeleton is the transformation from non-tumorigenic epithelial cells to their cancerous counterparts, or from nonmetastatic tumor cells to their metastatic counterparts. Metastatic tumor cells are characterized by their invasion through the basal membrane and the endothelial layer, which requires a highly deformable cytoskeleton. Therefore, cellular deformability could serve as a universal biophysical marker for detecting the propensity for tumor invasion, migration, and metastasis.
THE ROLE OF CELL BIOPHYSICS IN CANCER METASTASIS
Tumors are initiated by genetic alterations (e.g., mutations in TP53 or KRAS) in epithelial cells, which in turn confer epithelial cells with uncontrolled proliferative capacity; this process is called transformation into cancer cells (Hanahan and Weinberg, 2011). The process whereby tumor cells migrate and form a secondary tumor in a distant organ is called metastasis (Chaffer and Weinberg, 2011; Valastyan and Weinberg, 2011). Given that metastatic lesions often cause death of cancer patients, it is very important to understand this process and to discern the molecular and biophysical aspects of metastatic cancer cells (Chaffer and Weinberg, 2011; Valastyan and Weinberg, 2011). A cell’s molecular state is defined by the combination of at least 20,000 genes and 120,000 proteins, therefore, a single or a few molecular markers are often not sufficient to define and detect a specific cell phenotype.
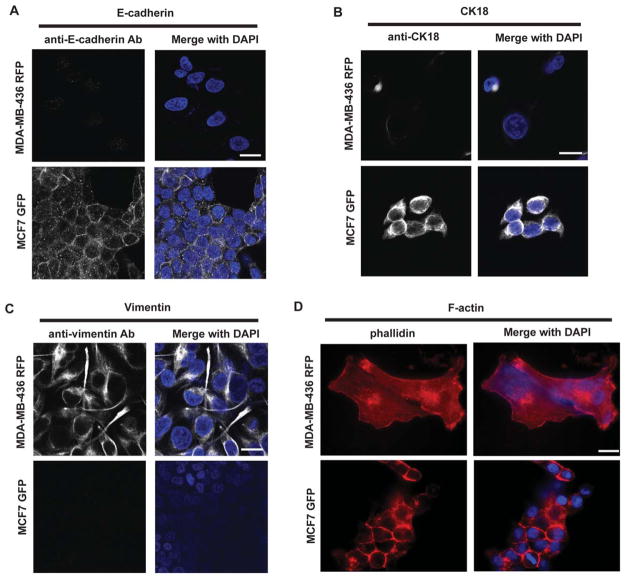
It is commonly believed that not all cancer cells have the ability to metastasize, and that a process similar to epithelial-mesenchymal transition (EMT) is required for cancer cell metastasis (Kumar and Weaver, 2009). Loss of adhesion and stiffness, both of which are biophysical properties, are thought to be hallmarks of EMT (Allure and Weinberg, 2009). One of the biochemical essentials for EMT is the loss of E-cadherin and cytokeratins, which change the physical and mechanical properties of cells; intercellular adhesion and cell morphology are also significantly changed during the EMT progress. In other words, metastatic tumor cells mechanically soften and modify their adhesion to extracellular matrix, which enhances their capacity to escape the primary tumor and survive in the circulation (Fig. 1). Fig. 2 shows the fluorescence images of labeled E-cadherin, CK18, vimentin, and F-actin staining. The greater deformability of the MDA-MB-436 cell cytoskeleton is indicated by the lower expression levels of F-actin and CK18 and the higher expression level of vimentin, compared to less aggressive MCF-7 cells. Unfortunately, there is at present no single biomarker that is able to differentiate or isolate mesenchymal cells to reveal their tumorigenicity. However, particular mechanical properties of epithelial and mesenchymal cells could be unique indicators of metastatic cancer (Cross et al., 2007; Darling et al., 2007; Remmerbach et al., 2009; Swaminathan et al., 2011; Wirtz et al., 2011), and characterization of the biomechanical properties, such as elastic stiffness, may allow a more accurate prognosis of metastasis.
Figure 2.
Cytoskeletal staining of MCF-7 and MDA-MB-436 cells. Staining of (A) E-cadherin, (B) CK18, (C) Vimentin, and (D) F-actin in MCF-7 and MDA-MB-436 cells. DAPI was used to visualize the nuclei. Scale bar: 20 μm.
New insight and understanding will arise when nontraditional approaches are applied to cancer biology by studying the physical principle of cancer (Smith, 2010). In the journey of cancer cells, the stiffness of these cells has been shown to directly indicate metastatic propensity (Kumar and Weaver, 2009). During intravasation and extravasation, metastatic cancer cells must undergo dramatic elastic deformations to penetrate endothelial cell–cell junctions. Cytoskeleton mechanics (stiffness) is usually regulated by a network of complex molecules with structural, cross-linking, and signaling functions (Fletcher and Mullins, 2010). Therefore, mechanical characterization of the cell is important for understanding the changes in these molecular networks.
On the clinical side, invasive spread is the primary cause of cancer death and limits curative therapeutic options. However, current diagnostic efforts in cancer screening, which depend largely on morphological and histological criteria, fail to identify some patients with a high risk of metastasis while it incorrectly targets others whose cancer may never spread. As a result, no accurate histopathology is available for determining metastatic potential. Biomechanical measurements may help to fill this void. From a pharmaceutical perspective, research advances in the biophysical characterization of metastatic cancer cells may lead to novel anticancer treatments by altering metastatic biomechanical changes.
Physiologically, each cancer cell can metastasize to distant organs in the following order: 1) detach from the primary tumor, 2) intravasation, 3) be transported through the blood stream, 4) become trapped in the endothelial walls of the distant organ, 5) extravasation into the parenchyma of the distant organ, 6) form secondary tumors at the distant organ. In each process, cancer cells must possess a particular biophysical property either on its own or by contact with other cells and/or extracellular matrix (ECM) (Suresh, 2007). For this review, we will focus on those biophysical properties presented by the cancer cell itself, or autonomous biophysics. The autonomous biophysics of individual cancer cells is most represented by cellular stiffness, which can be quantified by a number of methods, including microfluidics. Flexible cancer cells can pass through pores in an in vivo setting, a characteristic that makes them more metastatic; there are case studies proving the relationship between cellular deformity and metastatic potential (Suresh, 2007). In the next paragraph, we review these reports to establish this relationship and correlate this phenomenon with the prevailing molecular model of metastasis: EMT (Yang and Weinberg, 2008).
BIOPHYSICAL FEATURES OF METASTATIC CANCER CELLS
The study of metastatic cancer cells at the subcellular level reveals that their biophysical characteristics (e.g. cellular deformity) can be attributed to cytoskeletal compartments, such as actin filaments, intermediate filaments, and microtubules (Otten et al., 2012; Windoffer et al., 2011). Polymerization of filament monomers gives the resulting polymers viscoelasticity, which is assumed to provide cellular stiffness in epithelial cells (Beil et al., 2003; Yamada et al., 2003). Therefore, if the polymers destabilize in cancer cells, cancer cells can become more flexible, thus metastatic. This assumption is supported by published reports showing a positive correlation between destabilization of cytoskeletal filaments (e.. actin, keratin), cellular deformity, and metastatic activity (Guck et al., 2005). In these studies, the chemical compounds 12-O-tetradecanoylphorbol-13-acetate (TPA) and sphingosylphosphorylcholine (SPA) were used to promote the metastatic potential of breast cancer and pancreatic cancer cells, respectively. They then measured the stiffness of the cells with and without treatment, which have more or less metastatic potential, by using a microfluidic optical stretcher or laser micropipette puller. Both compounds destabilized actin or keratin polymers at the periphery of the cancer cells and made those cells flexible. These findings support a structural and physiological theory of metastasis, although this theory does not take into account other facets of metastatic cells’ biophysics [e.g., biophysics depending on cytoadhesion with other cancer cells or interaction with ECM (Kumar and Weaver, 2009)]. In summary, the cytoskeletal structure of cancer cells impacts the metastatic potential of those cells; the next step is to determine what molecules orchestrate the biophysical biphasic states of stiff or flexible. In the following paragraph, we discuss the relevance of the EMT model to the biophysical aspects of metastatic cells.
THE LIAISON BETWEEN EMT HALLMAKS AND BIOPHYSICAL HALLMARKS
EMT is a transcriptional factor (TF)-driven cellular program, allowing epithelial cells to transform into mesenchymal cells or migrating cells. This transition program can be seen at the embryonic phase of gastrulation (Yang and Weinberg, 2008). Gastrulation begins with an invagination of the primitive ectoderm at the ventral furrow of embryos, the beginning of mesoderm formation. When gastrulation begins, ectodermal cells (epithelial-like cells) at the leading edge of the invagination transform into mesenchymal cells so they can efficiently migrate into the primitive gastrula. On the other hand, in a wound healing process, the cells at the leading edge of the wound also transform into mesenchymal cells to help the wound close (Allure, 2009). Thus, EMT is the cellular program which can be seen even in physiological settings (e.g. embryogenesis, wound healing). Cancer cells are known to utilize the EMT program to allow them be become metastatic (Allure, 2009; Yang and Weinberg, 2008). Accumulating evidence has revealed that EMT is driven by TFs with a zinc-finger motif, allowing them to bind the E-box of promoter regions for genes encoding epithelial cadherin proteins, such as E-cadherin (Comijn et al., 2001; Thiery and Sleeman, 2006). Binding of TFs (e.g. Twist, Snal, Zeb1) at E-boxes downregulates the expression of epithelial cadherins, and these TFs also have other target genes to drive the overall EMT program. Characteristics of EMT include 1) loss of E-cadherin expression, 2) destabilization of actin filaments at the cellular periphery, and 3) switching of the intermediate filaments from keratins to vimentin, etc. (Thiery and Sleeman, 2006). In an epithelial mode, trans-binding of E-cadherins between adjacent epithelial cells stabilizes lipid membrane and is followed by polymerization of cytoskeletal filaments, including actin bundles. This sequence of events contributes to stasis or stiffness of epithelial-like cancer cells, in contrast to the disturbed state found in a mesenchymal cell. In case studies, including ours, which compared mesenchymal breast cancer cells (e.g. MDA-MB-436) with epithelial-like breast cancer cells (e.g. MCF7), mesenchymal breast cancer cells were more flexible (Guck et al., 2005; Zhang et al., 2012). This observation is consistent with the EMT sequence; MDA-MB-436 cells did not express E-cadherin or accumulate F-actin bundles at the cellular periphery and expressed vimentin, all of which were in contrast to the expression pattern of the MCF7 cells (Fig. 2). Therefore, it is likely that EMT induces cytoskeletal rearrangement in epithelial-like cancer cells and confers flexibility, which makes transformed mesenchymal cells more metastatic. To prove the cause-and-effect, along with the molecular and biophysical aspects of EMT, a dynamic model that allows us to monitor those two aspects of metastasis is warranted.
TECHNICAL APPROACHES TO PROBE CELL BIOPHYSICS
A sophisticated suite of technologies has been developed to identify the mechanical properties of cells, usually at the single cell level of analysis. These approaches include magnetic twisting cytometry, particle tracking rheometry, optical stretching rheometry, optical tweezers, micropipette aspiration, and AFM (Kumar and Weaver, 2009). These analyses of the mechanical phenotypes of cancer cells, both in vitro and ex vivo, have consistently revealed a general decrease in stiffness with the increasing migratory and invasive potential (Cross et al., 2007; Darling et al., 2007; Remmerbach et al., 2009; Swaminathan et al., 2011; Wirtz et al., 2011). In studying cell mechanics, the optically-induced deformability test introduced by Guck et al. is one of most successful technologies (Guck et al., 2005). Their study showed that the non-metastatic human breast cancer cell line MCF-7 was more deformable than its non-cancerous epithelial counterpart MCF-10. In 2013, Roth et al. developed high-throughput optical alignment compression cytometry to measure the mechanical properties of cells (Roth et al., 2013). Combining hydrodynamic drag with optical force, they measured a single cells’ deformability without destructive damage to the cell. AFM is another successful technology that has been used to characterize single cell mechanics (Fig. 3). AFM records strain-stress characteristics on single cells or tissues and provides precise force deformation curves. Assuming that the cell is a homogeneous and isotropic object, one can calculate the Young’s modulus of the measured cells. AFM measurements of cancer cell stiffness, quantified by the Young’s modulus, have shown a strong correlation between cell stiffness and cell malignancy. For example, the stiffness of metastatic cancer cells isolated from the pleural fluids of breast cancer patients was reported to be more than 70% less, with a standard deviation over five times narrower, than benign mesothelial cells (Cross et al., 2007). The same group also reported a significant increase in mechanical stiffness of green tea extract treated tumor cells (Cross, 2011). Xu et al. showed that ovarian cancer cells are generally softer and display lower variability in stiffness than benign ovarian epithelial cells (Xu et al., 2012). Plodinec et al. demonstrated that nanomechanical profiling by indentation-type atomic force microscopy provided quantitative indicators for clinical diagnostics of breast cancer with translational significance (Plodinec et al., 2012).
Figure 3.
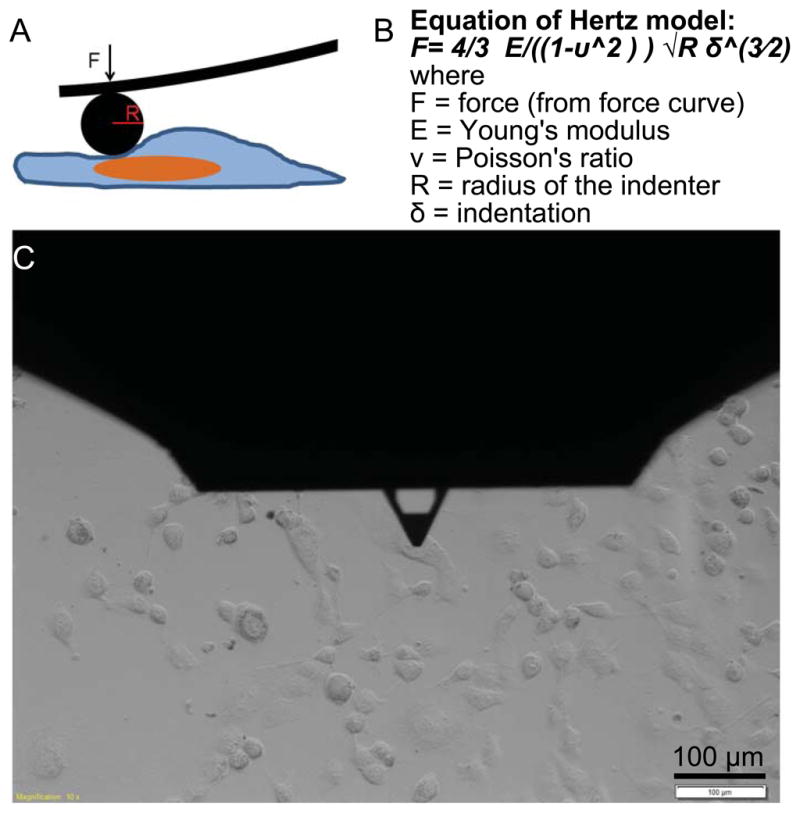
The AFM indentation method used for elastic modulus characterization. (A) Schematic showing Hertz model (spherical indenter) for measuring force curves. (B) Hertz model equation for compute Young’s modulus. (C) Optical microscopy image showing AFM tip approaching a cell.
Arguments may exist against the applicability of AFM, or other current methodologies, in cancer detection based on cellular deformability measurements. Such contentions include the statistic validity due to the low throughput and small sample size (n<30) and uncertainty due to the heterogeneity of actin-based fiber distribution (Janmey and McCulloch, 2007; Lekka and Laidler, 2009; Wirtz et al., 2011). In addition, what triggers and regulates mechanical properties currently remains unknown; the elasticity of the cytoskeleton and nuclei of a cell is the sum of many molecular factors, providing its integral biomechanical properties. To differentiate and study specific cancer cell types (such as disseminated or circulating tumor cells, which initiate metastasis), cell isolation based on the biomechanical signature has the potential to become a state-of-the-art technology. For this reason, there is an eagerness to discriminate and separate the elastic (flexible, deformable) mechanical cell phenotypes from heterogeneous cell populations. In addition, cell sorting methods should take into consideration the physical properties of the microenvironment including interstitial flow, porous and stiff ECM, endothelial cell wall, and external stimuli that force cells to squeeze through the ECM and endothelial cell walls.
MICROFLUIDICS AS A TOOL TO STUDY CELL BIOPHYSICS
Recent advances in microfluidics technologies have opened the door to cancer biophysics and the capture and downstream analyses of circulating tumor cells (Becker, 2009; Beebe et al., 2002; Bhagat et al., 2010; Gossett et al., 2012; Haeberle and Zengerle, 2007; Hansen and Quake, 2003; Kim et al., 2011; Qin, 2012; Qin et al., 2008). A number of groups have published microfluidic approaches for screening the biophysical properties of suspended cells. One of these strategies records transition time and velocity of a cell passing through microfluidic channels. Lee et al. demonstrated an on-chip erythrocyte deformability test under optical pressure using a microfluidics device to discriminate cancerous blood cells from normal blood cells. They first introduced the concepts of transit velocity, elongation index, and shape recovery time of erythrocytes in a confined microfluidic channel that measured 2 μm deep, 4 μm wide, and 100 μm long. In another study published by C. T. Lim’s group, microfluidic constriction channels were used to distinguish the deformability of MCF-10A and MCF-7 cells (Hou et al., 2009). The microfluidic device contained a 150×10×10 μm (L×W×H) microchannel and optical time-lapse images and distance-to-time records were used to measure the cells’ elongation ratios, transit velocities in channel, and times for entering channels. In 2008, D. Fletcher’s group reported a microfluidic device that measures a single cell’s transition times in blood cell populations passing through microfluidic networks. They used patient samples to demonstrate clinical relevance in two model disease states, sepsis and leukostasis. In 2012, Gossett et al. demonstrated an automated microfluidic platform capable of identifying malignant cells with a measurement speed of approximately 2000 cells per second (Gossett et al., 2012). This microfluidic technology uniformly delivered cells towards a narrow streamline center at a junction of two orthogonal channels, where two flows collide and stretch cells. The deformation of cells was recorded with a high-speed camera and analyzed computationally to extract quantitative parameters such as cell volume and deformation index. The authors rapidly characterized the deformability of leukocytes and malignant cells and accurately predicted disease states in patients with cancer and immune activation with a sensitivity of 91% and a specificity of 86%.
In our recent publication, we took advantage of the volume handling of microfluidics technology together with the integration of microstructures to manipulate cell stiffness analysis (Zhang et al., 2012). The mechanical separation chip (MS-chip) forced cells to flow through a maze of posts and separated cells based on their squishiness (Fig. 4). Subsequent analysis showed that the expression of motility genes in flexible cells could contribute to cancer metastasis. Abkarian et al. reported a technique to measure rapid variations in pressure drop between two points in a microfluidic manometer and demonstrated a pressure drop variation due to drug-induced changes in mechanical properties of the cytoskeleton. In another study, Guo et al. developed a microfluidic device to measure the deformability of red blood cells (Guo et al., 2012). Single blood cells were infused into microchannels and deformed through a series of funnel-shaped constrictions with openings ranging from 1 to 5 μm.
Figure 4.
Biophysical and microfluidics pathway to study the cancer metastasis.
CONCLUSION AND REMARKS
Physics has historically played an important role in the development of many modern biological theories (Gevaux, 2010). Here, a biophysical approach brings a fresh set of eyes to investigate metastasis by coupling the biophysical and biochemical properties of cancer cells. Importantly, biophysics can be used to explore molecular signatures and pathways that have been overlooked or are difficult to assess by traditional biological methods. Such studies allow for a different analysis of what events guide the development of metastasis and drive cancer biology to develop new hypotheses. An example of such hypotheses is the physical properties of the cancer cell, which can be altered either by pharmacological inhibition or through activation of proteins affecting cell mechanics, inherited over several generations. Microfluidic technology may offer new avenue to reveal this hypothesis, as well as an opportunities to identify the targeting molecules to inhibit this early stage of metastasis.
Impacts on Cancer Hallmarks.
The biophysical properties of cancer cells are key to the metastatic process of nearly all solid tumors, from breast to lung to pancreatic. Cancer cell deformability is defined as the ability of cells to change shape when passing through narrow spaces, such as circulating tumor cells passing through the microvasculature. A high level of deformability greatly aids cancer metastasis, allowing tumor cells to detach from a primary tumor and squeeze through stroma, penetrate blood vessel endothelium, survive the circulatory system, and eventually, successfully reach the secondary organ for colonization. This trait of metastatic cancer cells is one of the hallmarks of the metastasizing event and represents a newly emerging concept: the biophysical aspect of metastasis.
Acknowledgments
This study was funded by the Cancer Prevention and Research Institute of Texas (CPRIT-R1007), the Emily Herman Research Fund, the Golfers Against Cancer Foundation, and a grant from the State of Texas Rare and Aggressive Breast Cancer Research Program (to N.T.U. and K.K.).
References
- Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nat Mater. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- Becker H. Hype, hope and hubris: the quest for the killer application in microfluidics. Lab Chip. 2009;9:2119–2122. doi: 10.1039/b911553f. [DOI] [PubMed] [Google Scholar]
- Beebe DJ, Mensing GA, Walker GM. PHYSICS AND APPLICATIONS OF MICROFLUIDICS IN BIOLOGY. Annual Review of Biomedical Engineering. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, Van Veldhoven PP, Gern U, Wolff-Hieber E, Eggermann J, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- Bhagat AAS, Bow H, Hou HW, Tan SJ, Han J, Lim CT. Microfluidics for cell separation. Medical & Biological Engineering & Computing. 2010;48:999–1014. doi: 10.1007/s11517-010-0611-4. [DOI] [PubMed] [Google Scholar]
- Bosch FH, Werre JM, Schipper L, Roerdinkholder-Stoelwinder B, Huls T, Willekens FLA, Wichers G, Halie MR. Determinants of red blood cell deformability in relation to cell age. European Journal of Haematology. 1994;52:35–41. doi: 10.1111/j.1600-0609.1994.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nano. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- Cross SEJ, Yu-Sheng Lu, Qing-Yi Rao, Jian Yu, Gimzewski James K. Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology. 2011;22:215101. doi: 10.1088/0957-4484/22/21/215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Zauscher S, Block JA, Guilak F. A Thin-Layer Model for Viscoelastic, Stress-Relaxation Testing of Cells Using Atomic Force Microscopy: Do Cell Properties Reflect Metastatic Potential? Biophysical journal. 2007;92:1784–1791. doi: 10.1529/biophysj.106.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch A, Hockel M, Kiessling T, Nnetu KD, Wetzel F, Zink M, Kas JA. Are biomechanical changes necessary for tumour progression? Nat Phys. 2010;6:730–732. [Google Scholar]
- Fuchs E, Cleveland DW. A Structural Scaffolding of Intermediate Filaments in Health and Disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Gevaux D. Physics and the cell. Nat Phys. 2010;6:725. [Google Scholar]
- Gossett DR, Tse HTK, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao J, Clark AT, Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, et al. Optical Deformability as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic Competence. Biophysical journal. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Reiling SJ, Rohrbach P, Ma H. Microfluidic biomechanical assay for red blood cells parasitized by Plasmodium falciparum. Lab Chip. 2012;12:1143–1150. doi: 10.1039/c2lc20857a. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massagué J. Cancer Metastasis: Building a Framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Haeberle S, Zengerle R. Microfluidic platforms for lab-on-a-chip applications. Lab on a Chip. 2007;7:1094–1110. doi: 10.1039/b706364b. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hansen C, Quake SR. Microfluidics in structural biology: smaller, faster... better. Current Opinion in Structural Biology. 2003;13:538–544. doi: 10.1016/j.sbi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Hou HW, Li QS, Lee GYH, Kumar AP, Ong CN, Lim CT. Deformability study of breast cancer cells using microfluidics. Biomedical Microdevices. 2009;11:557–564. doi: 10.1007/s10544-008-9262-8. [DOI] [PubMed] [Google Scholar]
- Huang H, Kamm RD, Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. American Journal of Physiology - Cell Physiology. 2004;287:C1–C11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- Janmey PA, McCulloch CA. Cell Mechanics: Integrating Cell Responses to Mechanical Stimuli. Annual Review of Biomedical Engineering. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Yamamoto T, Fourmy D, Fujii T. An electroactive microwell array for trapping and lysing single-bacterial cells. Biomicrofluidics. 2011;5:24114. doi: 10.1063/1.3605508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Weaver V. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer and Metastasis Reviews. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda P, Pelling AE, Liu T, Baum B. Moesin Controls Cortical Rigidity, Cell Rounding, and Spindle Morphogenesis during Mitosis. Current biology: CB. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Lekka M, Laidler P. Applicability of AFM in cancer detection. Nat Nano. 2009;4:72–72. doi: 10.1038/nnano.2009.004. [DOI] [PubMed] [Google Scholar]
- Matzke R, Jacobson K, Radmacher M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nat Cell Biol. 2001;3:607–610. doi: 10.1038/35078583. [DOI] [PubMed] [Google Scholar]
- Michor F, Liphardt J, Ferrari M, Widom J. What does physics have to do with cancer? Nat Rev Cancer. 2011;11:657–670. doi: 10.1038/nrc3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten M, Nandi A, Arcizet D, Gorelashvili M, Lindner B, Heinrich D. Local motion analysis reveals impact of the dynamic cytoskeleton on intracellular subdiffusion. Biophys J. 2012;102:758–767. doi: 10.1016/j.bpj.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, LimRoderick YH, et al. The nanomechanical signature of breast cancer. Nat Nano. 2012;7:757–765. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Qin L. Studying Tumor Initiating Cell (TIC) Heterogeneity and Treatment Resistance by Single-Cell Microfluidics Technology. Single Cell Biology. 2012;1:1000, e1112. [Google Scholar]
- Qin L, Vermesh O, Shi Q, Heath JR. Self-Powered, Microfluidics Chip for Multiplexed Protein Assays from Whole Blood. Lab on a Chip. 2008 doi: 10.1039/b821247c. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmerbach TW, Wottawah F, Dietrich J, Lincoln B, Wittekind C, Guck J. Oral Cancer Diagnosis by Mechanical Phenotyping. Cancer Research. 2009;69:1728–1732. doi: 10.1158/0008-5472.CAN-08-4073. [DOI] [PubMed] [Google Scholar]
- Roth KB, Eggleton CD, Neeves KB, Marr DWM. Measuring cell mechanics by optical alignment compression cytometry. Lab on a Chip. 2013;13:1571–1577. doi: 10.1039/c3lc41253a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of Receptor Movement Alters Cellular Response: Physical Force Sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS. Physics challenged by cells. Nat Phys. 2010;6:726–729. [Google Scholar]
- Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan V, Mythreye K, O’Brien ET, Berchuck A, Blobe GC, Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Research. 2011 doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J, Gardner R, Boylan C, Carroll G, Chang K, Marvel J, Gonen B, Kilo C, Tran-Son-Tay R, Sutera S. Microrheologic investigation of erythrocyte deformability in diabetes mellitus. Blood. 1985;65:283–288. [PubMed] [Google Scholar]
- Windoffer R, Beil M, Magin TM, Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol. 2011;194:669–678. doi: 10.1083/jcb.201008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen G, Schwab B, Elson E, Downey G. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- Xu W, Mezencev R, Kim B, Wang L, McDonald J, Sulchek T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE. 2012;7:e46609. doi: 10.1371/journal.pone.0046609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Cork LC, Griffin JW, Cleveland DW. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993;73:23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- Yamada S, Wirtz D, Coulombe PA. The mechanical properties of simple epithelial keratins 8 and 18: discriminating between interfacial and bulk elasticities. J Struct Biol. 2003;143:45–55. doi: 10.1016/s1047-8477(03)00101-1. [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kai K, Choi DS, Iwamoto T, Nguyen YH, Wong H, Landis MD, Ueno NT, Chang J, Qin L. Microfluidics separation reveals the stem-cell–like deformability of tumor-initiating cells. Proceedings of the National Academy of Sciences. 2012;109:18707–18712. doi: 10.1073/pnas.1209893109. [DOI] [PMC free article] [PubMed] [Google Scholar]