Abstract
Rickettsia felis infection usually is a mild-to-moderate illness characterized by general signs and symptoms. Generally, patients do not require hospitalization. However, we detected 2 severe infections with R. felis. Our findings support the importance of R. felis infection as a potentially severe illness in humans.
Keywords: Rickettsia felis, Hepatitis, Flea-borne spotted fever, Rickettsioses, Emerging diseases
Introduction
In 1992, a bacterium was detected in cat fleas (Ctenocephalides felis) from a commercial colony (Adams et al., 1990). The bacterium was accepted as a new member of the Rickettsiaceae family and named Rickettsia felis. The first human case confirmed as an infection with R. felis was reported in Corpus Christi, Texas (USA), and since then new human cases were reported in México and worldwide (Pérez-Osorio et al., 2008; Schriefer et al., 1994; Zavala-Velazquez et al., 2000).
Usually, R. felis infection is the cause of a mild-to-moderate illness characterized by fever, headache, exanthem, and often neurologic and visceral signs and symptoms (Galvao et al., 2004). Although the patients seldom require hospitalization and the infection is self-limiting, the infection can have more severe manifestations involving pulmonary compromise (Zavala-Velázquez et al., 2006). In this work, we report on 2 patients with a severe illness caused by R. felis infection with significant hepatic and pulmonary manifestations.
Our findings support the importance of R. felis infection as a potentially severe disease of humans that should be considered, not only in the differential diagnosis for other febrile illnesses in general, but also in the differential diagnosis of diseases such as hepatitis and other infectious diseases which cause respiratory illness.
Case reports
Case 1
The first case was a 29-year-old male patient who lived in close contact with domestic animals (cats and dogs). He worked cleaning septic tanks and also had contact with mice and rats. The patient was hospitalized with high fever (40 °C) of 4 days evolution, headache, myalgia, generalized arthralgia, abdominal pain, nausea, vomiting, and hemorrhagic conjunctivitis. The patient was treated with antipyretics and cephalosporin as a primary treatment without remission of the symptoms. Two days after hospitalization, jaundice was observed in the sclerae and skin, and the patient experienced dyspnea. The dyspnea progressed to severe respiratory insufficiency caused by alveolar hemorrhage; the patient was intubated intratracheally and was ventilated mechanically. Hepatosplenomegaly was detected by abdominal ultrasound, with a hepatic border 2.0 cm below the costal arch. Chest radiographs showed a diffuse micronodular infiltrate with right basal consolidation with interstitial lesions.
Clinical laboratory studies revealed severe anemia (hemoglobin 8g/dl [normal range 12–18 g/dl], hematocrit 27% [normal range 37–51%]), severe thrombocytopenia (30 × 106 platelets/L [normal range 140–440 × 106 platelets/L]), elevated bilirubin (3.1 mg/dl) with cholestatic pattern, neutrophil leukocytosis (91% [normal range 55–68%]), elevated transaminases (AST 108 u/L [normal range 14–36 u/L], ALT 160 u/L [normal range 9–52 u/L]), and hematuria.
Several diseases with similar signs and symptoms such as dengue fever, malaria, brucellosis, human immunodeficiency virus infection (HIV), tuberculosis (TB), viral hepatitis A (HAV), B (HBV), and C (HVC), leptospirosis, and salmonellosis were excluded (Table 1). The diagnosis of rickettsiosis was established based on a single-step PCR amplification using genus-specific primers for the rickettsial genes ompA, ompB, and citrate synthase (gltA) (Table 2), using as controls DNA of R. felis (Ctenocephalides felis-LSU strain), R. rickettsii (Sheila Smith strain), R. akari (Kaplan strain), and R. typhi (Wilmington strain). The DNA of the controls and the patient were handled separately, and the PCR reactions were prepared in different PCR chambers to avoid contamination. Identification of R. felis as the causative agent was achieved by RFLP analyses of the amplified fragments of gltA and the comparison of the DNA sequences of the ompA, ompB and gltA PCR amplicons using the Blast software at the National Center for Biotechnology Information (NCBI) (Fig. 1) (Regnery et al., 1991; Altschul et al., 1997). All the sequences showed 100% identity with the corresponding R. felis genes (Table 2).
Table 1.
Pathogens excluded by serologic and molecular methods in both patients.
| Pathogen | Diagnostic method | Results | ||
|---|---|---|---|---|
|
|
|
|||
| Serologic | Molecular | Patient 1 | Patient 2 | |
| Leptospira interrogans | Microscopic agglutination test (MAT) | Neg. | Neg. | |
| Dengue virus | ELISAa | RT-PCR 5′-TCAATATGCTGAAACG CGCGAGAAACCG-3′ | Neg./Neg. | Neg./Neg. |
| 5′-TTGCACCAACAGTCAATGTCTT CAGGTTC-3′ | ||||
| Hepatitis A virus | Anti-HAVb | Neg. | Neg. | |
| Hepatitis B virus | HBcAGb, HBsAgb | Neg. | Neg. | |
| Hepatitis C virus | HCV Ag/Abb | Neg. | Neg. | |
| Human immunodeficiency virus | HIV Ag/Abb | Neg. | Neg. | |
| Mycobacterium tuberculosis | ELISAa | PCR 5′-CACATGCAAGTCGAACGG AAAGG-3′ | Neg./Neg. | Neg./Neg. |
| 5′-GCCCGTATCGCCCGCACGCTCACA-3′ Hybridizing probe 5-GGTGGA AAGCGCTTTAGCGGT-3′ | ||||
| Brucella abortus | ELISAa | Neg. | Neg. | |
| Salmonella typhi (Widal's test) | Detection of antigens O, Vi, and H | Neg. | Neg. | |
| Plasmodium falciparum, vivax, ovale, malariae | Malaria Abc | Neg. | Neg. | |
Diagnostic Automation Inc., Calabasas, CA, USA
Abbott Laboratories, Abbott Park, 1L, USA
Bio-Rad Laboratories Inc., Hercules, CA, USA
Table 2.
Molecular probes and positive controls used in the PCR reactions and sequence identity of the amplicons found in both patients.
| Gene | Primers | Size (bp) | Identity of PCR aimplicon | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Rickettsia felis, Ctenocephalides felis-LSU strain (%) | Rickettsia rickettsii Sheila Smith (%) | Rickettsia akari Kaplan strain (%) | Rickettsia typhi Wilmington strain (%) | |||
| ompB | Rp.330(2) 5′-atggctcaaaaaccaaattttc-3′ | 991 | 100 (AF182279) | 89(CP000848.1) | 89(CP000847.1) | 83 (AE017197.1) |
| Rp.330(2) 5′-agctctacctgctccattatct-3′ | 99(CP000053.1) | |||||
| Citrate synthase (gltA) | RpCS.1258 5′-attgcaaaaa gtaccgtaaaca-3′ | 382 | 100 (CP000053.1) | 95 (CP000848.1) | 96 (CP000847.1) | 95 (NC_006142) |
| RpCS.877 5′-gccccgccgtgggcaggccccc-3′ | ||||||
| ompA | Rrl90.70p 5′-atggcgaatatttctccaaaa-3′ | 512 | 100 (AJ563398) | 94 (CP000848.1) | 95 (CP000847.1) | |
| Rr190.602n 5′-agtgcagcattcgctccccct-3′ | 99 (CP000053.1) | |||||
Fig. 1.
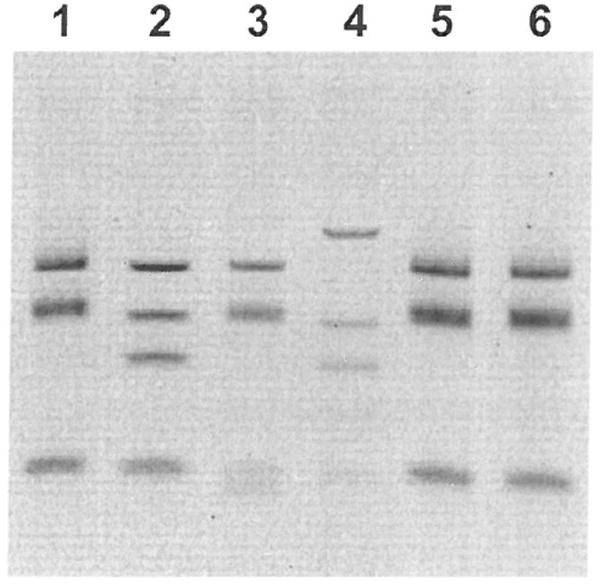
Restriction fragment length polymorphism (RFLP) of the gltA PCR product. Lane 1, R. felis positive control; lane 2, R. akari positive control; lane 3, R. rickettsii positive control; lane 4, R. typhi positive control; lane 5, 32-year-old patient; lane 6, 29-year-old patient.
IFA serology revealed antibodies at titers of 1:128 (IgM) and 1:256 (IgG) against R. rickettsii and 1:64 (IgM) and 1:128 (IgG) against R. akari. No antibodies against R. typhi were delected.
The patient was treated with intravenous chloramphenicol (75 mg/kg daily) for 10 days with symptom reduction within 72 h and a complete recovery after 5 days.
Case 2
A 33-year-old male who lived in close contact with animals (cats, dogs, goats, opossums), described flea bites 4 days before the onset of the illness. The patient was hospitalized with high fever (38.7 °C) of 5 days duration, headache, myalgia, generalized arthralgia, abdominal pain, nausea, and vomiting. After hospitalization, jaundice was observed in the sclerae and skin, and the patient experienced dyspnea. The patient had hepatosplenomegaly detected by abdominal ultrasound, with a hepatic border 1.5 cm below the costal arch. Chest radiographs showed a diffuse micronodular infiltrate.
Clinical laboratory studies revealed severe anemia (hemoglobin 9 g/dl [normal range 12–18 g/dl], hematocrit 29% [normal range 37–51%]); severe thrombocytopenia (40.5 × 106 platelets/L [normal range 140–440 × 106 platelets/L]); elevated bilirubin (2.7 mg/ dl), neutrophil leukocytosis (85% [normal range 55–68%]), and elevated transaminases (AST 95 u/L [normal range 14–36 u/L], ALT 120 u/L [normal range 9–52 u/L]).
The diagnoses of dengue fever, malaria, brucellosis, HIV, TB, HAV, HBV, HCV, leptospirosis, and salmonellosis were ruled out (Table 1). The diagnosis of rickettsiosis was established based on a single-step PCR amplification using genus-specific primers for the rickettsial genes ompA, ompB, and citrate synthase gene (gltA) (Table 2), using as controls DNA of R. felis, R. rickettsii (Sheila Smith strain), R. akari (Kaplan strain), and R. typhi (Wilmington strain). The DNA of the controls and the patient were handled separately to avoid contamination. Identification of R. felis as the causative agent was achieved by RFLP analyses of the amplified fragments of gltA and the comparison of the DNA sequences of the ompA, ompB, and gltA PCR amplicons using the Blast software at the National Center for Biotechnology Information (NCBI) (Fig. 1) (Regnery et al., 1991; Altschul et al., 1997). All the sequences showed 100% identity with the corresponding R. felis genes (Table 2).
IFA serology demonstrated antibodies at titers of 1:64 for IgM and 1:128 for IgG against R. rickettsii and 1:64 for IgM and 1:128 for IgG against R. akari. No antibodies reactive to R. typhi antigens were detected.
The patient was treated with intravenous chloramphenicol (75 mg/kg daily) for 10 days with symptom reduction within 72 h and a complete recovery after 5 days.
Discussion
Human rickettsiosis caused by R. felis has been documented around the world (Pérez-Osorio et al., 2008). In the last 12 years, rickettsioses caused by R. felis and R. rickettsii infections have been documented in Yucatan, Mexico (Zavala-Velazquez et al., 2000; Zavala-Castro et al., 2006). Usually, the clinical differences between these 2 infections are based on the severity of the signs and symptoms, with mild clinical manifestations for R. felis and moderate to severe clinical manifestations for R. rickettsii.
In spite of the moderate symptoms caused by some Rickettsia species, visceral and vascular involvement could have serious consequences for some patients, and even cause clinical manifestations usually not associated with the disease. Recently, hepatitis has been associated with rickettsialpox and is a well-documented manifestation of some cases of Rocky Mountain spotted fever but seldom occurs in R. akari infection which manifests moderate self-limiting signs and symptoms (Madison et al., 2008). Such an atypical clinical manifestation could also take place in other rickettsial infections and mislead the physician to an incorrect diagnosis.
As in other rickettsial infections, the course of illness caused by R. felis includes mild nonspecific signs and symptoms such as fever, arthralgia, myalgia, headache, and macular rush, traditionally confused with other febrile diseases especially with dengue fever, leptospirosis, and any other febrile exanthematous infection caused by a virus or another bacterium. However, other nonspecific but more severe clinical manifestations may occur in R. felis infection and other rickettsioses, such as thrombocytopenia, pulmonary compromise, and anemia and should stimulate the consideration of rickettsial infection in the differential diagnosis as well as other severe illnesses such as hepatitis, pneumonia, brucellosis, and malaria.
A grave clinical condition has not been reported in the illness caused by R. felis. The severe thrombocytopenia and anemia present in both patients were associated with bleeding represented by hematuria, alveolar hemorrhage, and hemorrhagic conjunctivitis almost certainly caused by the vascular damage that occurs in rickettsial infections. The vascular injury also caused significant hepatic injury in both patients and respiratory compromise in one. The elevated serum bilirubin and transaminases indicated hepatic involvement clinically represented by jaundice that required a workup for viral hepatitis.
Although R. felis infection has not been described as a cause of severe disease, some dissimilarity in the clinical features of the R. felis-infected patients from different countries has been reported (Galvao et al., 2004). Such reported dissimilarity and our findings suggest a potentially broader range of clinical manifestations in R. felis patients.
More investigation has to be done to elucidate the factors involved in the altered pathogenesis of some rickettsial diseases or to determine if the differences in the clinical features of rickettsial infections could be a consequence of genetic differences in the rickettsial strains as well as the result of differences in the genetics and environmental conditions of the host population and/or in rickettsial adaptation to diverse hosts and vectors. Differences in the amino acid composition of the outer membrane protein A of R. felis have been reported, and the changes may have implications in the immune recognition and even in the adaptation process to different environments, and could also be involved in the pathogenesis of rickettsial diseases (Pornwiroon et al., 2006: Zavala-Castro et al., 2005).
As in other rickettsial cases in our region, the titer of the immunofluorescence serology does not have a direct correlation with the severity of illness. Both patients had antibodies that were reactive with only spotted fever group rickettsiae, with a higher titer against R. rickettsii antigens.
It is important to reconsider the possibility that uncommon signs and symptoms of some rickettsial infections might lead to misdiagnosis and unnecessary deaths without timely and accurate diagnosis.
Acknowledgments
This research was supported by grants from the CONACyT (44064-M) to Jorge E. Zavala-Velázquez.
References
- Adams JR, Schmidtmann ET, Azad AF. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43:400–409. doi: 10.4269/ajtmh.1990.43.400. [DOI] [PubMed] [Google Scholar]
- Altscbul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped Blast and PSI-Blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao MA, Mafra CL, Chamone ChB, Calic SB, Zavala-Velázquez JE, Walker DH. Clinical and laboratorial evidence of Rickettsia felis infections in Latin America. Rev Soc Bras Med Trop. 2004;37:238–240. doi: 10.1590/s0037-86822004000300009. [DOI] [PubMed] [Google Scholar]
- Madison G, Kim-Schluger L, Braverman S, Nicholson WL, Wormser GP. Hepatitis in association with rickettsialpox. Vector-Borne Zoonotic Dis. 2008;8:111–115. doi: 10.1089/vbz.2007.0135. [DOI] [PubMed] [Google Scholar]
- Pérez-Osorio CE, Zavala-Velázquez JE, Arias JJ, Zavala-Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis. 2008;14:1019–1023. doi: 10.3201/eid1407.071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornwiroon W, Pourciau S, Foil LD, Macaluso KR. Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line. Appl Environ Microbiol. 2006;72:5589–5595. doi: 10.1128/AEM.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikatys BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriefer ME, Sacci JB, Jr, Dumler JS, Bullen MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994;32:949–954. doi: 10.1128/jcm.32.4.949-954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Castro JE, Small M, Keng C, Bouyer DH, Zavala-Velázquez JE, Walker DH. Transcription of the Rickettsia felis ompA gene in naturally infected fleas. Am J Trop Med Hyg. 2005;73:662–666. [PMC free article] [PubMed] [Google Scholar]
- Zavala-Castro JE, Zavala-Velázquez JE, Walker DH, Ruiz-Arcila EE, Laviada-Molina H, Olano JP, Ruiz-Sosa JA, Small MA, Dzul-Rosado KR. Fatal human infection with Rickettsia rickettsii, Yucatán, Mexico. Emerg Infect Dis. 2006;12:672–674. doi: 10.3201/eid1204.051282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Velazquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, Becerra-Carmona G, Walker DH. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356:1079–1080. doi: 10.1016/S0140-6736(00)02735-5. [DOI] [PubMed] [Google Scholar]
- Zavala-Velázquez JE, Laviada-Molina H, Zavala-Castro JE, Pérez-Osorio C, Becerra-Carmona G, Bouyer D, Walker D. Rickettsia felis, the agent of an emerging infectious disease: report of a new case in México. Arch Med Res. 2006;37:419–422. doi: 10.1016/j.arcmed.2005.08.003. [DOI] [PubMed] [Google Scholar]


