Abstract
Protein misfolding and aggregation underlie the pathogenesis of many neurodegenerative diseases. In addition to chaperone-mediated refolding and proteasomal degradation, the aggresome-macroautophagy pathway has emerged as another defense mechanism for sequestration and clearance of toxic protein aggregates in cells. Previously, the 14-3-3 proteins were shown to be indispensable for the formation of aggresomes induced by mutant huntingtin proteins. In a recent study, we have determined that 14-3-3 functions as a molecular adaptor to recruit chaperone-associated misfolded proteins to dynein motors for transport to aggresomes. This molecular complex involves a dimeric binding of 14-3-3 to both the dynein-intermediate chain (DIC) and an Hsp70 co-chaperone Bcl-2-associated athanogene 3 (BAG3). As 14-3-3 has been implicated in various neurodegenerative diseases, our findings may provide mechanistic insights into its role in managing misfolded protein stress during the process of neurodegeneration.
Keywords: 14-3-3, aggresomes, protein misfolding, protein aggregation, chaperones, inclusion bodies, neurodegeneration
Introduction
A common feature of various neurodegenerative diseases is the accumulation of misfolded and aggregated proteins. Protein misfolding can be caused by genetic mutations, environmental insults or oxidative stress. In neurodegenerative diseases, there is often a chronic imbalance between the generation and clearance of misfolded proteins. This leads to the failure of nerve cells to cope with excess aggregation-prone misfolded proteins, which impede cell functions and viability through a variety of mechanism, including pore formation, proteasome inhibition and disruption of intracellular transport.1-3
To prevent aggregation of misfolded protein, cells have evolved an elaborate protein quality-control system that includes molecular chaperone assisted refolding and proteasomal degradation. When the production of misfolded proteins exceeds the capacity of these cellular processes, misfolded and aggregated proteins can be actively transported into a perinuclear structure referred to as the aggresome and subsequently degraded by lysosome-dependent macroautophagy.4-6 This short review will discuss recent evidence in our understanding of the aggresome formation process with special emphasis on the 14-3-3 proteins, which we have shown to play an important role in this process.
Aggresome: An Active Cellular Response to Misfolded Protein Aggregates
Proteins must attain appropriate three-dimensional conformations to become functional molecules. As an inevitable byproduct of biogenesis, some proteins do not fold correctly. More than just being nonfunctional, misfolded proteins are prone to forming aggregates that perturb normal cellular functions, and ultimately lead to cell death. Therefore, cells of all kingdoms of life have developed sophisticated quality control systems to maintain protein homeostasis (proteostasis).7 Based on our current understanding, an important line of cellular defense appears to be mediated by the molecular chaperones, also known as heat-shock proteins (Hsps). Hsps are important for assisting the folding of nascent proteins in the endoplasmic reticulum (ER). They also associate with damaged proteins and help their refolding by acquiring energetically favorable conformations. The chaperone-assistance pathways function in both cytoplasm and intracellular trafficking processes, thereby ensuring that only the correctly folded proteins are involved in cellular activities. Once they fail to fold properly, however, proteins are then eliminated from cells through the ubiquitin-proteasome system.5,8,9 This cellular process begins with the recognition and modification of the misfolded protein (polyubiquitination) by a complex set of enzymes, and ends with the degradation of ubiquitinated proteins by the proteasome.10
In general, these protein quality-control systems are effective in maintaining the cellular proteostasis. However, the accumulation of misfolded protein does occur under certain pathological conditions. This could be a result of genetic mutation that renders the misfolded protein inaccessible to cytoplasmic proteasome proteolysis, or a defect in the proteolytic capacity of the cell. Once accumulated in the cell, misfolded proteins tend to interact with other unfolded or partially folded proteins, resulting in the formation of aggregates. While the cellular pathway for the clearance of protein aggregates is poorly understood, evidence accumulated in the last two decades suggests that a cellular structure, termed aggresome, may play an important role in managing misfolded protein aggregates in the cell.7,11
The aggresomes is a single prominent inclusion body localized at the perinuclear region of the cell. It has a poor solubility in aqueous or detergent solvents and is mainly composed of aggregated, undegraded misfolded proteins. The formation of aggresomes is an active cellular process, whereby misfolded and aggregated proteins are recruited to the dynein-dynactin motor and retrogradely transported on microtubules to the MTOC (microtubule organization center).12,13 It was previously proposed that aggresomes protect cells by sequestering cytotoxic misfolded proteins and small aggregates. More recently, emerging evidence indicates that aggresome formation is a cellular mechanism that helps concentrate misfolded and aggregated proteins for their eventual clearance by macroautophagy, a process that leads to the targeted degradation of aggresome contents through the formation of the autophagosome and subsequent fusion with the lysosome.14-16
14-3-3: A Molecular Adaptor for Aggresomal Targeting of Misfolded Proteins
14-3-3 refers to a family of ubiquitous proteins that are most abundantly expressed in the brain.17 They are highly conserved from yeast to human and consist of seven genetically distinct but structurally homologous isoforms (β, γ, ε, η, ζ, σ, and τ) in mammals.18 14-3-3 proteins exist as homo- and/or hetero-dimers and exert their functions by binding to and regulating target proteins containing specific phosphoserine motifs.19,20 To date, 14-3-3 proteins have been known to interact with over 200 proteins that are involved in a wide range of biological processes.21 The involvement of 14-3-3 proteins in the aggresome pathway was first indicated by an observation that 14-3-3 proteins colocalize with aggresome-like perinuclear inclusions in the cells ectopically expressing a mutant huntingtin protein.22 Subsequently, two studies showed that certain 14-3-3 isoforms are indispensable in the aggresome formation process: in yeast cells, deletion of the bmh1 gene, which encodes one of two yeast 14-3-3 homologs Bmh1, blocks aggresomal targeting of a disease-related huntingtin protein (Htt103QP).23 Likewise, suppression of 14-3-3ζ by siRNAs (small interfering RNA) in mammalian cells significantly inhibits aggresome formation induced by the expression of another polyglutamine-expanded huntingtin protein (Htt86Q).24 In both studies, 14-3-3 was found to interact with the mutant huntingtin proteins, but it was not yet clear how 14-3-3 might regulate the aggresome formation pathway at a molecular level.
We have recently performed a series of analyses in both yeast and mammalian cells to investigate the molecular mechanism underlying 14-3-3-dependent regulation of the aggresome formation process. In this study, we found that 14-3-3 is capable of promoting the formation of aggresomes induced by ectopic expression of several aggregation-prone proteins, including a GFP-tagged α-synuclein (α-Syn-EGFP), a mutant form of the cystic fibrosis transmembrane conducting regulator (CFTR-ΔF508), GFP-250, a mutant superoxide dismutase (SODG85R) and Htt103QP.25 Thus, 14-3-3 appears to have a broad function in the aggresome formation process, a role that is likely beyond its binding to a particular misfolded protein (e.g., the mutant Htt protein). Moreover, some of the substrates tested here (such as GFP-250 and SODG85R) are known to be non-ubiquitinated, suggesting that aggresome targeting by 14-3-3 does not dependent on the ubiquitination of misfolded proteins.26,27
Our results further revealed that 14-3-3 serves as a molecular adaptor that couples chaperone-associated misfolded and aggregated proteins to the dynein motor, and thereby facilitates their transport to the aggresome.25 First, we identified a novel protein-protein interaction between 14-3-3 and the dynein intermediate chain (DIC), and utilized truncation analyses to further define the region at DIC that is required for 14-3-3 binding. Second, we determined that dimerization of 14-3-3 is a functional requirement for aggresome formation in both yeast and mammalian cells. This finding is in line with published reports showing that the 14-3-3 dimer can function as a molecular adaptor to bridge the interaction of two different proteins.28,29 Furthermore, we discovered that 14-3-3 recruits misfolded proteins through its binding to an Hsp70 co-chaperone Bcl-2-associated athanogene 3 (BAG3). Previous studies have established the importance of the Hsp70 complex in targeted macroautophagic degradation of misfolded and aggregated proteins.30-32 Particularly, BAG3 has been shown to play a critical role in chaperone-based aggresome targeting and selective autophagy of misfolded proteins.5,30 Our biochemical analyses revealed that 14-3-3 binds directly to phosphorylated BAG3, thus forming a complex with Hsp70 as well as chaperone-associated misfolded proteins. Consistently, we found that 14-3-3 binding to BAG3 is crucial for aggresomal targeting of misfolded proteins. Together, these data suggest that 14-3-3 is a key linker between chaperone-associated misfolded proteins and the dynein motor.
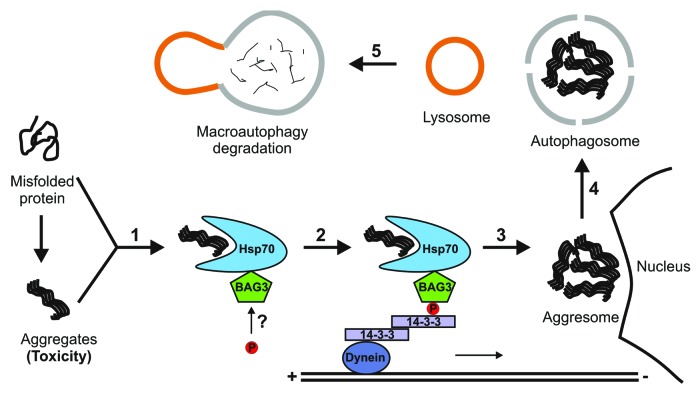
Based on these results, we propose a working model for a 14-3-3-mediated aggresome formation pathway. As depicted in Figure 1, misfolded proteins form toxic aggregates under conditions of genetic mutations or external stress. As a cellular stress response, misfolded and aggregated proteins are generally recognized and bound by molecular chaperones, such as the Hsp70 complex (Step 1). 14-3-3 interacts with the Hsp70 complex via a phosphorylation-dependent binding to the Hsp70 co-chaperone BAG3 and loads the misfolded protein cargo onto the cytoplasmic dynein complex. We postulate that this coupling is mediated by a dimeric binding of 14-3-3, in which each monomer interacts with BAG3 or DIC respectively (Step 2). The cargo complex is then transported along microtubule toward the MTOC to form the aggresome (Step 3). Subsequently, the aggresome can turn into an autophagosome through the recruitment of autophagic membrane (Step 4). Finally, fusions between the autophagosome and lysosome will result in the degradation and clearance of cytotoxic protein aggregates (Step 5).
Figure 1. Model for 14-3-3’s role in the aggresome-macroautophagy pathway. Numbers denote various steps during aggresome formation and clearance. A 14-3-3 dimer simultaneously binds to phosphorylated BAG3 and the dynein motor, thereby targeting chaperone-associated misfolded proteins and aggregates to the aggresome.
It is worth noting that several recent reports have provided evidence linking the chaperone-associated aggresome formation to targeted degradation of misfolded/aggregated proteins by selective macroautophagy.33,34 Specifically, BAG3 was shown to promote substrate degradation by macroautophagy in a p62/SQSTM1-dependent manner.35 In light of our identified protein-protein interaction between 14-3-3 and BAG3, future studies should be directed to investigate a potential role of 14-3-3 in the macroautophagy process. On the other hand, we have demonstrated that the phosphorylation of BAG3 is critical for 14-3-3 binding as well as the formation of aggresomes. This is consistent with previous studies showing the importance of protein kinase activation in promoting aggresome formation.36-39 It will be of interest to further identify the cellular factors (protein kinase/phosphatase) that regulate BAG3 phosphorylation, as these studies may potentially define novel cellular signaling cascades activated during misfolded protein stress.
14-3-3, Aggresomes, and Neurodegenerative Diseases
Many neurodegenerative diseases are characterized by the presence of intracellular inclusion bodies that share similar biochemical and morphological characteristics with the aggresome.40 As suggested by recent evidence, the formation of inclusion bodies is likely mediated by a cellular process that is analogous to the aggresome formation pathway, representing a cytoprotective mechanism for coping with accumulation of misfolded and aggregated proteins in pathological conditions.26 Interestingly, 14-3-3 proteins have previously been identified as a component of inclusion bodies in a number of neurodegenerative diseases, such as Lewy bodies in Parkinson disease, neurofibrillary tangles in Alzheimer disease, mutant huntingtin aggregates in Huntington’s disease, and aggresome-like inclusions of mutant superoxide dismutase in Amyotrophic lateral sclerosis.41-47 The observation that 14-3-3 is also present in the aggresome further establishes the similarity between inclusion bodies and aggresomes. As 14-3-3 protects cells by targeting misfolded proteins to aggresomes in the cell-based assays, we expect that this family of proteins is an important factor in promoting neuronal survival during the process of neurodegeneration. Indeed, such a function of 14-3-3 was proposed several years ago by Kaneko and Hachiya, who hypothesized that 14-3-3 may act as a sweeper to facilitate the sequestration and deposition of misfolded proteins in disease conditions.48
Considering the potential importance of 14-3-3 proteins in cellular responses to aggregation-prone misfolded proteins, it is conceivable that the 14-3-3-dependent molecular pathway may be a novel target for the prevention and therapy of neurodegenerative diseases, particularly those stem from protein aggregations.49 Encouragingly, recent effort has led to the discovery of some natural and synthetic compounds that enhance 14-3-3 functions by stabilizing its interactions with target proteins.50-52 A challenge for their in vivo application, however, lies in selective targeting of specific molecular complex (s) in which 14-3-3 operates. Based on our study, 14-3-3 bindings to BAG3 and DIC are crucial for depositing misfolded proteins to the aggresome. Thus, chemicals that selectively promote these two protein-protein interactions might have important implications for the treatment of some neurodegenerative diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grant NS50355 (to Y.Z.). We thank people in the Zhou lab for helpful discussions and critical reading of the manuscript.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Gregersen N. Protein misfolding disorders: pathogenesis and intervention. J Inherit Metab Dis. 2006;29:456–70. doi: 10.1007/s10545-006-0301-4. [DOI] [PubMed] [Google Scholar]
- 3.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–91. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 4.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–30. doi: 10.1016/S0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 5.Gamerdinger M, Carra S, Behl C. Emerging roles of molecular chaperones and co-chaperones in selective autophagy: focus on BAG proteins. J Mol Med (Berl) 2011;89:1175–82. doi: 10.1007/s00109-011-0795-6. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Mata R, Gao YS, Sztul E. Hassles with taking out the garbage: aggravating aggresomes. Traffic. 2002;3:388–96. doi: 10.1034/j.1600-0854.2002.30602.x. [DOI] [PubMed] [Google Scholar]
- 7.Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–88. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- 8.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–51. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci. 2006;31:137–55. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 11.Chin LS, Olzmann JA, Li L. Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans. 2010;38:144–9. doi: 10.1042/BST0380144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Mata R, Bebök Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol. 1999;146:1239–54. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–98. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamark T, Johansen T. Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int J Cell Biol. 2012;2012:736905. doi: 10.1155/2012/736905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–97. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JP, Tanaka F, Robitschek J, Sandoval CM, Taye A, Markovic-Plese S, Fischbeck KH. Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet. 2003;12:749–57. doi: 10.1093/hmg/ddg074. [DOI] [PubMed] [Google Scholar]
- 17.Moore BEP. V.J. Specific acidic proteins of the nervous system. In: F.D. Carlson EC, ed. Physiological and Biochemical Aspects of Nervous Integration. NJ: Prentice Hall, 1967:343–59. [Google Scholar]
- 18.Rosenquist M, Sehnke P, Ferl RJ, Sommarin M, Larsson C. Evolution of the 14-3-3 protein family: does the large number of isoforms in multicellular organisms reflect functional specificity? J Mol Evol. 2000;51:446–58. doi: 10.1007/s002390010107. [DOI] [PubMed] [Google Scholar]
- 19.Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–97. doi: 10.1016/S0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 20.Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–47. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 21.Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–62. doi: 10.1038/nrn1197. [DOI] [PubMed] [Google Scholar]
- 22.Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001;12:1393–407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Meriin AB, Zaarur N, Romanova NV, Chernoff YO, Costello CE, Sherman MY. Abnormal proteins can form aggresome in yeast: aggresome-targeting signals and components of the machinery. FASEB J. 2009;23:451–63. doi: 10.1096/fj.08-117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omi K, Hachiya NS, Tanaka M, Tokunaga K, Kaneko K. 14-3-3zeta is indispensable for aggregate formation of polyglutamine-expanded huntingtin protein. Neurosci Lett. 2008;431:45–50. doi: 10.1016/j.neulet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Graham K, Foote M, Liang F, Rizkallah R, Hurt M, Wang Y, Wu Y, Zhou Y. 14-3-3 protein targets misfolded chaperone-associated proteins to aggresomes. J Cell Sci. 2013;126:4173–86. doi: 10.1242/jcs.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–38. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 27.Olzmann JA, Chin LS. Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy. 2008;4:85–7. doi: 10.4161/auto.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braselmann S, McCormick F. Bcr and Raf form a complex in vivo via 14-3-3 proteins. EMBO J. 1995;14:4839–48. doi: 10.1002/j.1460-2075.1995.tb00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincenz C, Dixit VM. 14-3-3 proteins associate with A20 in an isoform-specific manner and function both as chaperone and adapter molecules. J Biol Chem. 1996;271:20029–34. doi: 10.1074/jbc.271.33.20029. [DOI] [PubMed] [Google Scholar]
- 30.Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011;12:149–56. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V, Moseley PL. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem. 2013;288:14959–72. doi: 10.1074/jbc.M113.462408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Qian SB. Chaperone-mediated hierarchical control in targeting misfolded proteins to aggresomes. Mol Biol Cell. 2011;22:3277–88. doi: 10.1091/mbc.E11-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broadley SA, Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009;583:2647–53. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol. 2011;23:184–9. doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meriin AB, Gabai VL, Yaglom J, Shifrin VI, Sherman MY. Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J Biol Chem. 1998;273:6373–9. doi: 10.1074/jbc.273.11.6373. [DOI] [PubMed] [Google Scholar]
- 37.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–77. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 38.Watabe M, Nakaki T. Protein kinase CK2 regulates the formation and clearance of aggresomes in response to stress. J Cell Sci. 2011;124:1519–32. doi: 10.1242/jcs.081778. [DOI] [PubMed] [Google Scholar]
- 39.Watabe M, Nakaki T. CK2 as anti-stress factor. Commun Integr Biol. 2012;5:278–80. doi: 10.4161/cib.19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran PB, Miller RJ. Aggregates in neurodegenerative disease: crowds and power? Trends Neurosci. 1999;22:194–7. doi: 10.1016/S0166-2236(99)01409-5. [DOI] [PubMed] [Google Scholar]
- 41.Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H. 14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol. 2002;61:245–53. doi: 10.1093/jnen/61.3.245. [DOI] [PubMed] [Google Scholar]
- 42.Ubl A, Berg D, Holzmann C, Krüger R, Berger K, Arzberger T, Bornemann A, Riess O. 14-3-3 protein is a component of Lewy bodies in Parkinson’s disease-mutation analysis and association studies of 14-3-3 eta. Brain Res Mol Brain Res. 2002;108:33–9. doi: 10.1016/S0169-328X(02)00510-7. [DOI] [PubMed] [Google Scholar]
- 43.Layfield R, Fergusson J, Aitken A, Lowe J, Landon M, Mayer RJ. Neurofibrillary tangles of Alzheimer’s disease brains contain 14-3-3 proteins. Neurosci Lett. 1996;209:57–60. doi: 10.1016/0304-3940(96)12598-2. [DOI] [PubMed] [Google Scholar]
- 44.Umahara T, Uchihara T, Tsuchiya K, Nakamura A, Iwamoto T, Ikeda K, Takasaki M. 14-3-3 proteins and zeta isoform containing neurofibrillary tangles in patients with Alzheimer’s disease. Acta Neuropathol. 2004;108:279–86. doi: 10.1007/s00401-004-0885-4. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto Y, Shirakashi Y, Ihara M, Urushitani M, Oono M, Kawamoto Y, Yamashita H, Shimohama S, Kato S, Hirano A, et al. Colocalization of 14-3-3 proteins with SOD1 in Lewy body-like hyaline inclusions in familial amyotrophic lateral sclerosis cases and the animal model. PLoS One. 2011;6:e20427. doi: 10.1371/journal.pone.0020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foote M, Zhou Y. 14-3-3 proteins in neurological disorders. Int J Biochem Mol Biol. 2012;3:152–64. [PMC free article] [PubMed] [Google Scholar]
- 47.Steinacker P, Aitken A, Otto M. 14-3-3 proteins in neurodegeneration. Semin Cell Dev Biol. 2011;22:696–704. doi: 10.1016/j.semcdb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Kaneko K, Hachiya NS. The alternative role of 14-3-3 zeta as a sweeper of misfolded proteins in disease conditions. Med Hypotheses. 2006;67:169–71. doi: 10.1016/j.mehy.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Bodner RA, Outeiro TF, Altmann S, Maxwell MM, Cho SH, Hyman BT, McLean PJ, Young AB, Housman DE, Kazantsev AG. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington’s and Parkinson’s diseases. Proc Natl Acad Sci U S A. 2006;103:4246–51. doi: 10.1073/pnas.0511256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milroy LG, Brunsveld L, Ottmann C. Stabilization and inhibition of protein-protein interactions: the 14-3-3 case study. ACS Chem Biol. 2013;8:27–35. doi: 10.1021/cb300599t. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J, Meyerkord CL, Du Y, Khuri FR, Fu H. 14-3-3 proteins as potential therapeutic targets. Semin Cell Dev Biol. 2011;22:705–12. doi: 10.1016/j.semcdb.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olzmann JA, Li L, Chin LS. Aggresome formation and neurodegenerative diseases: therapeutic implications. Curr Med Chem. 2008;15:47–60. doi: 10.2174/092986708783330692. [DOI] [PMC free article] [PubMed] [Google Scholar]