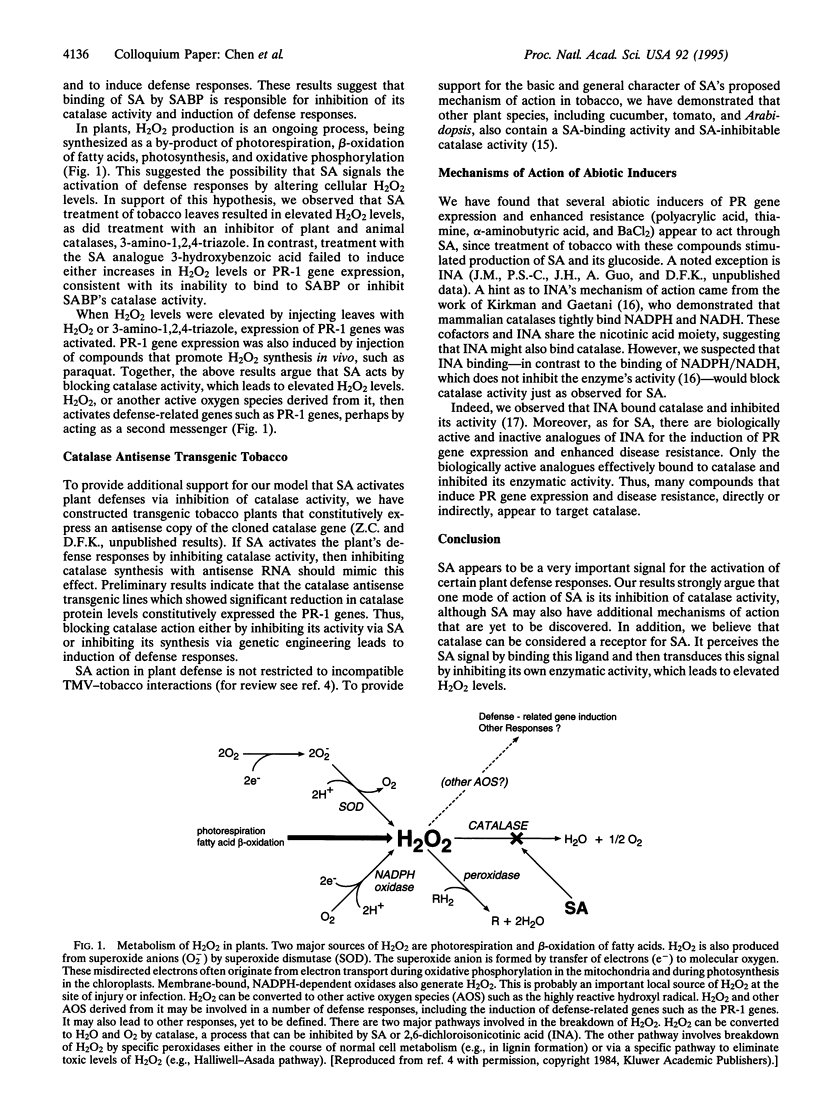
Abstract
Studies in our laboratory as well as others strongly suggest that salicylic acid (SA) plays an important signaling role in plant defense against pathogens. We have found that increases in endogenous SA levels correlates with both resistance of tobacco to infection with tobacco mosaic virus and induction of defense-related genes such as that encoding pathogenesis-related protein 1 (PR-1). Some of this newly synthesized SA was conjugated to glucose to form SA beta-glucoside. A cell wall-associated beta-glucosidase activity that releases SA from this glucoside has been identified, suggesting that SA beta-glucoside serves as an inactive storage form of SA. By purifying a soluble SA-binding protein and isolating its encoding cDNA from tobacco, we have been able to further characterize the mechanism of SA signaling. This protein is a catalase, and binding of SA and its biologically active analogues inhibited catalase's ability to convert H2O2 to O2 and H2O. The resulting elevated levels of cellular H2O2 appeared to induce PR-1 gene expression, perhaps by acting as a second messenger. Additionally, transgenic tobacco expressing an antisense copy of the catalase gene and exhibiting depressed levels of catalase also showed constitutive expression of PR-1 genes. To further dissect the SA signaling pathway, we have tested several abiotic inducers of PR gene expression and disease resistance for their ability to stimulate SA production. Levels of SA and its glucoside rose following application of all of the inducers except 2,6-dichloroisonicotinic acid. 2,6-Dichloroisonicotinic acid was found to bind catalase directly and inhibit its enzymatic activity. Thus, it appears that many compounds that induce PR gene expression and disease resistance in plants inactivate catalases directly or indirectly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Z., Klessig D. F. Identification of a soluble salicylic acid-binding protein that may function in signal transduction in the plant disease-resistance response. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8179–8183. doi: 10.1073/pnas.88.18.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Ricigliano J. W., Klessig D. F. Purification and characterization of a soluble salicylic acid-binding protein from tobacco. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9533–9537. doi: 10.1073/pnas.90.20.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Silva H., Klessig D. F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993 Dec 17;262(5141):1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Dempsey D. A., Klessig D. F. Salicylic acid, active oxygen species and systemic acquired resistance in plants. Trends Cell Biol. 1994 Sep;4(9):334–338. doi: 10.1016/0962-8924(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Enyedi A. J., Yalpani N., Silverman P., Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T., Friedrich L., Vernooij B., Negrotto D., Nye G., Uknes S., Ward E., Kessmann H., Ryals J. Requirement of salicylic Acid for the induction of systemic acquired resistance. Science. 1993 Aug 6;261(5122):754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Hennig J., Malamy J., Grynkiewicz G., Indulski J., Klessig D. F. Interconversion of the salicylic acid signal and its glucoside in tobacco. Plant J. 1993 Oct;4(4):593–600. doi: 10.1046/j.1365-313x.1993.04040593.x. [DOI] [PubMed] [Google Scholar]
- Kirkman H. N., Gaetani G. F. Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4343–4347. doi: 10.1073/pnas.81.14.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994 Dec;26(5):1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J., Hennig J., Klessig D. F. Temperature-Dependent Induction of Salicylic Acid and Its Conjugates during the Resistance Response to Tobacco Mosaic Virus Infection. Plant Cell. 1992 Mar;4(3):359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Casas P., Klessig D. F. A Salicylic Acid-Binding Activity and a Salicylic Acid-Inhibitable Catalase Activity Are Present in a Variety of Plant Species. Plant Physiol. 1994 Dec;106(4):1675–1679. doi: 10.1104/pp.106.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. R., Uknes S. J., Williams S. C., Dincher S. S., Wiederhold D. L., Alexander D. C., Ahl-Goy P., Metraux J. P., Ryals J. A. Coordinate Gene Activity in Response to Agents That Induce Systemic Acquired Resistance. Plant Cell. 1991 Oct;3(10):1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]



