Abstract
About 90% of individuals with multiple myeloma will develop osteolytic bone lesions from increased osteoclastic and decreased osteoblastic activity. Severe morbidities from pathologic fractures and other skeletal events can lead to poor circulation, blood clots, muscle wasting, compromised performance status, and overall poor survival. Supportive care targeting bone disease is an essential adjunct to antimyeloma therapy. In addition, the maintenance of bone health in patients with multiple myeloma can significantly improve quality of life. Oncology nurses and other healthcare providers play a central role in the management of bone disease and maintenance throughout the course of treatment. Safe administration of bisphosphonates, promotion of exercise, maintenance of adequate nutrition, vitamin and mineral supplementation, scheduled radiographic examinations, and monitoring of bone complications are among the important functions that oncology nurses and healthcare providers perform in clinical practice.
A main feature of multiple myeloma (MM) is bone destruction, and many patients will initially present with pain related to osteolytic lesions or vertebral compression fractures (Sezer, 2009). Destructive bone lesions and diffuse osteopenia are secondary to the stimulation of osteoclast-activating growth factors, cytokine release, and the lack of osteoblastic response (Esteve & Roodman, 2007). The clinical consequences of osteolytic bone lesions—fractures, severe bone pain, spinal cord compression, hypercalcemia, and renal insufficiency—can be devastating for patients, negatively affecting immediate and ongoing quality of life and worsening survival prospects (Kyle, Gertz, et al., 2003).
Pathophysiology of Myeloma Bone Disease
Bone disease is the major cause of morbidity and mortality in patients with MM (Coleman, 2006). Osteolytic lesions found with MM are caused by rapid bone turnover, which occurs as a result of increased osteoclastic resorption that is not accompanied by a comparable increase in bone formation (Berenson, Rajdev, & Border, 2006; Epstein & Walker, 2006). Normal bone formation is initiated by osteoblasts, and bone resorption is initiated by osteoclasts. Osteoprotegerin is a cytokine that inhibits production of osteoclasts. Osteoprotegerin maintains the balance between bone resorption and formation. Bone destruction in MM is believed to be multifactorial, resulting from an interaction of bone marrow stromal cells and myeloma tumor cells within the microenvironment of the bone marrow (Coleman, 2001). Bone destruction is characterized by overproduction of osteoclasts and reduced stimulation of osteoblasts resulting in unbalanced bone turnover.
Bone pain and the incidence of pathologic fracture are high among patients living with MM as a result of osteolytic bone lesions and bone turnover related to excess cytokine levels. Fractures typically occur near the time of diagnosis or relapse and are pathologic in nature. The most common locations of fracture are within the vertebral bodies (55%–70%), particularly in the lumbar vertebral bodies, flat and long bones (including the ribs), the extremities, and pelvis (Melton, Kyle, Achenbach, Oberg, & Rajkumar, 2005).
Long-Term Effects of Bone Disease
As noted, the majority of patients with MM will experience a pathologic fracture over the natural duration of their illness. As a result, 80% of patients will experience pain and altered quality of life (Roodman, 2008). The severity of bone disease and number of lesions present at the time of the diagnosis help to classify patients who are considered high risk. The Durie/Salmon PLUS staging system has integrated the quantification of bone lesions by magnetic resonance imaging (MRI) and positron-emission tomography (PET) to better define the treatment plan for patients newly diagnosed with early disease (Durie, 2006). Two staging systems are used to standardize treatment approaches. The International Staging System was introduced in 2005 by the International Myeloma Working Group and is based on two factors, serum beta-2 microglobulin and serum albumin levels (Greipp et al., 2005). This system divides cases of myeloma into three stages, providing a reliable prognostic tool. The Durie-Salmon staging system (Durie & Salmon, 1975) is based on the following criteria: percentage of marrow involved by monoclonal plasma cells; level of abnormal monoclonal immunoglobulin in the blood and/or urine; and level of serum calcium, renal dysfunction, degree of anemia, and presence of bone damage (CRAB) as later defined by the International Myeloma Working Group (Kyle, Child, et al., 2003). Although both systems provide valuable diagnostic and prognostic information, neither system describes how the severity of bone disease affects long-term outcomes.
The Durie/Salmon PLUS staging system (Durie, 2006) assimilates imaging methods into a new generation of anatomic and functional myeloma staging. Table 1 outlines the diagnostic imaging commonly used for patients with MM. With the use of MRI, whole body fluorodeoxyglucose (FDG) PET scanning and whole body computed tomography (CT), combined with PET directly or by fusion, the Durie-Salmon staging system has now been enhanced to include anatomic and functional staging. The new system may provide better classification of early disease. The Durie/Salmon PLUS staging system provides the following advantages.
Assessment of cell mass
More accurate staging for patients who lack traditional biomarkers because of hyposecretory or nonsecretory disease
Identifies patients at poor risk who have more than 20 focal lesions and/or extramedullary disease
Confirms stage I active disease for patients with negative x-rays
Provides a more detailed discernment between those diagnosed with stage II and III disease
Table 1.
Diagnostic Imaging in Multiple Myeloma
| DIAGNOSTIC IMAGING | PURPOSE |
|---|---|
| Bone density test | Useful in detecting osteopenia and osteoporosis |
| Bone scan | Not a useful tool in assessing myeloma bone disease |
| Magnetic resonance imaging | More sensitive than x-rays |
| Used when vertebral compression fracture or spinal cord compression is suspected | |
| Can detect edema and fluid surrounding active vertebral bone disease and marrow and soft tissue involvement | |
| Positron-emission tomography/computed tomography | Useful in assessing presence of lytic bone lesions and detecting occult plasmacytomas |
| Skeletal survey | Skeletal x-rays used to detect lytic bone lesions and bone fractures |
| Also used in staging of multiple myeloma |
Note. Based on information from Durie, 2006; Roodman, 2008.
The degree of bone involvement affects quality of life as well as prognosis. Compromised skeletal structure because of lytic lesions and vertebral compression fracture can result in altered mobility and function; therefore, early detection can offer potential benefits to patients. The life-altering mobility and functional aspects of myeloma are further described in Rome, Jenkins, Lilleby, and the International Myeloma Foundation Nurse Leadership Board (2011).
Assessment of Myeloma Bone Disease
Laboratory Testing
Laboratory tests to assess bone include calcium, vitamin D, fractionated alkaline phosphatase, and creatinine. Hypercalcemia, defined as a corrected serum calcium greater than 11.5 mg/dl, is seen in 10%–15% of patients at presentation and is considered an oncologic emergency. Alkaline phosphatase will be elevated with a high bone fraction and can indicate an increased rate of bone growth. Vitamin D levels can provide additional baseline evaluation of bone health because vitamin D deficiency can interrupt calcium metabolism, leading to weakened bones. Adequate vitamin D is crucial in preventing bone loss (Bischoff-Ferrari, 2007; Cashman, 2007; Guise, 2006; Mauck & Clarke, 2006; Roodman, 2004).
A variety of laboratory markers are used to monitor bone resorption in patients with MM. These markers can predict the development of new skeletal events. If the parathyroid hormone (PTH) level is low, bones will release more calcium in the blood, leading to hypercalcemia, and weakening of the bones, as well as resulting in fractures and pain. Some common symptoms of hypercalcemia include polyuria, polydipsia, constipation, confusion, somnolence, fatigue, vomiting, dehydration, weakness, and renal insufficiency. N-telopeptides are fragments of collagen released by the bone during bone turnover. When bone is broken down, this collagen is released in the urine; high levels of N-telopeptides in the urine can be indicative of active bone disease. Endocrine evaluation that includes thyroid, parathyroid, and testosterone levels may be indicated throughout the course (Bischoff-Ferrari, 2007; Cashman, 2007; Coleman, 2006; Guise, 2006; Mauck & Clarke, 2006; Roodman, 2004).
Diagnostic Imaging
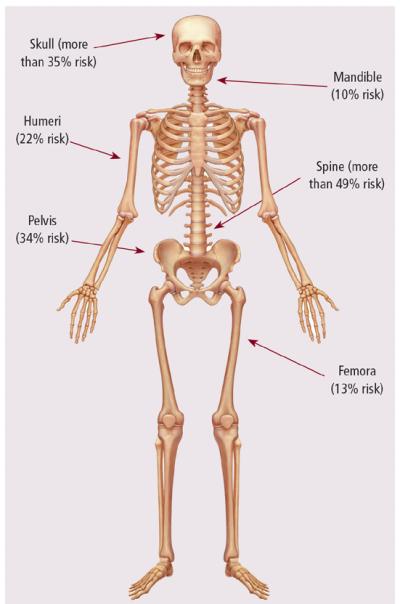
Bone imaging is an important tool for diagnosis and monitoring bone disease in patients with myeloma. Patients often are initially diagnosed because of the onset of acute bone pain or pathologic fracture. Any bone may be affected in MM; the bones with the highest chance of being affected are the spine with more than a 49% risk, the skull with more than a 35% risk, the pelvis at 34% risk, the humeri with 22% risk, the femora at 13% risk, and the mandible at 10% (Roodman, 2008) (see Figure 1). Of note, bone disease in myeloma is caused by an imbalance of osteoblast and osteoclast activity causing bone destruction and absence of bone formation. Therefore, bone scans are not the imaging technique of choice because the degree of bone disease may be underestimated (Roodman, 2008). A complete skeletal survey, the standard method of imaging in patients with MM, detects fractures, tumors, or degenerative changes in the bone. Metastatic bone surveys show that skeletal abnormalities are present in 79% of newly diagnosed patients. Osteosclerotic lesions are rarely seen (Kyle, Gertz, et al., 2003).
Figure 1. Bones at Highest Risk of Being Affected by Multiple Myeloma.
Note. Copyright 2011 by Bodell Communications, Inc./Phototake. All rights reserved. Used with permission.
Note. Based on information from Roodman, 2008.
Metastatic bone survey, the gold standard for assessing bone disease in myeloma, is able to only identity the lytic disease where a minimum of 30% of the trabecular bone has been destroyed, which basically renders the method somewhat insensitive. In addition, this technique does not demonstrate response to therapy (Roodman, 2008). These limitations of the skeletal survey have led to the use of three additional imaging techniques: CT, PET, and MRI. These three are not considered standard of care in the diagnosis of myeloma bone disease, but can be helpful in discerning suspicious lesions or areas of focal bone destruction. The more sensitive CT may reveal bone lesions not seen on a metastatic bone survey, particularly for patients who are experiencing pain. An MRI may reveal the presence of bone marrow involvement or spinal cord compression. For patients with nonsecretory or oligosecretory myeloma, MRI may reveal the presence and progression of disease and provide prognostic information as discussed in the Durie/Salmon PLUS staging system. For patients with renal insufficiency, which occurs in 20%–60% of patients throughout their disease, care should be taken to avoid the use of gadolinium (Angtuaco, Fassas, Walker, Sethi, & Barlogie, 2004; Kyle, Schreiman, McLeod, & Beabout, 1985; Mariette et al., 1999; Tariman & Faiman, 2011; Tryciecky, Gottschalk, & Ludema, 1997).
Although MRI has a higher sensitivity compared to a typical radiograph in providing anatomic information about the bone marrow, it lacks specificity because of the small signal strength on T1- and T2-weighted images. MRI is the diagnostic tool of choice for spinal cord compression. CT has a higher sensitivity for smaller lesions and is useful in detecting extraosseous extensions of the disease and to determine bone destruction. PET scans are useful in detecting bone disease, bone marrow infiltration, and extramedullary disease. Whole-body PET scans are much more sensitive than x-rays and other diagnostic imaging tools. The PET scan uses a radio-labeled sugar-based tracer called 18F-fluorodeoxyglucose (18F-FDG) that identifies metabolically active cells. FDG is absorbed but not metabolized in the cells and, therefore, accumulates. Areas of malignancy and infection are metabolically more active and typically uptake the tracer at greater rates. This provides a mechanism for imaging areas of infection or malignancy (Tryciecky et al., 1997). The use of whole-body 18F-FDG PET, combined with either CT alone or PET/CT, has been found to be useful in the detection of residual disease for myeloma that has been treated and is the imaging of choice for evaluation of extramedullary disease (Bartel et al., 2009). However, critics warn that the PET/CT tool requires additional study before it becomes part of the standard of care in patients with MM for the following reasons (Dimopoulos, Moulopoulos, & Terpos, 2009).
PET/CT may miss small lytic lesions and diffuse spinal involvement readily detected through use of an MRI.
PET/CT have been known to provide “false-positive” results, particularly in areas of inflammation and infection, post-surgical sites, and vertebroplasty or other metastatic disease.
Reimbursement remains an issue.
Treatment of Myeloma Bone Disease
The management of MM-related bone disease involves treatment of the disease itself, which controls the myeloma and the underlying manifestations of the disease. The most common forms of treatment are chemotherapy and autologous stem cell transplantation (for eligible patients). For patients with bone involvement who do not experience bone-related pain, systemic therapy often is the primary treatment choice. Adjuvant therapies include localized radiation, orthopedic surgical interventions including kyphoplasty or vertebroplasty for vertebral fractures, and the use of agents that can inhibit bone resorption, such as bisphosphonates.
Novel Antimyeloma Agents
Novel agents used today in the treatment of MM may positively affect bone metabolism. Management of active MM with effective antimyeloma therapies will help to decrease the risk of worsening bone structure and risk of fracture. However, these therapies are far from perfect, and it now is clear that skeletal disease may progress despite the achievement of stable disease (Kanis & McCloskey, 2000).
The proteasome inhibitor bortezomib appears to have an effect on myeloma bone disease. It inhibits nuclear factor kappa-B (NF-kB) activity, a critical factor for osteoclast formation and survival. It appears to act during the initial and ending stages of osteoclast differentiation. Several clinical trials conducted with bortezomib have demonstrated that it may increase osteoblast activity, leading to new bone formation, as researchers have reported an increase in bone formation markers in some patients (Pennisi, Li, Ling, Khan, Zangari, & Yaccoby, 2009).
The novel agent thalidomide may halt receptor activator of NF-kB ligand (RANKL)-induced osteoclast formation in vitro (Terpos et al., 2008). Lenalidomide, an immunomodulatory drug (IMiD) and an analog of thalidomide that is more active with an improved side-effect profile, decreases osteoclast formation and activity (Bolzoni et al., 2010; Breitkreutz et al., 2008; Terpos et al., 2009). In vitro studies demonstrate that thalidomide and the currently investigational immunomodulatory drug pomalidomide can inhibit the osteoclast activation process (Bolzoni et al., 2010). The effect of thalidomide combined with dexamethasone reduces bone resorption (Roodman, 2008). The combination of bortezomib, thalidomide, and dexamethasone reduces bone resorption and the RANKL/osteoprotegerin ratio; however, no impact on the formation of bones was observed (Terpos et al., 2009). Whether combining lenalidomide with bortezomib would enhance the osseous effects of each agent alone is unknown; potentially this is a topic for future study. As the understanding of myeloma bone biology develops, more targeted therapies have the potential to emerge (Yeh & Berenson, 2006).
Bisphosphonates
Bisphosphonates are strong inhibitors of bone resorption and are effective in the management of hypercalcemia of malignancy, decrease risk of fractures, and may decrease pain in some patients. Bisphosphonates bind to mineralized bone throughout resorption as well as inhibit osteoclast maturation. They cause osteoclast apoptosis as well as prevent attachment to the bone. The bisphosphonates that generally are administered to patients with MM in the United States include pamidronate and zoledronic acid. Both are given via IV, but the time of infusion differs for each. Skeletal-related events can be reduced with bisphosphonates, which decrease bone complications and improve quality of life (Fitch & Maxwell, 2008). It also has been suggested that pamidronate has an antimyeloma effect (Terpos & Dimopoulos, 2005); an antimyeloma effect has recently been shown for zoledronic acid (Morgan et al., 2010).
Radiation
Myeloma cells have a high level of sensitivity to radiation, and radiation to affected areas of bone is a useful management modality for some patients with MM, providing local pain and tumor control as well as preventing or treating skeletal fractures. Radiation may be curative in patients with solitary bone plasmacytomas (Yeh & Berenson, 2006). Unfortunately, solitary bone plasmacytoma will evolve to MM in the majority of patients within two to three years. Fortunately, about 15%–45% of patients will remain disease free at 10 years following diagnosis with an overall median survival of 7–12 years (Weber, 2005). Total body irradiation was used in the past to treat MM but, because of its severe toxicities and modest benefit, this treatment modality is rarely used today (Hu & Yahalom, 2000). The use of radiation should be limited as it may cause permanent bone marrow damage in the treatment areas and compromise organ function within the treatment fields (National Comprehensive Cancer Network, 2010; Roodman, 2008; Yeh & Berenson, 2006).
Surgical Procedures
A large lytic lesion that is present but has not yet progressed to a pathologic fracture may require stabilization to prevent a fracture. A surgical procedure may become a necessity to decrease the size of the tumor, control pain, and prevent or treat fractures. The spine, long bones, and pelvis are the most common areas affected. Patients with tumors affecting the long bones or weight-bearing joints may benefit from prophylactic surgery to stabilize and restore function to the affected bone. Intramedullary rod placement in the femur or pins and screws may be used for surgical fixation if the bone quality is good enough to support such hardware. Open surgical repairs, despite the invasiveness of these procedures, may be considered even at an advanced stage of disease to prevent impending fracture (Cady, Easson, Aboulafia, & Ferson, 2005). Stabilizing bone before a fracture occurs is far easier, and doing so will result in less pain and morbidity (Awan, Azer, Harrani, & Cogley, 2002).
Vertebral compression fractures are common among patients with MM. Two additional surgical techniques specific to the spine include percutaneous vertebroplasty and balloon kyphoplasty. Both are minimally invasive procedures and are performed on an outpatient basis by an interventional radiologist, orthopedic surgeon, or a neurosurgeon. Both procedures involve using contrast guided-imaging under CT or CT fluoroscopy and an incision of less than 1 cm to reach the anterior and paramedian aspect of the vertebral body. In vertebroplasty, a hollow needle is passed through the skin into the fractured vertebra and bone cement (polymethylmethacrylate) is injected through the hollow needle into the compressed or fractured area. Kyphoplasty is similar to verteboplasty, but a balloon tamp is inserted into the fractured vertebral body, which then is inflated in an attempt to restore vertebral height. The balloon is then deflated and withdrawn, forming a cavity in the vertebral body; the cavity is then filled with bone cement. Using balloon kyphoplasty instead of vertebroplasty can decrease the chance of cement extravasation; vertebroplasty does not involve restoring the structure of the collapsed vertebral body and requires a high-pressure cement fill that can lead to leakage (Roodman, 2008). Both procedures can provide immediate pain relief in some patients along with improvements in functional stability and spine stabilization. A recent randomized, controlled, open-label trial compared balloon kyphoplasty with usual nonsurgical care in patients with cancer, including patients with MM, who had vertebral compression fractures. Patients receiving kyphoplasty had improved back-specific physical function, more rapid back pain relief, and improved quality of life. Potential serious complications include cement leakage (Berenson et al., 2011). The risk for future fractured vertebral bodies rises in those opting for vertebroplasty or kyphoplasty as compared with individuals not undergoing either procedure (Ludwig & Zojer, 2007).
Emerging Treatments
RANKL is required for production and survival of osteoclasts and appears to play a role in the process of bone destruction (Roodman, 2008). The human monoclonal antibody denosumab binds to RANKL, preventing it from activating its receptor, RANK, thereby blocking its effects. Denosumab is approved for use in preventing skeletal-related events in solid tumors and is indicated for the management of postmenopausal osteoporosis in women at increased risk for fractures. Denosumab is not approved in the United States for administration to patients with MM, although it currently is used in clinical trials, as are Dickkopf-related protein 1 (DKK1) inhibitors. DKK1 and other members of the Wnt signaling pathway are logical therapeutic targets (Roodman, 2008).
Risk Factors Adversely Affecting Bone Health
In addition to the diagnosis of MM, patients can have comorbid conditions that may place them at risk for poor bone health, such as osteoporosis, metastatic malignancies, immobility, side effects from long-term steroid and other drug use, and hormone changes. Factors contributing to osteoporosis include renal disease, natural or therapy-induced gonadal failure, depression, diabetes, and vitamin deficiencies (Mauck & Clarke, 2006; Mezuk, Eaton, & Golden, 2008).
Osteoporosis and Risk of Fracture
Risk factors for osteoporosis are numerous and can be classified as primary or secondary (Mauck & Clarke, 2006) (see Table 2). Primary causes are those that cannot be modified (Cashman, 2007), and include female gender, increased age, family history of osteoporosis or fracture, small or thin frame, and low levels of sex hormones (Mauck & Clarke, 2006). Secondary causes are potentially modifiable through medical intervention and behavioral changes; they include nutritional deficits, chronic medical conditions, inactivity, smoking, alcohol abuse, and certain medications (Cashman, 2007; Mauck & Clarke, 2006). Thirty to 60% of men experience osteoporosis related to decreased gonadal function, use of glucocorticosteroids, and alcoholism; 50% of women will experience osteoporosis related to primary and secondary risk factors such as decreased estrogen, glucocorticosteroid use, and hyperthyroidism (Mauck & Clarke, 2006).
Table 2.
Risk Factors Contributing to Bone Loss, Osteoporosis, and Risk of Fracture
| CATEGORY | RISK FACTORS |
|---|---|
| Primary risk factors | Older than age 50 for men and women, female gender, postmenopausal at any age, men with lower testosterone and estrogen levels, personal and first-degree relative with a history of fracture as an adult, dementia, and ethnicity (Caucasians and Asian Americans have a higher incidence than Hispanics and African Americans.a) |
| Secondary risk factors | Low body weight (less than 127 lbs.), smoking, certain medications, hypogonadism (hormone deficiency and being younger than age 45), comorbid conditions, poor health and frailty, recent falls, inactivity and immobility (i.e., prolonged bed rest or wheelchair bound), and diet (more than two alcoholic drinks per day, low calcium intake, vitamin D deficiency of any cause, and excess consumption of vitamin A, caffeine, sodium, and protein) |
Difference in ethnic prevalence may be related to underreporting in some ethnic groups.
About 55% of the U.S. population older than age 50 is at high risk for the development of osteoporosis. An estimated 10 million individuals are affected by the disease, with more than 34 million at risk as evidenced by low bone mass. Osteoporosis is a chronic and progressive disease characterized by decreased bone mass, leading to structural changes within the bone, placing an individual at a higher risk for developing a fracture, particularity of the spine, wrist, or hip. Osteoporosis affects four times as many women as it does men, with significant risk seen among all ethnic backgrounds (Mauck & Clarke, 2006).
The National Osteoporosis Foundation ([NOF], 2010) estimates that 80% of women and 20% of men older than age 50 are affected by osteoporosis. In addition, one in two women and one in four men will experience a fracture secondary to osteoporosis. Studies have shown that not only does the risk for subsequent fractures increase after the first event, but the quality of life and impact on independent activities of daily living is dramatically reduced following a fracture (NOF, 2010). The median age for diagnosis of MM is 66 years (Kyle & Rajkumar, 2007). MM and other comorbid conditions (see Figure 2) increase the risk of age-related osteoporosis and fracture.
Figure 2. Comorbid Conditions Associated With Osteoporosis.
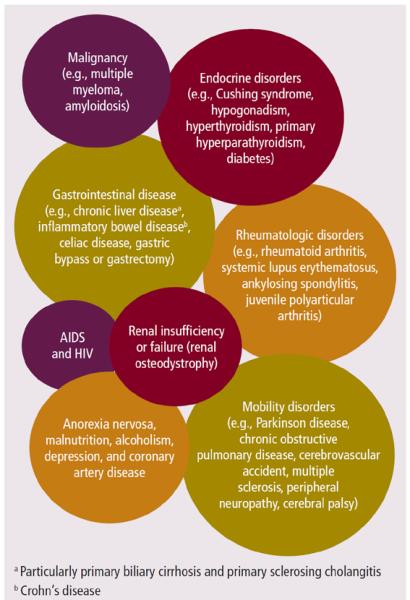
Note. Based on information from Adami, 2009; Everitt et al., 2006; Mauck & Clarke, 2006; Mezuk, Eaton, & Golden, 2008; Mezuk, Eaton, Golden, Wand, et al., 2008; Szulc et al., 2008)
Other Comorbid Conditions
Renal osteodystrophy
Bone loss associated with chronic renal disease is one of the most common osseous complications of patients with MM. The bone changes from chronic kidney disease or renal osteodystrophy can begin in adults several years prior to the appearance of any symptoms. Management of myeloma-related renal complications are described in Faiman, Mangan, Spong, Tariman, and the International Myeloma Foundation Nurse Leadership Board (2011). Patients with either osteoporosis or renal osteodystrophy experience increased risk of fractures and resultant joint and bone pain, but renal osteodystrophy does not necessarily respond to bisphosphonates (Legg, 2005).
Kidneys have a significant role in the maintenance of bone mass throughout one's life by maintaining calcium and phosphorus levels in the blood. Patients with kidney disease may develop hypocalcemia leading to increased stimulation of the parathyroid glands to release PTH. The excess PTH results in osteopenia, and constant removal of calcium over time will weaken the bones (Legg, 2005).
The kidneys also regulate serum calcium by producing calcitriol, a form of vitamin D produced by healthy kidneys that helps the body absorb dietary calcium. In kidney failure, calcitriol production is decreased and the resulting hypocalcemia stimulates the parathyroid gland to release more PTH, which contributes to further osseous calcium loss. Nurses need to be aware that routine monitoring for serum PTH and vitamin D should take place in patients with chronic kidney disease (Legg, 2005).
Gonadal insufficiency
Gonadal failure can be a natural part of aging or therapy related and can affect both men and women. Postmenopausal women have a higher risk of developing osteoporosis and risk of fracture. The loss of estrogen results in a higher rate of bone loss (Everitt et al., 2006). Bone mineral density (BMD) in women is estimated to decrease at over 2% annually for the first five years following menopause, and then the rate slows, whereas the rate of bone loss in men begins in midlife and is further reduced from 0.5%–1% per year (Guise, 2006). Selected therapies, both surgical and medical, that result in gonad failure and contribute to the risk of osteoporosis and risk of fracture are listed in Table 3.
Table 3.
Bone Loss and Cancer Therapy
| THERAPY | TUMOR |
|---|---|
| Chemotherapy (cyclophosphamide, methotrexate or ifosfamide, and alkylating agents) | Various malignancies (e.g., breast cancer, multiple myeloma, osteosarcoma, Hodgkin or non-Hodgkin lymphoma) |
| Glucocorticoids | Various malignancies and autoimmune disorders |
| Hormone therapy (androgen-deprivation therapy, selective estrogen-receptor modulators, and aromatase inhibitors) | Prostate, testicular, ovarian, and breast cancer |
| Radiation therapy | Various malignancies |
| Stem cell transplantation | Various malignancies |
| Surgical (bilateral orchiectomy and oophorectomy) | Prostate, testicular, ovarian, and breast cancer |
Note. From “Bone Loss and Fracture Risk Associated With Cancer Therapy,” by T.A. Guise, 2006, Oncologist 11, p. 1122. Copyright 2006 byAlphaMed Press. Adapted with permission.
Depression
Major depressive disorders occur in about 16% of the general population, but more frequently in those diagnosed with cancer (Mezuk, Eaton, & Golden, 2008; Mezuk, Eaton, Golden, Wand, & Lee, 2008). People who suffer from major depressive disorders have a lower BMD than those in control groups. The association of depression, osteoporosis, and risk of fracture is unclear. Physiologically, hormone levels that promote osteoclastic function and decrease osteoblastic function, including interleukin-6, tumor necrosis factor-alpha, PTH, C-reactive protein, and cortisol, also are elevated in those who have major depressive disorders. Secondary risk factors may play the greatest role because an association exists between depression and unhealthy behaviors (e.g., smoking, alcohol use, fatigue resulting in inactivity) (Mezuk, Eaton, & Golden, 2008).
Diabetes
People suffering from type 1 diabetes have decreased BMD and increased risk of fracture, and those with type 2 diabetes also are at a higher risk for suffering a fracture even if their BMD is normal or increased (Adami, 2009). However, the mechanism for bone loss in these patients is not well understood (Chau & Edelman, 2002). Patients with MM undergoing treatment with high-dose steroids have an increased risk of developing steroid-induced diabetes which, in turn, increases the risk of osteoporosis (Faiman, Bilotti, Mangan, Rogers, & the International Myeloma Foundation Nurse Leadership Board, 2008).
Cardiovascular disease
The association of osteoporosis and cardiovascular disease in men is not well understood and may be a culmination of comorbid conditions that place a person at risk for both conditions (e.g., inactivity, low testosterone levels, end-stage renal disease) (Szulc, Kiel, & Delmas, 2008).
Medications
Many cancer treatment regimens can have adverse effects on bones, resulting in more rapid and severe bone loss than seen in primary causes of osteoporosis in men and women (Guise, 2006; Melton et al., 2005). In addition, many comorbid conditions are treated with pharmacotherapy, which also may contribute to osteoporosis and risk of fracture. Figure 3 lists drugs that may predispose patients to osteoporosis, putting them at a higher risk for fracture.
Figure 3. Checklist of Drugs Associated With Osteoporosis and Risk of Fracture.

Note. Based on information from Guise, 2006; Mauck & Clarke, 2006.
Steroids
Glucocorticoids (dexamethasone or prednisone) remain a backbone of antimyeloma therapy. Steroids kill myeloma cells directly and may enhance the efficacy of other myeloma drugs when used in combination. However, steroid use may result in osteopenia or osteoporosis by inhibiting or killing osteoblasts, stimulating bone resorption, inhibiting calcium absorption, and increasing calcium excretion. Steroid use also is associated with avascular necrosis or osteonecrosis (Faiman et al., 2008).
Antidepressants
Antidepressant medications such as selective serotonin reuptake inhibitors and tricyclic antidepressants have been implicated in increased risk of fractures. Current use of antidepressants plays a greater role in risk of fracture than previous use and may be age- and life-stage dependent (Mezuk, Eaton, & Golden, 2008).
Bone Marrow Transplantation
Multiple small studies evaluating BMD following autologous and allogeneic bone marrow transplantation report that bone loss is a common side effect. Few long-term studies are available, but disturbance in bone metabolism and reduced BMD is measurable years after the procedure. The mechanism is not completely understood, however, alteration of the osteoprotegerin/RANKL system may play a significant role (Ebeling et al., 1999; Kang et al., 2000; Kerschan-Schindl et al., 2004; Lee, Cho, et al., 2002, Lee, Kang, et al., 2002). Interestingly, one small study (Kielholz et al., 1997) of 29 patients who underwent autologous bone marrow or peripheral blood transplantation following high-dose chemotherapy showed that they did not have significant osteopenia despite high-dose steroids, prolonged inactivity, and decreased estrogen levels. The lowest BMD was seen in men who had the lowest testosterone levels. Because this was a follow-up study of patients who were five years post-transplantation, these unexpected results may be from recovery of endocrine function during that time. With such a small cohort of patients, however, additional investigation is warranted before making any conclusions (Kielholz et al., 1997).
Diet
Although diet is considered a secondary risk factor for osteoporosis and risk of fracture, the peak bone mass, which is reached in youth, is not modifiable in adults. About 90% of bone mass is established within the first two decades of life and, most significantly, during puberty (Cashman, 2007). Vitamins and nutrients important for bone mineralization and skeletal development are listed in Table 4. Vitamin D and calcium are required during development and throughout life to promote bone health (Everitt et al., 2006). Maximizing serum vitamin D levels, as measured by 25-hydroxyvitamin D (25-OHD), contributes to an increase in BMD and is associated with improved muscle strength, resulting in a 20% decreased risk of fracture in older adults. Bischoff-Ferrari (2007) stated that optimal levels of serum 25-OHD are between 90 and 100 nmol/L. Inadequate levels of vitamin D cause elevation of PTH that can lead to decreased BMD (Bischoff-Ferrari, 2007; Mauck & Clarke, 2006). Evidence shows that increased levels of vitamin K reduce the risk of fracture. Phytoestrogens, found naturally in plant-based products such as soy, are a nonsteroidal compound that may act as a safe hormone replacement therapy for postmenopausal women (Cashman, 2007). Smoking and use of alcohol (more than two drinks per day) are both associated with decreased bone cell proliferation related to impaired absorption of calcium in the intestine (Mauck & Clarke, 2006; Mezuk, Eaton, Golden, Wand, et al., 2008). Consumption of excess or insufficient amounts of protein, vitamin A, and phosphorus can have positive or negative effects on bone health, depending on their renal effects. Paradoxically, obesity has a positive effect on BMD (Cashman, 2007; Everitt et al., 2006), even in those patients considered to be inactive. Weight-bearing activity promotes bone density, so the additional body mass contributes to bone strength (Everitt et al., 2006; Mauck & Clarke, 2006; Mezuk, Eaton, & Golden, 2008). However, the comorbid conditions associated with obesity make this a counterproductive approach to promoting bone health.
Table 4.
Factors That Reduce Osteoporosis and Risk of Fracture
| INTERVENTION | ITEMS |
|---|---|
| Diet or dietary supplements | Calcium, copper, magnesium, phytoestrogens, vitamin C (with caveats about potential interaction with bortezomib), vitamin D, vitamin K, and zinc |
| Pharmacotherapy | Estrogen, statins, and thiazides |
| Weight-bearing activity | – |
Note. Based on information from Adami, 2009; Mauck & Clarke, 2006; Mezuk, Eaton, & Golden, 2008; Mezuk, Eaton, Golden, Wand, et al., 2008.
MM occurs more frequently in African Americans than in other racial or ethnic groups (Kyle & Rajkumar, 2007). African Americans have lower serum levels of vitamin D, which may place individuals at increased risk of fracture. Although the risk of falling is similar to Caucasians, the risk of fracture is lower. This process is not fully understood, but possible elements protecting African Americans include increased rates of obesity, bone composition, remodeling, and inherited factors (Aloia, 2008).
Evidence-Based Recommendations to Manage and Maintain Bone Health
Comorbidities and concomitant risk factors, such as age, gender, medications, and mobility, should be considered when developing a treatment plan and will influence the approach taken for each patient (see Figure 4). In addition to mobility and exercise, which are discussed in depth in another article in this supplement (Rome et al., 2011), nurses can assist patients to manage their bone health by maintaining proper diet and nutrition, undergoing radiation treatment or surgical procedures when necessary, and safely taking bisphosphonates and pain medications where appropriate.
Figure 4.
Long-Term Survivor Bone Health Plan for Clinicians Treating Patients With Multiple Myeloma
Diet and Supplements
Only general recommendations exist in regard to diet, supplements, and bone health in MM. Clinicians should encourage patients to eat well-balanced diets comprising fruits, vegetables, protein, and carbohydrates. Most nutritional supplements are safe in moderation; however, based on preclinical evidence, the Nurse Leadership Board suggests the following compounds be used with caution by patients receiving bortezomib. Vitamin C interferes with the ability of bortezomib to kill human cancer cell lines in culture, apparently by binding to bortezomib and inactivating it (Zou et al., 2006). Alpha lipoic acid, often recommended for the treatment of peripheral neuropathy, has been shown to interfere with antimyeloma effects of bortezomib in myeloma cell lines (Steinberg et al., 2009). In addition, preclinical (in vitro and in vivo) research demonstrated tumor cell death caused by bortezomib may be negated by the use of green tea (Golden et al., 2009). Although additional research is warranted, and no clinical evidence exists, the International Myeloma Foundation Nurse Leadership Board recommends avoidance of vitamin C, alpha lipoic acid, and green tea on the day of bortezomib therapy.
Calcium and vitamin D supplementation is advised for all patients with osteopenia or osteoporosis, particularly if they are receiving bisphosphonates such as pamidronate or zoledronic acid. The NOF's recommendations for calcium and vitamin D supplementation are provided at http://nof.org/aboutosteoporosis/prevention/calcium.
In summary, for people older than age 50, 1,200 mg of calcium and 800–1,000 IU of vitamin D daily are recommended. Those younger than age 50 do no require as much, with 1,000 mg of calcium and 400–800 IU of vitamin D daily recommended. Of note, however, individuals with hypercalcemia or renal insufficiency should not take calcium replacement (NOF, 2010), both of which may be concerns for patients with MM. A discussion with a healthcare provider should take place.
Radiation as Treatment for Bone Disease
Care of patients receiving radiation for local pain and tumor control includes alleviating side effects related to the radiated field. The potential for side effects is dose dependent. External beam doses can range from 600–800 cGy hemi-body irradiation for palliation of generalized pain, 3,000 cGy for a localized painful bone lesion, and to 4,500 cGy for tumor control. Patients receiving lower-dose radiation for pain palliation have less potential for side effects, but all should be monitored closely (Terpos & Dimopoulos, 2005). For example, patients with head and neck radiation may have trouble eating and swallowing and may experience mouth sores. For these patients, good oral hygiene is necessary to prevent mouth sores and decrease the risk of infections. Pain medication may be indicated if severe mouth sores or radiation burns to the skin develop. Individuals who receive mediastinal, thoracic, or lumbar spine radiation may develop nausea, loss of appetite, or vomiting. Assessment of hydration and calorie counts and evaluation by a dietitian may be necessary if intake is compromised. All patients receiving cumulative external beam radiation therapy may be at risk for radiation dermatitis and are encouraged to keep their skin well hydrated with nonalcohol lotions applied after radiation (Berkey, 2010).
Other common side effects of radiation include fatigue and pain. Patients should be made aware of fatigue as a side effect and be educated about managing their activities. Pain medication may be warranted because individuals are required to remain in an uncomfortable position during the radiation treatment. Opioid or nonopioid analgesics may enhance comfort during and after radiation treatment. A baseline and ongoing pain assessment is integral to the patient's plan of care.
Vertebroplasty or Kyphoplasty
Several studies suggest balloon kyphoplasty and vertebroplasty are effective in reducing pain scores associated with vertebral compression fracture, but each procedure has associated risks. Potential risk is related to the cement injection resulting in extravasation and causing nerve damage or other neurologic issues. General risks include those associated with any surgical procedure, namely increased risk of bleeding and infection. Although minimal blood loss occurs with vertebroplasty and balloon kyphoplasty, this risk increases if patients are thrombocytopenic or are receiving low molecular weight heparin, heparin, warfarin, or antiplatelet agents, aspirin, or nonsteroidal anti-inflammatory drugs (NSAIDs). Although specific recommendations are not documented, practices followed by Nurse Leadership Board members at their institutions include stopping aspirin and NSAIDs seven days prior to the procedure. Management should be patient specific. Therapeutic anticoagulation should be bridged from warfarin to low molecular weight heparin or heparin, and this should be stopped within six hours of the procedure. Baseline complete blood count, including platelets, white blood count differential, and coagulation studies such as prothrombin time, international normalized ratio, and partial thromboplastin time may be indicated.
Infection risk could rise because of a low white blood cell count as a result of marrow infiltration by myeloma or as a result of the treatment itself. Clinicians should assess complete blood count with white blood cell differential if patients are receiving myelosuppressive antimyeloma treatment.
Mobility and exercise can be encouraged almost immediately after the procedure. The cement used creates an internal cast that hardens within minutes. Patients can resume their usual activities and, if this procedure proves effective at decreasing pain, patients can be weaned off pain medications gradually. The use of slings and orthotic braces are not usually encouraged unless used in preparation for planned surgical intervention because they may reduce mobility and lead to osteopenia (Tariman & Estrella, 2005).
Bisphosphonates
Bisphosphonates should be administered monthly for a total duration of two years in individuals with lytic lesions or osteoporosis. The International Myeloma Working Group suggests that using pamidronate or zoledronic acid decreases the risk of skeletal-related events, but the optimal duration of bisphosphonates therapy is unknown (Durie et al., 2007). In patients achieving very good partial response or better from their initial treatment as defined as greater than a 90% reduction in serum or urine paraprotein, bisphosphonates should be given on a monthly basis for one year. Although clear evidence does not exist for modified administration, the International Myeloma Working Group members have agreed that patients should continue bisphosphonates use if they have active bone disease or achieve less than a very good partial response to treatment. If, after two years, the patient does not have evidence of active bone disease, bisphosphonate use may be discontinued. Bisphosphonates should be resumed at the time of relapse if they were discontinued (Durie et al., 2007; Kyle et al., 2007).
Although the aim is to reduce skeletal-related events, bisphosphonates can have adverse effects. Side effects of bisphosphonates include acute phase reactions, a small but increased risk of osteonecrosis of the jaw (ONJ), and renal impairment (Maxwell, Swift, Goode, Doane, & Rogers, 2003). Acute phase reactions present as flu-like symptoms after the bisphosphonate infusion. These symptoms can be decreased with the use of acetaminophen. Other side effects include nausea, fatigue, and bone pain.
Monitoring and surveillance for ONJ is necessary. ONJ is an uncommon but serious condition usually involving the maxilla or mandible that may occur with prolonged use of bisphosphonates. The incidence is relatively low, at 2%–10%. Signs of ONJ may include jaw or tooth pain, and exposed bone may be identified on physical examination. If ONJ symptoms occur, initial treatment should be with antibiotic therapy and not surgical procedures (Cafro et al., 2008). ONJ is thought to be an infectious process, and the use of antibiotic prophylaxis for dental procedures has been studied. The Italian Myeloma Group evaluated patients receiving either amoxicillin-clavulanate 2 g per day by mouth, levofloxacin 500 mg per day by mouth, beginning a day prior, up to three days after a dental procedure, or standard care (no antibiotics). In patients receiving antibiotic prophylaxis with amoxicillin-clavulanate, a decreased incidence of infection with dental procedures such as cleaning, implants, and extractions was noted. More studies are required to validate these findings (Montefusco et al., 2008).
Prevention of ONJ is important, and good dental hygiene is integral to the prevention of ONJ in patients with MM. Patients should receive baseline and routine dental examinations every six months. In addition, dentures should fit well because poor-fitting dentures increase the risk of ONJ (Vahtsevanos et al., 2009). Patients should advise their dental care providers when they are receiving bisphosphonates.
Renal impairment is common in patients with MM. Precautions concerning the use of bisphosphonates in patients with renal impairment are discussed in Faiman et al. (2011).
Pain Management
Damage to bones as a result of MM often causes pain; effective doses of analgesia should be administered for patient comfort and to increase mobility and quality of life. A good pain history is essential to assessing the quality and character of a patient's
 pain. The pain assessment includes asking a patient about the following.
pain. The pain assessment includes asking a patient about the following.
Onset: When did it start? Was trauma involved?
Location: Where is it located?
Quality: Dull, sharp, burning, or stabbing?
Duration: How long have you had this pain?
Character: Is it present when you sit or move? All the time or some of the time?
In addition, patients should be asked to rate their pain according to a pain scale. One of the most common scales is a 1–10 rating system with 1 as minimal pain and 10 as the worst pain. Treatment of the pain with analgesics can be accomplished by using the World Health Organization (2009) pain ladder. Additional management strategies include the use of bisphosphonates and systemic antimyeloma therapy that may have a marked effect on bone pain.
Bone pain from MM can be challenging to control because it usually occurs when patients change position or walk and is often called “incident pain.” Three main types of analgesia are used to treat bone pain and other types of pain related to MM and include nonopioid analgesics, opioid analgesics, and adjunct medications. Nonopioid analgesia includes acetaminophen, NSAIDs, and aspirin. Use of NSAIDs is discouraged because of the risk of renal injury. Many opioid analgesics exist and also can decrease pain, but at the expense of sedation and the risk of constipation. Neuropathic pain often is present after nerve-related injuries such as in peripheral neuropathy, post-herpetic neuralgia, or spinal canal compromise and often is described as “burning or shooting” in character. Medications such as gabapentin or pregabalin, or tricyclic antidepressants such as amitriptyline, can be helpful in decreasing this type of pain (Levy, 1996). Systemic antimyeloma therapy and the use of bisphosphonates will not be effective in reducing pain in some patients, and this group may require bracing or surgical intervention.
Effective pain management is imperative because the psychosocial aspects of pain can lead to depression and anxiety. In addition, uncontrolled pain will result in immobility and muscle and bone wasting, in turn leading to increased risks of atelectasis, pneumonia, and venous thromboembolism. Healthcare providers must recognize this, diligently monitor pain scores, and prescribe analgesia to combat these deleterious side effects (Coleman, 2000).
Patient and Family Education
Nurses play an important role in educating patients as well as promoting functional independence. Activity can decrease fatigue as well as improve mood and prevent insomnia. However, even simple activities can be difficult for patients with pain. All patients must have effective pain management. Patients should be educated regarding the use of NSAIDs and narcotics as educated patients are more likely to adhere to their therapy. Because bone disease can be debilitating throughout the continuum of treatment, a dire need exists to instruct and educate patients and their caregivers regarding the signs and symptoms of depression and anxiety. Interdisciplinary collaboration may be required in the education of the patient. All patients will have varying needs for education regarding their bone health; emphasis on the benefits of treatment will assist patients in adhering to their therapy.
Educational opportunities include the following.
Using evidence-based recommendations to promote behaviors that enhance bone health
Instructing patients on how to rate pain using a pain scale
Informing patients about symptoms of hypercalcemia, a complication of bone disease that includes lethargy, nausea, confusion, thirst, and constipation
Enforcing the need for bisphosphonate use; advocating good oral hygiene while patients are receiving bisphosphonates
Referring patients to a clinical social worker or psychiatrist to identify anxiety and depression if necessary
Counseling to encourage continued adherence to pharmacologic regimens and exercise programs
Useful resources and tools for patients and caregivers, as well as nurses and other healthcare providers, can be obtained from the International Myeloma Foundation at www.myeloma.org.
Conclusion
Recent research in myeloma and the advent of novel agents has led to increased response rates and improved survival. Bone disease as a prognostic indicator and therapies directed at bone disease may alter future treatment regimens and outcomes. Supportive care and the management of bone disease are of the utmost importance to alleviate bone-related sequelae, enhance mobility and disease response, and promote quality of life. Oncology nurses and other healthcare providers play a crucial part in assessing and managing myeloma, along with educating patients, as they live longer and continue to experience both disease- and treatment-related complications.
At a Glance.
-
✦
Most patients with multiple myeloma will develop osteolytic bone lesions. Pathologic fractures and other skeletal events can result in poor circulation, blood clots, muscle wasting, and decreased survival.
-
✦
Supportive care targeting bone disease is an essential part of myeloma therapy.
-
✦
Oncology nurses play a key role in the management of bone disease and maintenance of adequate bone health throughout the course of myeloma.
Acknowledgments
The authors take full responsibility for the content of this article. Publication of this supplement was made possible through an unrestricted educational grant to the International Myeloma Foundation from Celgene Corp. and Millennium: The Takeda Oncology Company. Colson is a consultant with Merck & Co., Inc., and Millennium: The Takeda Oncology Company; Faiman is a consultant and on the speakers bureau at Celgene Corp. and Millennium: The Takeda Oncology Company; Miller is on the advisory board and speakers bureau at Celgene Corp. and Millennium: The Takeda Oncology Company; and Tariman is a consultant with Millennium: The Takeda Oncology Company. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by independent peer reviewers or editorial staff.
Digital Object Identifier: 10.1188/11.S1.CJON.9-23
The authors gratefully acknowledge Brian G.M. Durie, MD, and Robert A. Kyle, MD, for critical review of the manuscript; Lynne Lederman, PhD, medical writer for the International Myeloma Foundation, for preparation of the manuscript; and Lakshmi Kamath, PhD, at ScienceFirst, LLC, for assistance in preparation of the manuscript.
References
- Adami S. Bone health in diabetes: Considerations for clinical management. Current Medical Research and Opinion. 2009;25:1057–1072. doi: 10.1185/03007990902801147. doi:10.1185/03007990902801147. [DOI] [PubMed] [Google Scholar]
- Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: A paradox. American Journal of Clinical Nutrition. 2008;88:545S–550S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angtuaco EJ, Fassas AB, Walker R, Sethi R, Barlogie B. Multiple myeloma: Clinical review and diagnostic imaging. Radiology. 2004;231:11–23. doi: 10.1148/radiol.2311020452. doi:10.1148/radiol.2311020452. [DOI] [PubMed] [Google Scholar]
- Awan N, Azer A, Harrani K, Cogley D. Intramedullary spread of tumour cells during IM nailing: A histological diagnosis. European Journal of Orthopaedic Surgery and Traumatology. 2002;12:53–55. doi:10.1007/s00590-002-0013-x. [Google Scholar]
- Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr., van Rhee F, Anaissie E, Barlogie B. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. doi:10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schedchtmann K, Tillman JT, Cancer Patient Fracture Evaluation Investigators Balloon kyphoplasty versus nonsurgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: A multicentre, randomized controlled trial. Lancet Oncology. 2011 doi: 10.1016/S1470-2045(11)70008-0. Advanced online publication. doi:10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Rajdev L, Border M. Bone complications in multiple myeloma. Cancer Biology and Therapy. 2006;5:1082–1085. doi: 10.4161/cbt.5.9.3307. [DOI] [PubMed] [Google Scholar]
- Berkey FJ. Managing the adverse effects of radiation therapy. American Family Physician. 2010;82:381–388. [PubMed] [Google Scholar]
- Bischoff-Ferrari HA. The 25-hydroxyvitamin D threshold for better health. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3–5):614–619. doi: 10.1016/j.jsbmb.2006.12.016. doi:10.1016/j.jsbmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Bolzoni M, Abeltino M, Storti P, Bonomini S, Agnelli L, Todoerti K, Giuliani N. The immunomodulator drugs lenalidomide and pomalidomide inhibit multiple myeloma-induced osteoclast formation and RANKL/OPG ratio in myleoma microenvironment targeting the expression of adhesion molecules [Abstract 448] 2010 doi: 10.1016/j.exphem.2012.11.005. Retrieved from http://ash.confex.com/ash/2010/webprogram/Paper29811.html. [DOI] [PubMed]
- Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, Anderson KC. Lenalidomide inhibits osteoclastogenesis, survival factors, and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–1932. doi: 10.1038/leu.2008.174. doi:10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- Cady B, Easson A, Aboulafia AJ, Ferson PF. Part 1: Surgical palliation of advanced illness—What's new, what's helpful. Journal of the American College of Surgeons. 2005;200:115–127. doi: 10.1016/j.jamcollsurg.2004.10.007. doi:10.1016/j.jamcollsurg.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Cafro AM, Barbarano L, Nosari AM, D'Avanzo G, Nichelatti M, Bibas M, Andriani A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Definition and management of the risk related to zoledronic acid. Clinical Lymphoma and Myeloma. 2008;8:111–116. doi: 10.3816/clm.2008.n.013. doi:10.3816/CLM.2008.n.013. [DOI] [PubMed] [Google Scholar]
- Cashman KD. Diet, nutrition, and bone health. Journal of Nutrition. 2007;137(11, Suppl):2507S–2512S. doi: 10.1093/jn/137.11.2507S. [DOI] [PubMed] [Google Scholar]
- Chau DL, Edelman SV. Osteoporosis and diabetes. Clinical Diabetes. 2002;20:153–157. doi:10.2337/diaclin.20.3.153. [Google Scholar]
- Coleman RE. Management of bone metastases. Oncologist. 2000;5:463–470. doi: 10.1634/theoncologist.5-6-463. doi:10.1634/theoncologist.5-6-463. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Metastatic bone disease: Clinical features, pathophysiology, and treatment strategies. Cancer Treatment Reviews. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. doi:10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical Cancer Research. 2006;12(20, Pt. 2):6243S–6249S. doi: 10.1158/1078-0432.CCR-06-0931. doi:10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Moulopoulos LA, Terpos E. A new pet for myeloma. Blood. 2009;114:2007–2008. doi: 10.1182/blood-2009-06-224196. doi:10.1182/blood-2009-06-224196. [DOI] [PubMed] [Google Scholar]
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. doi:10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Durie BGM. The role of anatomic and functional staging in myeloma: Description of Durie/Salmon PLUS staging system. European Journal of Cancer. 2006;42:1539–1543. doi: 10.1016/j.ejca.2005.11.037. doi:10.1016/j.ejca.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Durie BGM, Attal M, Beksac M, Belch A, Bensinger W, Blade J, Zonder J. Use of bisphosphonates in multiple myeloma: IMWG response to Mayo Clinic consensus statement. Mayo Clinic Proceedings. 2007;82:516–517. doi: 10.4065/82.4.516. doi:10.4065/82.4.516. [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP. Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. Journal of Bone Mineral Research. 1999;14:342–350. doi: 10.1359/jbmr.1999.14.3.342. doi:10.1359/jbmr.1999.14.3.342. [DOI] [PubMed] [Google Scholar]
- Epstein J, Walker R. Myeloma and bone disease: “The dangerous tango.”. Clinical Advances in Hematolology and Oncology. 2006;4:300–306. [PubMed] [Google Scholar]
- Esteve FR, Roodman GD. Pathophysiology of myeloma bone disease. Best Practice in Research and Clinical Haematology. 2007;20:613–624. doi: 10.1016/j.beha.2007.08.003. doi:10.1016/j.beha.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Hilmer SN, Brand-Miller JC, Jamison HA, Truswell AS, Sharma AP, Le Couteur DG. Dietary approaches that delay age-related diseases. Clinical Interventions in Aging. 2006;1:11–31. doi: 10.2147/ciia.2006.1.1.11. doi:10.2147/ciia.2006.1.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman B, Bilotti E, Mangan PA, Rogers K, The International Myeloma Foundation Nurse Leadership Board Consensus statement for the management of steroid-associated side effects in patients with multiple myeloma. Clinical Journal of Oncology Nursing. 2008;12(3, Suppl):53–62. doi: 10.1188/08.CJON.S1.53-62. doi:10.1188/08.CJON.S1.53-62. [DOI] [PubMed] [Google Scholar]
- Faiman B, Mangan PA, Spong J, Tariman JD, International Myeloma Foundation Nurse Leadership Board Renal complications in multiple myeloma and related disorders: Survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clinical Journal of Oncology Nursing. 2011;15(4, Suppl):66–76. doi: 10.1188/11.CJON.S1.66-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MI, Maxwell C. Bisphosphonate therapy for metastatic bone disease: The pivotal role of nurses in patient education. Oncology Nursing Forum. 2008;35:709–713. doi: 10.1188/08.ONF.709-713. doi:10.1188/08.ONF.709-713. [DOI] [PubMed] [Google Scholar]
- Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Schonthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–5937. doi: 10.1182/blood-2008-07-171389. doi:10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BGM, Crowley JJ, Barlogie B, Blade J, Westin J. International staging system for multiple myeloma. Journal of Clinical Oncology. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. doi:10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Guise TA. Bone loss and fracture risk associated with cancer therapy. Oncologist. 2006;11:1121–1131. doi: 10.1634/theoncologist.11-10-1121. [DOI] [PubMed] [Google Scholar]
- Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology (Williston Park) 2000;14:101–108. [PubMed] [Google Scholar]
- Kang MI, Lee WY, Oh KW, Han JH, Song KH, Cha BY, Kim CC. The short-term changes of bone mineral metabolism following bone marrow transplantation. Bone. 2000;26:275–279. doi: 10.1016/s8756-3282(99)00265-3. doi:10.1016/S8756-3282(99)00265-3. [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV. Bisphosphonates in multiple myeloma. Cancer. 2000;88(12, Suppl):3022–3032. doi: 10.1002/1097-0142(20000615)88:12+<3022::aid-cncr19>3.0.co;2-r. doi:10.1002/1097-0142(20000615)88:12+<3022::AID-CNCR19>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kerschan-Schindl K, Mitterbauer M, Fureder W, Kudlacek S, Grampp S, Bieglmayer C, Kalhs P. Bone metabolism in patients more than five years after bone marrow transplantation. Bone Marrow Transplant. 2004;34:491–496. doi: 10.1038/sj.bmt.1704618. doi:10.1038 /sj.bmt.1704618. [DOI] [PubMed] [Google Scholar]
- Kielholz U, Max R, Scheibenbogen C, Wuster C, Korbling M, Haas R. Endocrine function and bone metabolism 5 years after autologous bone marrow/blood-derived progenitor cell transplantation. Cancer. 1997;79:1617–1622. doi: 10.1002/(sici)1097-0142(19970415)79:8<1617::aid-cncr27>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Child JA, Anderson K, Barlogie B, Bataille R, Bensinger W, International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma, and related disorders: A report of the International Myeloma Working Group. British Journal of Haematology. 2003;121:749–757. doi:10.1046/j.1365-2141.2003.04355.x. [PubMed] [Google Scholar]
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Greipp PR. Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proceedings. 2003;78:21–33. doi: 10.4065/78.1.21. doi:10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Epidemiology of the plasma-cell disorders. Best Practice and Research Clinical Hematology. 2007;20:637–664. doi: 10.1016/j.beha.2007.08.001. doi:10.1016/j.beha.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Schreiman JS, McLeod RA, Beabout JW. Computed tomography in diagnosis and management of multiple myeloma and its variants. Archives of Internal Medicine. 1985;145:1451–1452. doi:10.1001/archinte.145.8.1451. [PubMed] [Google Scholar]
- Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, Anderson K. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology. 2007;25:2464–2672. doi: 10.1200/JCO.2007.12.1269. doi:10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- Lee WY, Cho SW, Oh ES, Oh KW, Lee JM, Yoon KH, Kim CC. The effect of bone marrow transplantation on the osteoblastic differentiation of human bone marrow stromal cells. Journal of Clinical Endocrinology and Metabolism. 2002;87:329–335. doi: 10.1210/jcem.87.1.8135. doi:10.1210/jc.87.1.329. [DOI] [PubMed] [Google Scholar]
- Lee WY, Kang MI, Baek KH, Oh ES, Oh KW, Lee KW, Kim CC. The skeletal site-differential changes in bone mineral density following bone marrow transplantation: Three-year prospective study. Journal of Korean Medical Sciences. 2002;17:749–754. doi: 10.3346/jkms.2002.17.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg V. Complications of chronic kidney disease. American Journal of Nursing. 2005;105(6):40–49. doi: 10.1097/00000446-200506000-00024. [DOI] [PubMed] [Google Scholar]
- Levy MH. Pharmacologic treatment of cancer pain. New England Journal of Medicine. 1996;335:1124–1132. doi: 10.1056/NEJM199610103351507. doi:10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Zojer N. Supportive care in multiple myeloma. Best Practice and Research in Clinical Haematology. 2007;20:817–835. doi: 10.1016/j.beha.2007.10.001. doi:10.1016/j.beha.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mariette X, Zagdanski AM, Guermazi A, Bergot C, Arnould A, Frija J, Fermand JP. Prognostic value of vertebral lesions detected by magnetic resonance imaging in patients with stage I multiple myeloma. British Journal of Haematology. 1999;104:723–729. doi: 10.1046/j.1365-2141.1999.01244.x. doi:10.1046/j.1365-2141.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- Mauck KF, Clarke BL. Diagnosis, screening, prevention, and treatment of osteoporosis. Mayo Clinic Proceedings. 2006;81:662–672. doi: 10.4065/81.5.662. doi:10.4065/81.5.662. [DOI] [PubMed] [Google Scholar]
- Maxwell C, Swift R, Goode M, Doane L, Rogers M. Advances in supportive care of patients with cancer and bone metastasis: Nursing implications of zoledronic acid. Clinical Journal of Oncology Nursing. 2003;7:403–408. doi: 10.1188/03.CJON.403-408. doi:10.1188/03.CJON.403-408. [DOI] [PubMed] [Google Scholar]
- Melton LJ, III, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: A population-based study. Journal of Bone and Minereral Research. 2005;20:487–493. doi: 10.1359/JBMR.041131. doi:10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Golden SH. Depression and osteoporosis: Epidemiology and potential mediating pathways. Osteoporosis International. 2008;9:1–12. doi: 10.1007/s00198-007-0449-2. doi:10.1007/s00198-007-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Golden SH, Wand G, Lee HB. Depression, antidepressants, and bone mineral density in a population-based cohort. Journal of Gerontology A, Biologic Science and Medical Science. 2008;63:1410–1415. doi: 10.1093/gerona/63.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefusco V, Gay F, Spina F, Miceli R, Maniezzo M, Ambrosini MT, Corradini P. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leukemia and Lymphoma. 2008;49:2156–2162. doi: 10.1080/10428190802483778. doi:10.1080/10428190802483778. [DOI] [PubMed] [Google Scholar]
- Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, National Cancer Research Institute Haematological Oncology Clinical Study Group First-line treatment with zoledronic acid as compared with clodonic acid in multiple myeloma (MRC Myeloma IX): A randomized controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. doi:10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology™: Multiple myeloma [v.3.2010] 2010 Retrieved from http://www.nccn.org/professionals/physician_gls/f_guide lines.asp.
- National Osteoporosis Foundation Fast facts on osteoporosis, 2010. 2010 Retrieved from http://www.nof.org/osteoporosis/diseasefacts.htm#top.
- Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib, suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. American Journal of Hematology. 2009;84:6–14. doi: 10.1002/ajh.21310. doi:10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome S, Jenkins B, Lilleby K, International Myeloma Foundation Nurse Leadership Board Mobility and safety in the multiple myeloma survivor: Survivorship care plan of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing. 2011;15(4, Suppl):41–52. doi: 10.1188/11.S1.CJON.41-52. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. New England Journal of Medicine. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. doi:10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Skeletal imaging and management of bone disease. Hematology: American Society of Hematology Education Program. 2008:313–319. doi: 10.1182/asheducation-2008.1.313. [DOI] [PubMed] [Google Scholar]
- Sezer O. Myeloma bone disease: Recent advances in biology, diagnosis, and treatment. Oncologist. 2009;14:276–283. doi: 10.1634/theoncologist.2009-0003. doi:10.1634/theoncologist.2009-0003. [DOI] [PubMed] [Google Scholar]
- Steinberg JA, Shen J, Sanchez E, Chen H, Li ZW, Hilger J, Berenson JR. Alpha lipoic acid (ALA) inhibits the antimyeloma effects of bortezomib [Abstract 3832] 2009 Retrieved from http://ash.confex.com/ash/2009/webprogram/Paper19795.html.
- Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. Journal of Bone and Mineral Research. 2008;23:95–102. doi: 10.1359/jbmr.070903. doi:10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- Tariman J, Faiman B. Multiple myeloma. In: Yarbro CH, Wujcik D, Gobel BH, editors. Cancer nursing principles and practice. 7th ed. Jones and Bartlett; Boston, MA: 2011. pp. 1513–1545. [Google Scholar]
- Tariman JD, Estrella SM. The changing treatment paradigm in patients with newly diagnosed multiple myeloma: Implications for nursing [Online exclusive] Oncology Nursing Forum. 2005;32:E127–E138. doi: 10.1188/05.ONF.E127-E138. doi:10.1188/05.ONF.E127-E138. [DOI] [PubMed] [Google Scholar]
- Terpos E, Christoulas D, Kastritis E, Katodritou E, Kyrtsonis MC, Papanikolaou X, Dimopoulos MA. Effect of lenalidomide-based regimens on bone remodeling in patients with relapsed/refractory multiple myeloma [Abstract 0192]. Presented at the European Hematology Association 13th Congress in Copenhagen; Denmark. Jun, 2008. [Google Scholar]
- Terpos E, Christoulas D, Migkou M, Gavriatopoulou M, Papatheodorou A, Eleutherakis-Papaiakouvou E, Dimopoulos MA. Consolidation therapy with bortezomib, thalidomide, and dexamethasone (VTD) regimen after ASCT in myleoma patients who do not receive bisphosphonates reduces bone resorption and RANKL/OPG ratio but seems to have no effect on bone formation and angiogenesis [Abstract: 3863] 2009 Retrived from http://ash.confex.com/ash/2009/webprogram/Paper18577.html.
- Terpos E, Dimopoulos MA. Myeloma bone disease: Pathophysiology and management. Annals of Oncology. 2005;16:1223–1231. doi: 10.1093/annonc/mdi235. doi:10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- Tryciecky EW, Gottschalk A, Ludema K. Oncologic imaging: Interactions of nuclear medicine with CT and MRI using the bone scan as a model. Seminars in Nuclear Medicine. 1997;27:142–151. doi: 10.1016/s0001-2998(97)80044-x. [DOI] [PubMed] [Google Scholar]
- Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, Antoniades K. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. Journal of Clinical Oncology. 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. doi:10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology. 2005;1:373–376. doi: 10.1182/asheducation-2005.1.373. [DOI] [PubMed] [Google Scholar]
- Wick JY. Spontaneous fracture: Multiple causes. Consultant Pharmacologist. 2009;24:100–102. 105–108, 110–102. doi: 10.4140/tcp.n.2009.100. [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO's pain ladder. 2009 Retrieved from http://www.who.int/cancer/palliative/painladder/en. [Google Scholar]
- Yeh HS, Berenson JR. Treatment for myeloma bone disease. Clinical Cancer Research. 2006;12(20, Part 2):6279s–6284s. doi: 10.1158/1078-0432.CCR-06-0681. doi:10.1158/1078-0432.CCR-06-0681. [DOI] [PubMed] [Google Scholar]
- Zou W, Yue P, Lin N, He M, Zhou Z, Lonial S, Sun SY. Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. Clinical Cancer Research. 2006;12:273–280. doi: 10.1158/1078-0432.CCR-05-0503. doi:10.1158/1078-0432.CCR-05-0503. [DOI] [PubMed] [Google Scholar]