Abstract
The purple pigment violacein is well known for its numerous biological activities including antibacterial, antiviral, antiprotozoan, and antitumor effects. In the current study we identify violacein as the antinematode agent produced by the marine bacterium Microbulbifer sp. D250, thereby extending the target range of this small molecule. Heterologous expression of the violacein biosynthetic pathway in E. coli and experiments using pure violacein demonstrated that this secondary metabolite facilitates bacterial accumulation in the nematode intestine, which is accompanied by tissue damage and apoptosis. Nematodes such as Caenorhabditis elegans utilise a well-defined innate immune system to defend against pathogens. Using C. elegans as a model we demonstrate the DAF-2/DAF-16 insulin/IGF-1 signalling (IIS) component of the innate immune pathway modulates sensitivity to violacein-mediated killing. Further analysis shows that resistance to violacein can occur due to a loss of DAF-2 function and/or an increased function of DAF-16 controlled genes involved in antimicrobial production (spp-1) and detoxification (sod-3). These data suggest that violacein is a novel candidate antinematode agent and that the IIS pathway is also involved in the defence against metabolites from non-pathogenic bacteria.
Introduction
Parasitic nematodes are an important group of human, animal and plant pathogens representing a major threat not only to public health, but also to livestock and agricultural industries around the globe [1]. Yet heavy reliance on the few available chemotherapeutic agents has resulted in the development of nematode resistance and little progress has been made in the search for new treatments [2], [3]. Thus there is an urgent requirement for the discovery of new antinematode compounds that can be developed into chemotherapeutic drugs.
The nematode C. elegans is a powerful model organism used broadly across the fields of cellular biology, developmental biology and neurobiology, and more recently also as a model organism for the study of host-microbial interactions with a focus on pathogenesis and drug discovery [4], [5]. In a recent functional screen of genomic libraries of marine bacteria, a number of fosmid clones expressing high toxicity towards C. elegans were identified [6]. One of the highly toxic clones (designated 20G8) with a sequence-insert originating from the marine bacterium Microbulbifer sp. D250, expressed a violet pigment. Genetic analysis of the insert revealed that the clone 20G8 contained genes encoding for the synthesis of the indole-antibiotic violacein (vioA-E) [6], suggesting that this metabolite is responsible for its toxic phenotype.
Violacein is produced by several bacterial species thriving in a range of habitats such as terrestrial, marine, fresh water and glacier environments. Some of the known violacein producing organisms include Chromobacterium violaceum [7], Collimonas sp. [8], Duganella sp. [9], Janthinobacterium lividum [10], [11] and Pseudoalteromonas spp. [12], [13]. Violacein exhibits several biological activities with ecological relevance. Firstly, violacein has been suggested to be involved in oxidative stress resistance in C. violaceum [14]. Secondly, violacein producing bacteria (namely J. lividum) have been implicated in the natural defence of amphibians to fungal disease [15]. Finally, Matz and colleagues [12] have demonstrated that in the marine bacterium Pseudoalteromonas tunicata, violacein production can act as an antipredator defence mechanism against protozoan grazers. In addition to its ecological significance, violacein has also gained increasing importance for its potential medical and industrial applications. The biological activities of this compound include antioxidant, leishmanicidal, trypanocidal, antifungal, antiviral, antibacterial and antiprotozoal effects, as well as antitumoral and apoptosis-inducing activities in mammalian cancer cells (reviewed in [16]).
Although violacein has a broad range of activities, the direct molecular and cellular targets remain unknown. In addition there is limited understanding of its activity in multicellular eukaryotes and whether metazoans have the capacity to mount a defence to neutralize its activity or not. Whilst lacking adaptive immune strategies, nematodes, such as C. elegans, are suitable models to investigate metazoan defence as they posses a sophisticated innate immune system that protects against toxic microorganisms [17]. Key features of the nematodes defence include the conserved immune regulatory pathways, i.e., the p38 mitogen activated protein kinase (MAPK) and the insulin/IGF-1 signalling (IIS) pathways [18]. In particular the IIS pathway with the gene regulators DAF-2 and DAF-16, is increasingly recognised for its important role in stress response, aging and immune homeostasis across nematodes, insects and mammals [18], [19]. Furthermore, the IIS pathway is known to play a key role in the innate immune response against different pathogen-induced stresses, including colonization [17], [20], [21] and bacterial virulence factors [22].
In C. elegans the binding of insulin to DAF-2 triggers a phosphorylation cascade that results in activation of PDK-1 (3-phosphoinositide-dependent kinase 1) and eventual retention of the DAF-16 transcriptional activator in the cytoplasm [23]. De-activation or loss of DAF-2 function allows DAF-16 to move to the nucleus where it enhances the expression of genes including among others sod-3 (superoxide dismutase), spp-1 (SaPosin-like Protein) and lys-7, which are involved in detoxification, antimicrobial peptide expression and antimicrobial lysozyme production, respectively [24], [25], [26]. Moreover recent data indicates that the canonical IIS signalling diverges at PDK-1 into a second arm of the pathway mediated by the protein WWP-1 (WW domain protein 1) [22]. In the present study, we hypothesised that C. elegans makes use of this immune response pathway not only in the situation of infection by pathogenic organisms, but also to neutralize the effect of toxic bacterial secondary metabolites, such as violacein that originate from non-pathogens. Furthermore studying the mechanisms in which C. elegans mediates resistance to bacterial metabolites may shed further light into their molecular/cellular targets. To address this hypothesis, we first confirm that violacein is responsible for the toxic activity against C. elegans in clone 20G8 and its parental strain Microbulbifer sp. D250. We further show that the expression of enzymes that synthesize violacein in E. coli facilitates bacterial accumulation in the host intestine and induces apoptosis in the nematode. Finally we demonstrate that the IIS immune pathway modulates C. elegans sensitivity to violacein toxicity, most likely via the control of genes involved in detoxification and antimicrobial production.
Materials and Methods
Strains and culture conditions
All bacterial strains and vectors used in this study are listed in Table 1. Bacteria were grown in Luria broth (LB10), nematode growth medium (NGM) [27] or marine broth (Difco Laboratories, Maryland) [28] as indicated, and stored in 30% (v/v) glycerol at −80°C. Solid medium was prepared by the addition of 19 g of agar (Oxoid, Australia) per litre of culture fluid. All strains were grown at 25°C. Where required (see Table 1), chloramphenicol (12.5 µg/ml), kanamycin (100 µg/ml), and L-arabinose (0.02%, w/v) were added to the media. C. elegans strains (listed in Table 2) were maintained at 20°C on NGM agar plates spread with E. coli OP50 as a food source [29], [30]. C. elegans strains were stored in glycerol (70:30 vol/vol) at −80°C [30].
Table 1. Bacterial strains and vectors used in this study.
| Strain/Vector | Relevant characteristic or genotype | Source or reference |
| E. coli EPI300-T1R | F-mcrA Δ(mrrhsdRMSmcrBC) φ80dlacZΔM15ΔlacX 74 recA1 endA1 araD139 Δ(ara, leu) 7697galU galK λ- rpsL nupG trfA tonA dhfr | Epicentre |
| E. coli 20G8 | Fosmid 20G8 cloned in EPI300-T1R; Cmr | This study |
| E. coli 20G8vioA- | Fosmid 20G8 mutated in vioA gene and cloned in EPI300-T1R; Cmr, Kanr | This study |
| E. coli 20G8vioB- | Fosmid 20G8 mutated in vioB gene and cloned in EPI300-T1R; Cmr, Kanr | This study |
| E. coli 20G8vioC- | Fosmid 20G8 mutated in vioC gene and cloned in EPI300-T1R; Cmr, Kanr | This study |
| E. coli 20G8vioD - | Fosmid 20G8 mutated in vioD gene and cloned in EPI300-T1R; Cmr, Kanr | This study |
| E. coli OP50 | Uracil auxotroph | [29] |
| Microbulbifer sp. D250 | Wild type strain | [62] |
| Microbulbifer sp. D250 dv2 | D250 strain mutated in vioB gene; Kanr | This study |
| pLof/Tn10 KM | Mini-Tn10 (Kanr); Amp r | [34] |
| pCC1FOSa | Fosmid backbone for genomic library; Cmr | Epicentre |
Copy number inducible by arabinose.
Table 2. C. elegans strains used in this study.
| Strain name | Genotype/allele designation | Relevant characteristics | Source or reference |
| N2 Bristol | C. elegans wild isolate | Wild type isolate | CGCa |
| CU1546 | smIs34 | ced-1p::ced-1::GFP + rol-6(su1006) | CGCa |
| CB1370 | daf-2(e1370) III | Mutated in the insulin-like receptor DAF-2. Temperature sensitive dauer constitutive | CGCa |
| IU10 | daf-16(mgDf47) I; rrf-3(pk1426) II | Mutated in the FOXO-family transcription factor DAF-16 | CGCa |
| TJ356 | zIs356 IV | Integrated DAF-16::GFP roller strain. Daf-c, Rol, fluorescent DAF-16::GFP. Overexpression of DAF-16 | CGCa |
| JT9609 | pdk-1 (Sa680) x | Mutation in the gene encoding for 3-phosphoinositide-dependent protein kinase | CGCa |
| RB1178 | wwp-1(ok1102) I. | Mutation in the gene encoding for the WW domain protein 1 | CGCab |
| TM127 | daf-2(e1370) III; sod-3(sj134) X | Double mutant in the insulin-like receptor DAF-2 and in the superoxide dismutase SOD-3 | CGCa |
| MQ876 | daf-2(e1370) III; lys-7(ok1384) V | Double mutant in the insulin-like receptor DAF-2 and in the putative antimicrobial lysozyme LYS-7 | CGCa |
| MQ513 | daf-2(e1370) III; spp-1(ok2703) III | Double mutant in the insulin-like receptor DAF-2 and in the antimicrobial peptide caenopore SPP-1 | CGCa |
| GA186 | sod-3(tm760) X | Mutated in the iron/manganese superoxide dismutase SOD-3 | CGCa |
| RB1286 | lys-7(ok1384) V | Mutated in the putative antimicrobial lysozyme LYS-7 | CGCa |
| RB2045 | spp-1(ok2703) III | Mutated in the antimicrobial peptide caenopore SPP-1 | CGCa |
Caenorhabditis Genetics Center, the University of Minnesota.
C. elegans Gene Knockout Project http://www.celeganskoconsortium.omrf.org.
Fosmid analysis and transposon mutant library screening
A transposon mutant library of the antinematode fosmid clone 20G8 was generated using an in vitro transposon mutagenesis kit (EZ-Tn5 insertion kit; Epicentre) following the manufacturers' instructions. The DNA fosmid sequence for clone 20G8 is available from the National Center for Biotechnology Information (NCBI) public database (GenBank) via accession number JX523957. The subsequent library of 96 E. coli transposon mutants was replicated on LB10 Omnitray plates (Nunc, Denmark), and screened for loss of toxic activity towards C. elegans as previously described [31]. Clones that were partially or totally grazed by the nematodes were chosen for further characterization in the nematode killing assay (below). The disrupted genes were identified by outward sequencing from the transposon using the KAN-2 forward and reverse primers (Epicentre) (KAN-2 Forward Primer 5' ACCTACAACAAAGCTCTCATCAACC 3', KAN-2 Reverse Primer 5' GCAATGTAACATCAGAGATTTTGAG 3') and sequences were subjected to BLAST analysis [32].
Purification and identification of violacein as the antinematode agent produced by Microbulbifer sp. D250
Violacein was purified from an overnight culture of Microbulbifer sp. D250, cells were collected by centrifugation and the cell pellets repeatedly extracted with 100% methanol. The resulting (pooled) crude extract was applied to a C18 solid phase extraction column (pre-packed C18 columns, 10 g, Alltech); after several washing steps with methanol:water (20 to 60% methanol), the violacein was eluted with 100% methanol. The violacein fraction from the solid phase extraction was further purified by preparative high performance liquid chromatography (HPLC), when using a Prodigy ODS3 column (Phenomenex, 150×4.6 mm, 5 µm particle size) and a methanol gradient from 0–100% methanol.
Liquid chromatography electrospray ionisation ion-trap mass spectrometry (LC-MS/MS) was employed to confirm that the purified purple pigment (see above) is violacein. Briefly, the LC-MS/MS was performed on a LCQ Deca SP Iontrap-MS/MS system (Thermo Finnigan). Up to 20 µl of the extract were loaded on a Nucleosil C18 column (125×3 mm, 5 µm particle size, Macherey-Nagel, Germany). The mobile phases used were (A) water acidified with 0.1% formic acid and (B) acetonitrile, at a flow rate of 0.2 ml/min. The gradient program was started at 20% B, and after 3 minutes, increased to 100% B over 13 min, and maintained at 100% B. For the MS conditions, the electrospray voltage was -5kV with a current of 12 µA; the sheath gas flow rate was 34l/min; the capillary was maintained at 275°C. For the MS/MS fragmentation, the mass width for isolation of precursor ions was 1.0Da, and the relative collision energy set at 40%. The MS chromatograms were recorded in the positive ion mode. The purified purple pigment eluted at 13.9 min, with an absorption scan corresponding to violacein as observed by HPLC-diode array detection (maxima at 260, 378 and 570 nm), and this peak in the MS exhibited a protonated molecule ([M+H]+) corresponding to violacein (MW 343Da; mass of the observed [M+H]+ ion, 344 Da); the MS/MS fragmentation pattern of this [M+H]+ ion also corresponded to violacein, with ions observed at (% basepeak) 344 (64), 326 (45), 316 (100), 301 (49), 299 (24), 273 (5), 251 (20), 211 (4), 183 (2), 158 (1) and 132 (1) Da (e.g, due to loss of water, elimination of CO and nitrogen species, and cleavages at the rings).
Transposon mutagenesis of Microbulbifer sp. D250
To generate mutants of strain D250 that are unable to produce violacein, a random transposon mutagenesis was performed using the Tn-10-KmR mini-transposon systems as described previously [33]. Briefly, a spontaneous streptomycin (Sm) resistant mutant of Microbulbifer sp. D250 was generated (D250-SmR) and used as the recipient. E. coli containing the Tn10 based delivery plasmid pLOF/Km, encoding a kanamycin (Km) resistance gene marker [34], was used as a transposon donor. Donor cells were conjugated with Sm resistant recipient cells of isolate D250 (D250-SmR) on filter discs in 1∶1 ratio and incubated for 12 hours at 30°C. The conjugation mix was resuspended in marine broth and serial dilutions of this mixture were spread on, marine agar medium supplemented with 200 µg/ml Sm and 100 µg/ml Km. Transposon mutants were allowed to grow at room temperature for 48 to 72 hours and visually screened for the loss of purple pigmentation; the selected transposon mutants and wild type cells were extracted with methanol (as described above) and loss of pigmentation in the mutants confirmed by measuring the absence of absorbance at 575 nm. The DNA flanking the transposon insertion in the relevant mutants was sequenced using a “pan-handle” method as described previously [33].
Nematode killing assay
E. coli clones were pre-grown overnight at 37°C in LB10, and 10 µl of the cultures were spread onto 3.5 cm diameter LB10 agar plates supplemented with selective antibiotic and L-arabinose as required, followed by incubation at 25°C for four days. L4-stage nematodes were added to the bacterial lawns (30 to 40 per plate), incubated at 20°C, and scored for live and dead nematodes every 24 hours for 20 days. In order to avoid multiple generations of nematodes on the same plate, which may have lead to errors when scoring, the nematodes where transferred to a fresh plate each day. A random non-toxic E. coli clone was used as a negative control under the same conditions. A nematode was considered dead when it failed to respond to touch. Since the C. elegans daf-2 and pdk-1 mutants are temperature sensitive (at 20°C 15% of the population enter in the resistant dauer stage), all the daf-2 and pdk-1 mutant assays were carried out at 15°C. Control C. elegans strains were also tested at 15°C in order to avoid temperature bias in nematode's life span. Each assay was carried out in independent triplicate plates.
Since marine agar does not support C. elegans growth due to its high osmolarity, the standard nematode killing assay had to be modified in order to assess the toxicity of the marine isolates (i.e., strain D250 and violacein deficient mutant dV2). The marine bacteria were pre-grown overnight at 20°C in liquid marine broth, and 10 µL of the cultures were spread onto 2 cm paper filters (0.22 µm, Millipore, Maryland) and the filters placed on 3.5 cm marine agar plates supplemented with selective antibiotics as required, followed by incubation at 20°C for four days. On day four, the paper filters were removed from marine agar plates and placed onto 3.5 cm LB10 agar plates, to which the L4-stage nematodes were added (30 to 40 per plate). p values were calculated on the pooled data of all of the experiments done in each set by using the log- rank (Mantel–Cox) method [35], [36] with the Prism software version 6.0c (GraphPad Software, La Jolla, CA, USA). A p<0.05 was considered significant.
Violacein dose response assay
In order to estimate the dose response of C. elegans towards violacein, a nematode killing assay was carried out using 96 well agar plates as previously described [31]. E. coli clone 20G8 mutated in vioA gene (hereafter referred to as 20G8vioA− mutant, see Table 1) was pre-grown overnight in LB10 at 37°C and inoculated (2 µL) to each LB10 agar well supplemented with selective antibiotic and L-arabinose as required. Plates were thereafter incubated at 25°C for four days to grow the bacterial lawns, and then each 5 µL of the purified violacein preparation (in methanol, see above) was added at various concentrations to the side of the bacterial lawn and the methanol was allowed to evaporate before the violacein was gently mixed with the lawn by the addition of 10 µl of sterile water. E. coli mutant clone 20G8vioA − alone, and pure methanol added to a 20G8vioA − lawn, were used as negative controls. Methanol was allowed to evaporate before L4-stage nematodes were added (5–15 per well). The lethal concentration at which 50% of the nematodes were killed (LC50) was calculated by inference with the Prism software version 6.0c (GraphPad Software, La Jolla, CA, USA). The assays were carried out in independent triplicate plates. In order to determine if there was a significant difference in the survival of nematodes, a Students t-test was performed on the number of surviving nematodes at day seven. A p<0.05 was considered significant.
Heat killing of bacterial strains
Bacterial lawns pre-grown on agar plates were heat killed at 65°C for one hour, and the plates cooled to 20°C before adding nematodes. In order to confirm that heat killed bacteria were dead, samples of the bacterial lawns were streaked on LB10 agar plates before the nematode killing assay, and 20 days after the start of the incubation with nematodes. These plates were each incubated at 37°C for 24 to 48 hours and subsequently checked for an absence of bacterial colonies.
Microscopy
Overnight liquid cultures of each bacterial strain were spread on 3.5 cm LB10 agar plates and grown for four days. Nematode strains were exposed to bacteria for 24 hours and up to four days, depending on the assay, and placed on a microscope slide that had been immersed in 0.1 M sodium azide solution. Nematodes were examined under an Olympus DP70 digital camera system (Japan) with differential interference contrast (DIC) microscope optics. In order to detect the GFP signal indicative of an apoptosis induction, the CED-1::GFP reporter nematodes (strain CU546, Table 2) were exposed to a bacterial lawn of the 20G8 clone and 20G8vioA − and 20G8vioC − mutants for 24 hours. Nematodes were visualized under epifluorescence microscopy, and the percentage of nematodes with a GFP signal calculated for each treatment.
Results
Violacein is a toxic metabolite that mediates antagonistic interactions between the bacterium Microbulbifer sp. D250 and C. elegans
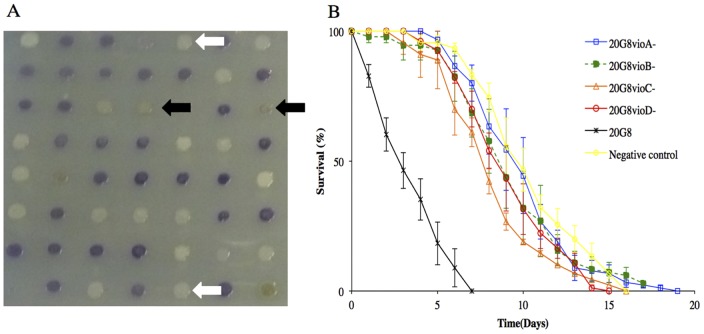
Screening of a random transposon mutant library of the fosmid clone 20G8 for the loss of toxic activity towards C. elegans resulted in four mutant clones (Tables 1 and 3 and Figure 1). The nematode killing assay demonstrated that the killing phenotype of the four mutant clones was significantly reduced (p<0.0001) when compared to the wild type clone 20G8 (Figure 1). The activity of mutant 20G8VioA − was similar to the negative control (p = 0.803) and was therefore considered non-toxic for C. elegans. Sequencing of the four non-active mutants revealed that in all cases transposons had inserted in open reading frames with high sequence identity to the vioABCDE gene cluster of C. violaceum, which has previously been shown to be involved in the synthesis of violacein [37] (Table 3). Specifically, two mutants that had lost the violet pigmentation, had insertions in the vioA and vioB genes, and the two clones that expressed a grey pigmentation typical of violacein precursors, were mutated in the vioC and vioD genes (Table 3 and Figure 1) [37].
Table 3. Summary of the effects of violacein-producing clone 20G8 and its violacein deficient mutants on C. elegans.
| Compound predicted to be expressed by the mutanta | L-tryptophan | prodeoxyviolacein | proviolacein | violacein |
| Proposed gene function involved in the biosynthesisa , b | vioAB: tryptophan 2-monooxygenase | vioD: hydroxylase | vioC: monooxygenases | N/A |
| Mutant/clone name and NCBI accession number | 20G8vioA −(AFT64169) 20G8vioB −(AFT64168) | 20G8vioD− (AFT64166) | 20G8vioC− (AFT64167) | 20G8 (JX523957) |
| BLASTp analysisc | VioA tryptophan 2-monooxygenase; VioB polyketide synthase | VioD hydroxylase | VioC monooxygenase | N/A |
| Nematode survival (p values)d | p<0.0001 | p<0.0001 | p<0.0001 | N/A |
| Colonizatione | 0% | N/A | 0% | 76%±1.6 |
| Apoptosisf | 0% | N/A | 0% | 70.3%±5.7 |
| Clone pigmentation | white | grey | grey | violet |
Genes were ordered based on enzymatic activity in violacein biosynthesis and not on the locus position within the cluster [37].
Only hits with 100% query coverage, 100% identity and E value of 0 were considered.
p values of the survival of nematodes exposed to violacein mutants compared to the wild type clone 20G8 are reported.
Percentage of alive nematodes colonized after four days.
Percentage of CU1546 C. elegans strain displaying GFP signal indicative of apoptosis induction. All studies were carried out in C. elegans strain N2 except for apoptosis studies where CU1546 strain was employed. Each data represents means ± the standard error of three replicates. N/A = not applicable.
Figure 1. Characterization of the transposon mutant library of violacein producing clone 20G8.
(A) Several mutants exhibit changes in violet pigmentation typical of the presence of violacein: 20G8vioA − and 20G8vioB − mutants have lost pigmentation (white arrows). 20G8vioC− and 20G8vioD− mutants express green-grey pigmentation (black arrows). (B) Nematode killing assay (N2 animals vs bacterial strains 20G8 clone and 20G8vioABCD − mutants). Survival kinetics of nematodes fed with either the 20G8 clone or the 20G8vioABCD − mutants deficient in violacein production. The killing phenotype of the four mutant clones is significantly reduced (p<0.0001) when compared to the wild type clone 20G8. A randomly chosen clone from the library with no activity is used as a negative control. Each data point represents means ± the standard error of three replicate plates. p values were calculated on the pooled data of all of the plates in each experiment by using the log-rank (Mantel–Cox) method.
To further support the involvement of violacein in nematode toxicity, Microbulbifer sp. D250, the parent organism for the fosmid clone 20G8, was shown to rapidly kill C. elegans (p<0.0001) when compared to negative control OP50 (Figure 2A). In contrast, the nematode's life span was significantly improved when exposed to a violacein deficient mutant dV2 (p<0.0001, Figure 2A). Sequencing of the transposon insertion site in strain dV2 supported the loss of violacein production, by demonstrating that the homologue to the violacein biosynthesis gene vioB (GenBank AFT64168) had been disrupted in the dV2 mutant. Notably, survival of the nematodes fed with the dV2 mutant strain was significantly reduced (p<0.0001) compared to the negative control using E. coli OP50 (see Figure 2A), which might indicate that other antinematode activities are also present in this bacterium. However, the involvement of violacein in toxicity towards C. elegans was further confirmed by the direct chemical identification of the purified violet pigment produced by Microbulbifer sp. D250 wild type strain. The pigment was extracted from cultures, purified and analysed by liquid chromatography-mass spectrometry (LC-MS/MS). The purified purple pigment eluted as one peak in the liquid chromatography, and this peak represented violacein, as was identified by the matching mass of the protonated molecule ([M+H]+) and its characteristic MS/MS fragmentation pattern (see Material and Methods).
Figure 2. Nematode killing assay and dose response assay (N2 animal vs the alive and heat killed 20G8 clone and D250 strains).

(A) Killing kinetics of Microbulbifer sp. D250 and dV2 mutant deficient in violacein production. Negative control OP50 is a non-pathogenic strain of E. coli. (B) Dose response of C. elegans to pure violacein added to 20G8vioA− mutant bacteria alive and heat killed. Each point on the graph represents the average survival of worms after seven days exposure to violacein (C) Kinetics of nematode killing when nematodes are fed either the live or heat killed 20G8 clone or 20G8vioA− mutant. Each data point represents means ± the standard error of three replicate plates. p values were calculated on the pooled data of all of the plates in each experiment by using the log-rank (Mantel–Cox) method.
Finally, we used pure violacein directly in the toxicity assays (see Material and Methods). The 50% survival (LC50) of nematodes exposed to violacein falls between the range of 7.5 µM and 75 µM of pure violacein (LC50 = 31.13 µM calculated from Figure 2B), when added to a viable bacterial lawn of the violacein non-producing mutant 20G8vioA − (Figure 2B). Interestingly, the survival of nematodes improved when violacein preparations were added to lawns of heat killed 20G8vioA − bacteria compared to violacein added to lawns of viable 20G8vioA − cells (i.e. p = 0.001 and p = 0.043 for nematodes exposed to 0.75 µM and 7.5 µM of pure violacein, respectively).
Bacterial accumulation in the nematode intestine is involved in the killing activity of the violacein-producing E. coli clone
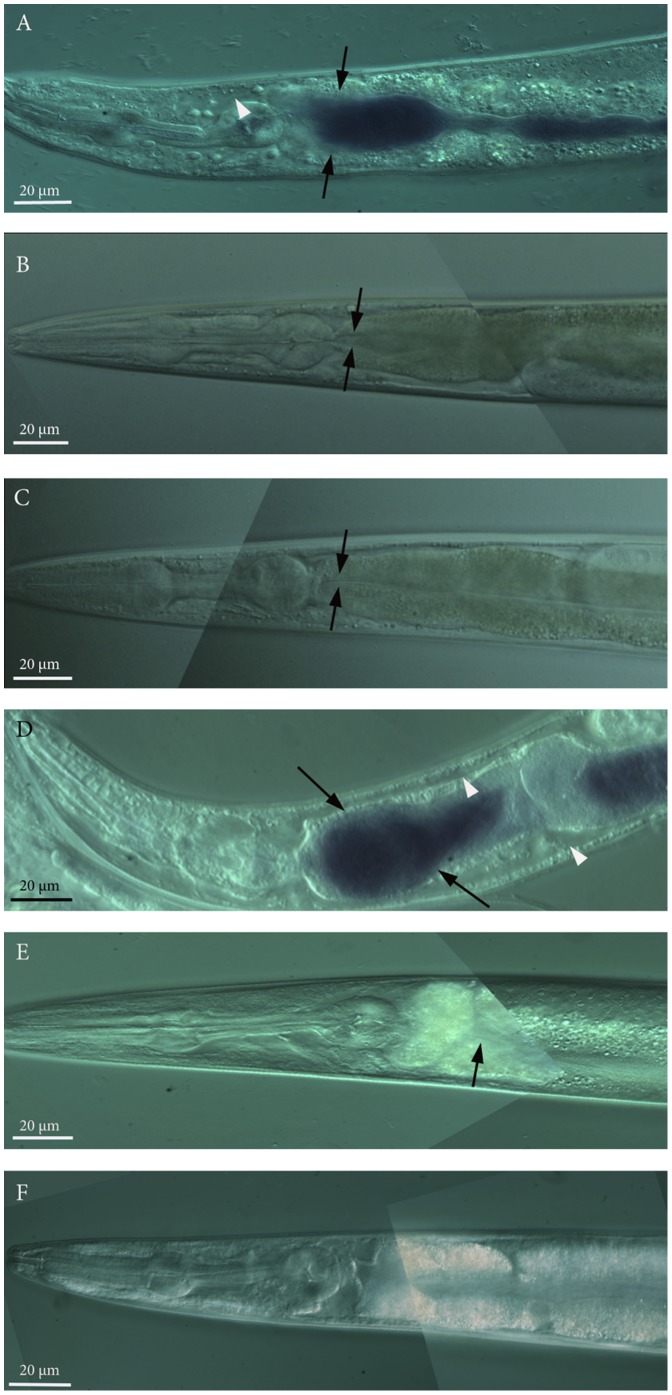
Given that the presence of live bacteria contributed to the decrease in the survival of nematodes in the presence of violacein (see above), we looked for evidence of bacterial accumulation in the nematodes intestine. The gut of 76% (n = 47) of the nematodes exposed to violacein-producing E. coli clone 20G8 had an accumulation of bacterial cells expressing a violet pigmentation in their intestine (Figure 3A). In contrast, nematodes exposed for four days to the violacein deficient mutants 20G8vioA− (n = 42) and 20G8vioC− (n = 57) showed no accumulation of bacteria in the intestinal lumen (Figure 3B and C). Microscopic analysis further showed tissue damage with enlargement of the intestinal lumen and enlargement of extracellular regions in nematodes fed with the 20G8 clone (arrowheads in Figure 3A). These data suggest that accumulation of bacteria in the intestinal lumen is one factor in the killing activity of clone 20G8. In order to further assess this, a nematode killing assay was performed using live and heat killed 20G8 cells. Heat inactivation of bacterial cells showed that nematode survival significantly increases (p<0.0001) in the presence of heat killed 20G8 cells compared to viable 20G8 bacteria (Figure 2C). In contrast, there was no significant effect (p>0.05) of heat killing on the survival of nematodes exposed to the violacein deficent mutant 20G8vioA− (Figure 2C). In order to confirm that bacterial cells were successfully inactivated by heat, they were streaked on LB10 agar plates and monitored for growth. Bacterial growth was not detected confirming that the heat treatment was sufficient to kill the bacteria. Together these data suggest that the toxic effect of violacein is significantly increased by the presence of live violacein-producing bacteria that accumulate in the intestine.
Figure 3. Visualization of bacterial accumulation in the nematode intestine by the E. coli clones (wild type animals, the daf-2 and daf-16 null mutant animals and the DAF-16 overexpressing nematodes vs the E. coli clone 20G8 and 20G8vioA− and 20G8vioC− mutant clones).
All images present C. elegans anterior to the left and show the pharynx and first part of the intestinal lumen by differential interference contrast (DIC) microscopy. Accumulation by 20G8 cells in the nematodes intestine (arrows in panels A and D) and extensive enlargement of extracellular regions (white arrowheads panels A and D) in N2 wild type (panel A) and daf-16 mutant (panel D) animals. No change in phenotype was observed in N2 nematodes fed with violacein deficient mutant clones 20G8vioA− (negative control-panel B) and 20G8vioC− (panel C). Similarly, no bacterial cells or enlargement of extracellular regions were detected in daf-2 and DAF-16 over-expressing mutant nematodes exposed to the violacein producing clone 20G8 (arrows in panel E and F respectively). Each panel was assembled from multiple photomicrographs taken with the same magnification and same acquisition settings.
Violacein-producing bacteria induce apoptosis in C. elegans
To further elucidate the process of killing, transgenic nematodes with a GFP marker for apoptosis (CU1546 strain, Table 2) were exposed to a bacterial lawn of the violacein producing clone 20G8 and the violacein deficient mutant clones 20G8vioA− and 20G8vioC − for 24 hours, and thereafter visualised using differential interference contrast (DIC) and epifluorescence microscopy. The GFP signal was detected in somatic cells only in animals incubated with the 20G8 clone (70.3% of animals, n = 20), but not with 20G8vioA− and 20G8vioC − mutants (0%, n = 13 and n = 15 respectively) (Figure 4) showing that only the violacein-producing bacteria are capable of inducing apoptosis.
Figure 4. Apoptosis analysis in CED-1::GFP transgenic nematode strain CU1546.
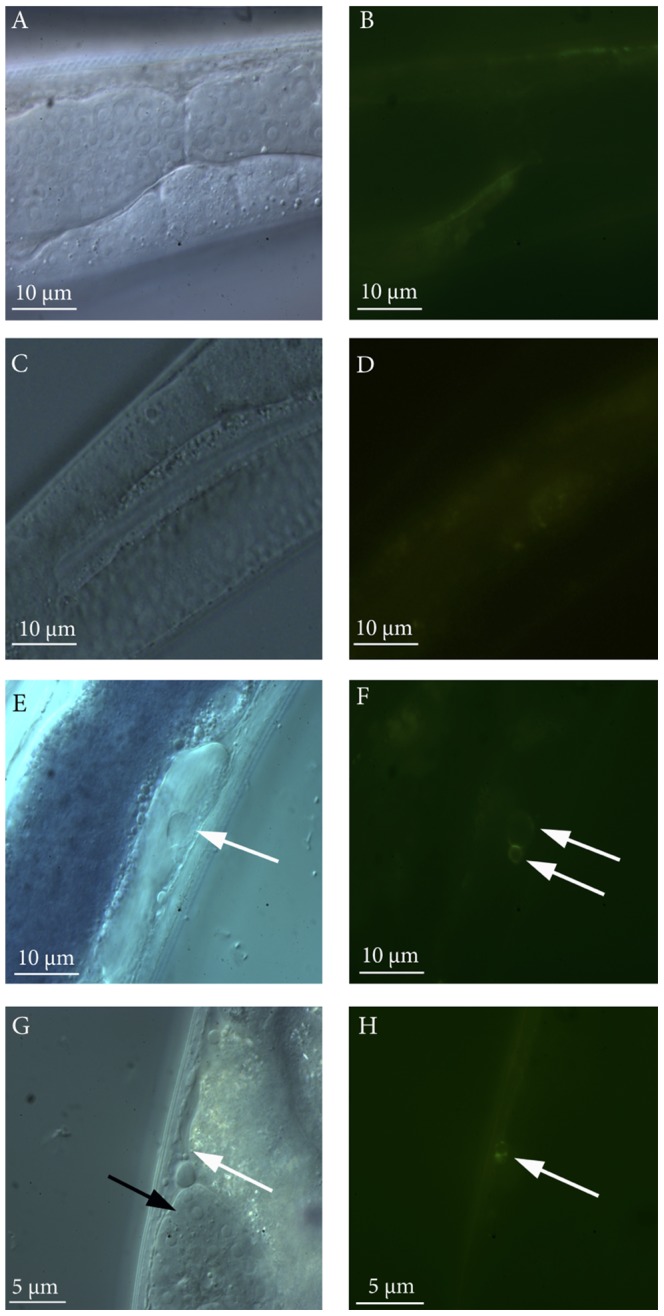
Visualization of GFP apoptosis marker inside the nematodes exposed to the violacein deficient mutant clones (20G8vioA − panels A and B; 20G8vioC− panels C and D) or violacein producing clone (20G8, panels E and F and G and H). Images show a section of the intestine and the gonads of C. elegans by DIC (panels A, C, E, G) and by epifluorescence microscopy (panels B, D, F, H). A ring-shaped GFP signal was detected in somatic cells of CU1546 nematodes exposed to 20G8 clone (two white arrows in panel F and white arrow in panel H) but not to 20G8vioA − and 20G8vioC− mutants (panel B and D). The ring-shaped GFP signal is associated with the expression of CED-1 receptor on the cell wall of engulfing cells only when phagocytosis of apoptotic cells is taking place. The ring-shaped GFP signal is apoptosis-specific and is visible in somatic cells of the nematodes (white arrows in E and G), the germ line (gonad) of the nematode is shown in panel G (black arrow). A non-specific fluorescence signal is visible in panels B and D.
Mutations in the IIS pathway influence the sensitivity of C. elegans to violacein-producing bacteria and pure violacein
The IIS pathway with gene regulators DAF-2 and DAF-16 plays an important role in C. elegans pathogen defence [18]. We therefore questioned if C. elegans uses this pathway to also protect itself against violacein-mediated killing. To address this question, C. elegans loss of function mutants in the genes daf-2 (CB1370), pdk-1 (JT9609), daf-16 (IU10) and wwp-1 (RB1178), and a DAF-16 over-expressing strain (TJ356) (Table 2) were fed with the violacein-producing clone 20G8 in the nematode killing assay. The life span of C. elegans strains carrying loss of function mutations in daf-2 and pdk-1 and wwp-1 were significantly increased compared to both wild type animals and daf-16 mutant (p<0.0001) (Figure 5A, 5B and 5C). In contrast the C. elegans IU10 strain with loss of function mutation in the FOXO-family transcription factor DAF-16 displayed significantly reduced (p<0.0005) survival compared to wild type N2 animals (Figure 5A). Whereas viability of transgenic DAF-16::GFP nematodes that overexpress DAF-16 was significantly improved compared to both wild type and daf-16 mutant animals (p<0.0001) (Figure 5A).
Figure 5. Nematode killing assay and dose response assay (wild type animals and daf-2, daf-16, pdk-1, wwp-1 and DAF-16 mutant nematodes vs 20G8 clone).
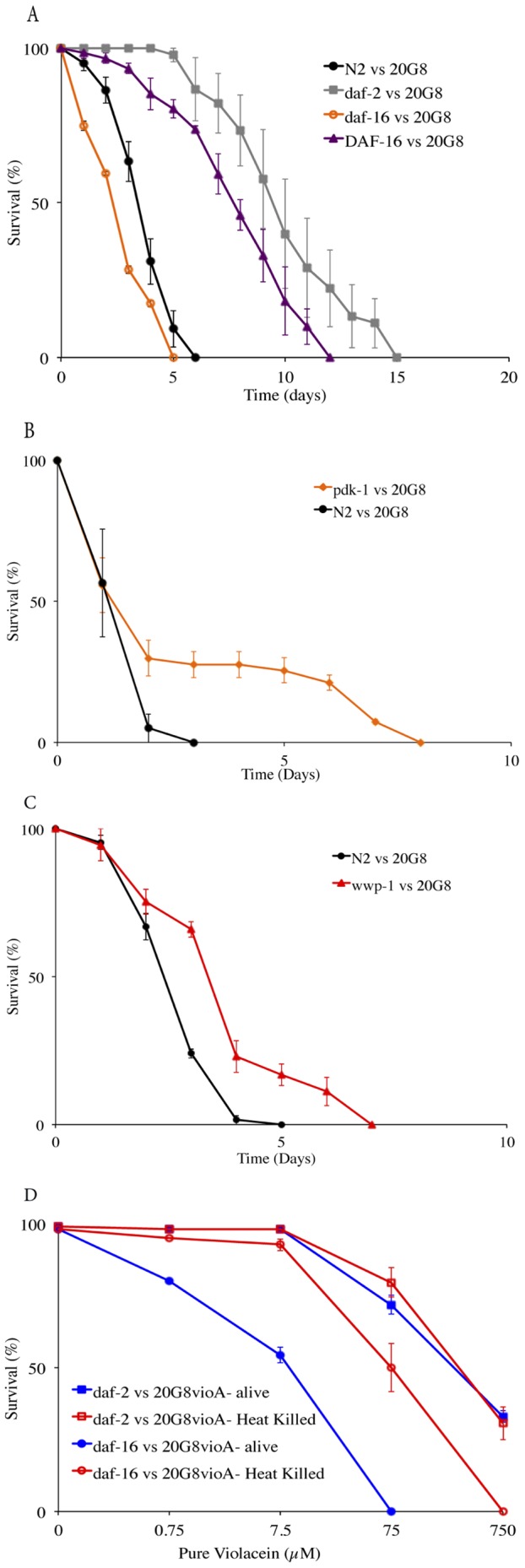
The survival of nematodes fed with 20G8 clone was measured for (A) daf-2, daf-16 and DAF-16 mutant animals. (B) pdk-1 mutant animals (C) wwp-1 mutant animals (D) Dose response of C. elegans daf-2 and daf-16 strains to pure violacein added to 20G8vioA− mutant bacteria alive and heat killed. Each data point represents means ± the standard error of three replicate plates p values were calculated on the pooled data of all of the plates in each experiment by using a Students t-test on the number of surviving nematodes at day seven.
Similar results were observed for the C. elegans strains fed with the violacein deficient mutant (20G8vioA −) supplemented with pure violacein in a dose response assay (Figure 5D). The toxic effect of violacein was dose dependent in wild type N2 nematodes and in daf-2 and daf-16 mutant animals (significant difference when nematodes of the same strain were exposed to 7.5, 75 or 750 µM of pure violacein, p<0.05). In these assays the lethal dose of violacein required to kill 50% of the population was higher for daf-2 mutants (75 µM<LC50<750 µM) than for the wild type (7.5 µM<LC50<75 µM) or the daf-16 mutant animals (7.5 µM<LC50<75 µM) (Figures 2B and 5D), further indicating that sensitivity or resistance to violacein is at least partially mediated by the IIS pathway.
C. elegans daf-2 mutant and DAF-16 overexpressing strains are resistant to intestinal accumulation by the violacein-producing clone 20G8
Given that bacterial intestinal accumulation is in part responsible for the toxic phenotype of violacein producing cells and that the IIS pathway has previously been shown to influence bacterial accumulation in the nematode intestine [21], we aimed to determine if elements of the IIS pathway mediate resistance to violacein by preventing bacterial accumulation.
Firstly, we compared the sensitivity of various C. elegans strains to violacein when fed viable or heat killed bacteria. The presence of viable bacterial cells significantly increased the sensitivity of daf-16 mutant animals to violacein compared to when the mutant was exposed to heat killed bacteria (significant difference when pure violacein was added to viable or heat killed bacteria p<0.05, Figure 5D). In contrast, heat killed bacteria had no or little impact on the survival of daf-2 mutant (p>0.05, Figure 5D).
Secondly, we assessed the ability of E. coli clone 20G8 to accumulate in the intestine of the various C. elegans strains. Ninety one percent of daf-16 mutant nematodes (n = 35) showed evidence of bacterial accumulation when exposed to 20G8 cells, in contrast no or little bacterial accumulation was present in the daf-2-loss of function and DAF-16 over-expressing mutants under the same treatment (0% n = 45 and 1.1% n = 31, respectively). Enlargement of extracellular regions was present in wild type and daf-16 mutant nematodes (90% n = 20 and 92% n = 25, respectively) fed with clone 20G8 (arrowheads in Figure 3 panel A and panel D respectively) while daf-2-loss of function and DAF-16 over-expressing mutant animals showed normal anatomical structures and no tissue injury was detected (Figure 3 panel E 0% n = 19 and Figure 3 panel F 0% n = 16, respectively). Together these data indicate that the resistance to violacein by daf-2 null mutants and DAF-16 over-expressing C. elegans strains is, in part, due to their improved ability to prevent accumulation of bacteria in the presence of violacein.
Loss of function in IIS controlled genes results in increased sensitivity to violacein
In C. elegans DAF2/DAF16 controls the expression of various effector genes including those relevant for detoxification and antimicrobial activity such as the superoxidase dismutase gene sod-3 and antimicrobial genes spp-1 and lys-7 [23], [38]. Thus given that the precise molecular target/s for violacein in C. elegans are unknown we sought to determine which, if any, of these relevant downstream genes are required for the increased resistance to violacein observed in daf-2 null and DAF-16 over-expressing strains. Specifically we chose to test violacein sensitivity in C. elegans mutants defective in sod-3, spp-1 and lys-7 (Table 2) because of the previous reported involvement of these genes in immunity to bacterial accumulation [39], [40], [41]. We found that daf-2;spp-1 and daf-2;sod-3 double mutants displayed significantly reduced survival compared to the single mutant daf-2 (p<0.0001, Figure 6A) when exposed to the 20G8 clone in a nematode killing assay. No reduction in viability was detected in the daf-2;lys-7 double mutant when compared to the single mutant daf-2 (p = 0.937, Figure 6A). Interestingly a single mutation in gene spp-1 significantly reduced the nematode's life span when compared to wild type animals (p<0.0001), while the viability of the nematode was not affected by mutations in the lys-7 and sod-3 genes (p>0.05, Figure 6B). These data indicate that resistance to violacein in daf-2 mutants is at least in part driven by SPP-1 and SOD-3, with the antimicrobial LYS-7 having little or no involvement.
Figure 6. Nematode killing assay (wild type animals and daf-2, daf-2;spp-1, daf-2;lys-7, daf-2;sod-3, spp-1, lys-7, sod-3 mutant nematodes vs the 20G8 clone).
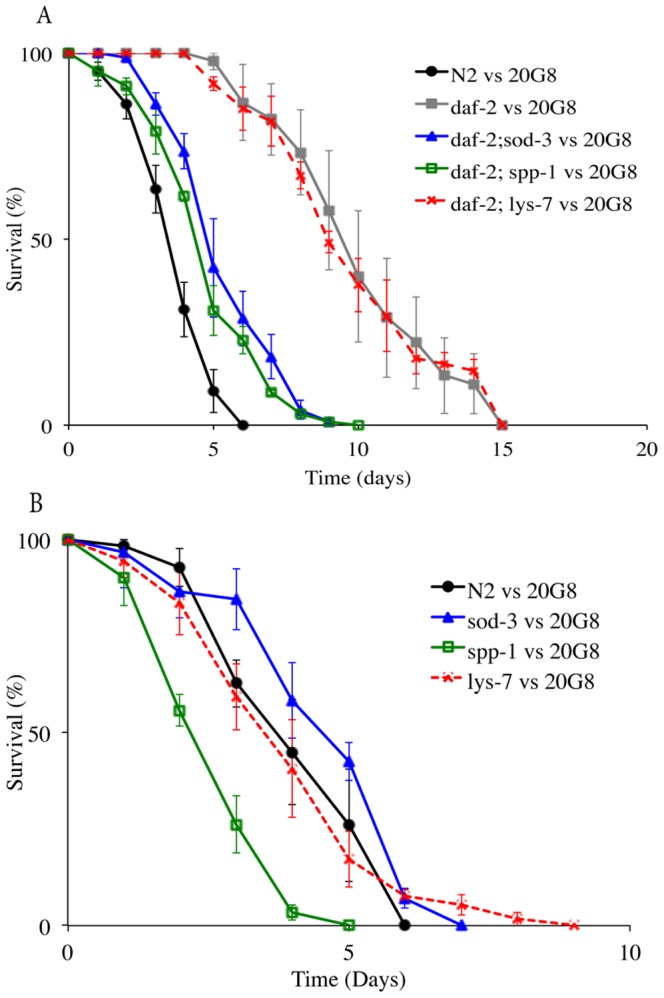
(A) The survival of the nematode was tested using C. elegans double mutants daf-2;sod-3, daf-2;spp-1, daf-2;lys-7 and (B) the single mutant animals sod-3, spp-1, and lys-7. Each data point represents means ± the standard error of three replicate plates. p values were calculated on the pooled data of all of the plates done in each experiment by using the log-rank (Mantel–Cox) method and the values are provide in the text.
Discussion
Violacein has antinematode activity
In this study, we identified violacein as the metabolite responsible for the antinematode activity of Microbulbifer sp. D250. Violacein is arguably best known for its antibacterial properties and its activity as a potentially novel therapeutic against a range of tumors [16]. However, to the best of our knowledge, and with the exception of studies related to cancer therapy, this is the first report of violacein toxicity towards a multicellular eukaryote, thus adding to the list of biological functions for this natural metabolite.
Genetic analysis identified an operon of five conserved biosynthesis genes vioA-E that have been identified across all violacein-producing strains studied to date [7], [12], [42], [43]. Interestingly, mutations in vioC or vioD result in a grey colony pigmentation, reminiscent of the accumulation of the violacein precursor pro-violacein [7], [43], [44]. Pro-violacein differs from violacein by the absence of one oxo-group in the C15 position (indolyl instead of indolone, see Table 3 and reference [7]). This minor chemical difference, however, has a substantial impact on the toxicity, as the grey-pigmented vioC mutant did not kill nematodes in our study. Similar observation have also been recently made for E. coli K12 strains producing either violacein or pro-violacein, which were and were not resistant, respectively, to protozoan predation [45].
C. elegans is intrinsically more resistant to the effects of violacein (LC50>30 µM) than other bacterial grazers such as flagellates and amoebae, which were found to be effective at concentrations of 10 µM [46] and 1 µM [12] respectively. Nevertheless, violacein appears to be more potent towards C. elegans than toxins derived from known bacterial pathogens. For example, small phenazine molecules, including phenazine-1-carboxylic acid recently identified from Pseudomonas aeruginosa, are toxic to C. elegans only at concentrations greater than 70 µM. The effective concentration of violacein against C. elegans observed here is also similar to that of recent studies investigating novel antihelminthic therapies. For example, in a screen of existing drug leads Taylor et al [47] identified 18 candidate compounds having detectable phenotypes against C. elegans with EC50 ranging between 0.7 µM and >192 µM, including those already approved as cancer therapeutics such as Dasatinib (LC50 22.3 µM) and Flavopiridol (LC50 48.3 µM). Despite its many biological activities, the toxicity of violacein on (non-tumoral) mammalian cells is quite low. Recently, it has been reported that intraperitoneal doses of violacein of up to 1 mg kg−1 are not toxic to mouse blood, kidneys, or liver, thus enabling a potential in vivo use of violacein and its derivatives as a therapeutic compound with few side effects [48]. Given the relatively low effective concentration of violacein towards C. elegans determined here, we propose that the investigation of violacein as an antiparasitic compound is a reasonable prospect and that further studies regarding the activity of violacein against model parasitic nematodes, could reinforce the therapeutic potential of this drug.
Bacterial accumulation in the intestine of C. elegans is a key factor involved in the toxicity of violacein
We observed that in the presence of violacein the otherwise non-pathogenic E. coli has the ability to accumulate in the intestine and eventually kill C. elegans. The exact mechanism by which violacein treatment leads to bacterial accumulation and reduced nematode viability is yet to be determined, however recent reports have demonstrated a link between nematode longevity and intestinal colonization [21]. Specifically, Portal-Celhay et al [21] showed that the capacity to control bacterial accumulation in the gut was dependent on the immunological status and age of the individual animal. Heavy bacterial accumulation has also been shown to reduce the lifespan of the nematodes depending on the bacterial strain used [21], [49]. Thus it is possible that exposure to violacein compromises the nematode's defence resulting in a reduced capacity to control bacteria in the gut and, thus, increasing the mortality rate. This is supported by similar observations recently made in various Bacillus species, in which treatment with the Bacillus pore-forming crystal protein (Cry PFP) seemingly sensitizes C. elegans to bacterial infection [50]. An alternative explanation is that the presence of violacein allows bacteria to penetrate the intestinal tissue resulting in a lethal infection. Whilst we did not observe bacteria within the tissue of nematodes exposed to violacein, previous reports have suggested internal infection as a possible cause of death in older nematodes [49], [51] and so this alternative possibility should not be dismissed.
Violacein induces apoptosis in C. elegans
In addition to increased susceptibility to bacterial accumulation, violacein is capable of inducing apoptosis in C. elegans (Figure 4). Previous studies have revealed that apoptosis is also involved in violacein-mediated cell death in mammalian cell lines [16] and amoebae [12]. Thus although the exact molecular target of violacein in the eukaryotic cell is yet to be elucidated, the induction of an apoptosis-like cell death mechanism in multiple, distantly related eukaryotic systems (mammalian cells, amoeba and nematodes) suggest that an ancient, common eukaryotic cell process may be an additional target of violacein-driven toxicity.
The insulin/IGF-1 signalling pathway contributes to the native defence against violacein
The innate immune response, including the IIS pathway with regulators DAF-2 and DAF-16, is a key component of the nematodes first line of defence particularly against pathogens [18], [19], [52]. Here we add to that knowledge by demonstrating that the IIS pathway also contributes to the nematodes native defence against secondary metabolites derived from non-pathogenic bacteria. The C. elegans daf-2 and pdk-1 deficient animals were significantly more resistant to the toxicity of violacein and the associated bacterial accumulation as compared to the wild type strain. These finding are consistent with the “long-lived” phenotype of the C. elegans daf-2 mutant [53], which is also known to mediate the immune defence to bacterial infections [52], [54].
Since the molecular target of violacein-mediated toxicity in C. elegans remains to be elucidated the mechanisms involved in the nematode immune response towards violacein is unknown. However recent studies using E. coli expressing the Pseudomonas aeruginosa translational inhibitor exotoxin A (ToxA), have demonstrated that C. elegans induces an immune response towards ToxA, which the nematode detects indirectly via the toxin-mediated damage [55]. Others have also demonstrated activation of immunity and detoxification genes in response to damage to a variety of cellular functions [56], many of which could result from exposure to bacterial toxins. Thus such an effector-triggered immunity is likely to be widespread in animals and may function to enable bacteriovorus organisms such as C. elegans to discriminate between commensal and pathogenic bacteria [57]. Therefore once the molecular target of violacein is established it will be of interest to determine if C. elegans responds directly to the presence of violacein or rather to the associated inhibition of, or damage to, specific cellular functions.
Identifying genes under DAF-2/DAF-16 control that are involved in the increased resistance to violacein may provide further insight into the molecular/cellular target of this compound. Indeed assessment of violacein sensitivity of selected C. elegans daf-2 double mutant strains in the current study indicates that while the antimicrobial lysozyme (LYS-7) is not involved in violacein resistance, both the superoxide dismutase SOD-3 and the antimicrobial peptide SPP-1 seem to play a role in host defence against violacein. The potential role of SOD-3 is consistent with previous findings that violacein can cause oxidative stress in human cancer cell lines [58]. However other studies have indicated that violacein acts as an antioxidant for the producing bacteria [59]. This apparent contradiction may result from the same molecule producing different responses depending on the target cells. Spp-1 is expressed in the nematode intestinal cells and has previously been shown to be involved in the immune response against pathogens Salmonella typhimurium [41] and P. aeruginosa [60]. Results from this study support the idea that SPP-1 could also control the violacein induced intestinal accumulation of E. coli in C. elegans and further suggest a wider target spectrum for this peptide.
Despite the large body of knowledge surrounding the IIS pathway of the innate immune response in C. elegans and its role in stress resistance and pathogenesis, there is a paucity of information regarding its role in response to bacterial toxins. In one of the few studies to date, Chen et al. [22] demonstrated that the IIS pathway is involved in the cellular defence of C. elegans against Bacillus thuringensis crystal pore-forming toxins (Cry PFT). It appears likely that C. elegans uses this conserved pathway as a general bacterial toxin defence mechanism, as, similar to our findings for violacein, reduction in DAF-2 signalling confers resistance to Cry PFT, which is at least in part, dependent on DAF-16 function. However the newly described second branch of the IIS pathway mediated by WWP-1 appears to play a different role in defence against violacein, as in contrast to the hypersensitivity towards Cry PFT [22], wwp-1 mutant animals were more resistant to violacein than wild type nematodes (Figure 5C). These contrasting observations may speak to differences in how the two toxins affect target cells and will be the subject of future studies investigating the cellular response of C. elegans to bacterial toxins.
Conclusion
In their natural habitat nematodes, including C. elegans, are major predators for bacteria that, in turn, have evolved a number of predatory defence mechanism including the production of inhibitory metabolites [61]. This work presents, for the first time, violacein as an antinematode compound and assesses toxicity and the cellular response to violacein exposure in a C. elegans animal model. We found that this small molecule facilitates intestinal accumulation of the E. coli host cells and stimulates apoptotic activity. Mutations in a number of genes in the IIS pathway (daf-2 and pdk-1) and over-expression of another (daf-16) can confer significant resistance to violacein toxicity, providing evidence that C. elegans uses the DAF-2/DAF-16 innate immune signalling pathway to defend itself against this and potentially other noxious bacterial metabolites. Finally we demonstrate that defence against violacein requires, at least in part, the function of the antimicrobial peptide SPP-1 and possibly superoxide dismutase SOD-3. It is expected that the added knowledge will assist in the design of future mutational and/or gene expression studies aimed at determining the mechanisms of violacein toxicity in the nematode model including its direct molecular target/s. Such studies will not only prove important for further understanding the molecular basis for microbial antagonistic interactions but also for the development of microbial secondary metabolites such as violacein as novel chemotherapeutics.
Acknowledgments
We thank the IGB Open Laboratory for facilities and resources, the C. elegans gene knockout project and the Caenorhabditis Genetics Center (CGC) at the University of Minnesota for provision of C. elegans samples. We further thank Silvana Castro for technical assistance and A/Prof. Torsten Thomas for revision of the draft manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All sequencing files are available from the National Center for Biotechnology Information (NCBI) public database via accession number JX523957.
Funding Statement
These authors have no support or funding to report.
References
- 1. Holden-Dye L, Walker RJ (2007) Anthelmintic drugs. WormBook 2: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan RM (2004) Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 20: 477–481. [DOI] [PubMed] [Google Scholar]
- 3. Prichard RK, Geary TG (2008) Drug discovery: Fresh hope to can the worms. Nature 452: 157–158. [DOI] [PubMed] [Google Scholar]
- 4. Jones AK, Buckingham SD, Sattelle DB (2005) Chemistry-to-gene screens in Caenorhabditis elegans . Nat Rev Drug Discov 4: 321–330. [DOI] [PubMed] [Google Scholar]
- 5. Sifri CD, Begun J, Ausubel FM (2005) The worm has turned-microbial virulence modeled in Caenorhabditis elegans . Trends Microbiol 13: 119–127. [DOI] [PubMed] [Google Scholar]
- 6. Penesyan A, Ballestriero F, Daim M, Kjelleberg S, Thomas T, et al. (2012) Assessing the effectiveness of functional genetic screens for the identification of bioactive metabolites. Mar Drugs 11: 40–49 doi:10.3390/md11010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoshino T (2011) Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: biosynthetic mechanism and pathway for construction of violacein core. Appl Microbiol Biotechnol 91: 1463–1475. [DOI] [PubMed] [Google Scholar]
- 8. Hakvag S, Fjarvik E, Klinkenberg G, Borgos S, Josefsen K, et al. (2009) Violacein producing Collimonas sp. from the sea surface microlayer of costal waters in trondelag, Norway. Mar Drugs 7: 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aranda S, Montes-Borrego M, Landa BB (2011) Purple-pigmented violacein-producing Duganella spp. inhabit the rhizosphere of wild and cultivated olives in southern Spain. Microb Ecol 62: 446–459. [DOI] [PubMed] [Google Scholar]
- 10. Pantanella F, Berlutti F, Passariello C, Sarli S, Morea C, et al. (2007) Violacein and biofilm production in Janthinobacterium lividum . J Appl Microbiol 102: 992–999. [DOI] [PubMed] [Google Scholar]
- 11. Lu Y, Wang L, Xue Y, Zhang C, Xing X-H, et al. (2009) Production of violet pigment by a newly isolated psychrotrophic bacterium from a glacier in Xinjiang, China. Biochem Eng J 43: 135–141. [Google Scholar]
- 12. Matz C, Webb JS, Schupp PJ, Phang SY, Penesyan A, et al. (2008) Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS One 3: e2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang LH, Xiong H, Lee OO, Qi SH, Qian PY (2007) Effect of agitation on violacein production in Pseudoalteromonas luteoviolacea isolated from a marine sponge. Lett Appl Microbiol 44: 625–630. [DOI] [PubMed] [Google Scholar]
- 14. Konzen M, De Marco D, Cordova CA, Vieira TO, Antonio RV, et al. (2006) Antioxidant properties of violacein: possible relation on its biological function. Bioorg Med Chem 14: 8307–8313. [DOI] [PubMed] [Google Scholar]
- 15. Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KP (2009) The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl Environ Microbiol 75: 6635–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durán M, Ponezi A, Faljoni-Alario A, Teixeira MS, Justo G, et al. (2012) Potential applications of violacein: a microbial pigment. Med Chem Res 21: 1524–1532. [Google Scholar]
- 17. Marsh EK, May RC (2012) Caenorhabditis elegans, a model organism for investigating immunity. Appl Environ Microbiol 78: 2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan MW, Shapira M (2011) Genetic and molecular analysis of nematode-microbe interactions. Cell Microbiol 13: 497–507. [DOI] [PubMed] [Google Scholar]
- 19. Yanase S, Yasuda K, Ishii N (2002) Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev 123: 1579–1587. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Zhang Y (2009) Neural-immune communication in Caenorhabditis elegans . Cell Host Microbe 5: 425–429. [DOI] [PubMed] [Google Scholar]
- 21. Portal-Celhay C, Bradley ER, Blaser MJ (2012) Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans . BMC Microbiol 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CS, Bellier A, Kao CY, Yang YL, Chen HD, et al. (2010) WWP-1 is a novel modulator of the DAF-2 insulin-like signaling network involved in pore-forming toxin cellular defenses in Caenorhabditis elegans . PLoS One 5: e9494 doi:9410.1371/journal.pone.0009494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukhopadhyay A, Oh SW, Tissenbaum HA (2006) Worming pathways to and from DAF-16/FOXO. Exp Gerontol 41: 928–934. [DOI] [PubMed] [Google Scholar]
- 24. Back P, Matthijssens F, Vlaeminck C, Braeckman BP, Vanfleteren JR (2010) Effects of sod gene overexpression and deletion mutation on the expression profiles of reporter genes of major detoxification pathways in Caenorhabditis elegans . Exp Gerontol 45: 603–610. [DOI] [PubMed] [Google Scholar]
- 25. Durai S, Karutha Pandian S, Balamurugan K (2011) Changes in Caenorhabditis elegans exposed to Vibrio parahaemolyticus . J Microbiol Biotechnol 21: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 26. Roeder T, Stanisak M, Gelhaus C, Bruchhaus I, Grotzinger J, et al. (2010) Caenopores are antimicrobial peptides in the nematode Caenorhabditis elegans instrumental in nutrition and immunity. Dev Comp Immunol 34: 203–209. [DOI] [PubMed] [Google Scholar]
- 27.Sulston J, Hodgkin J (1988) The nematode Caenorhabditis elegans. New York: Cold Spring Harbor Lab Press.
- 28. Marden P, Tunlid A, Malmcrona-Friberg K, Odham G, Kjelleberg S (1985) Physiological and morphological changes during short term starvation of marine bacteria isolates. Arch Microbiol 142: 326–332. [Google Scholar]
- 29. Brenner S (1974) The genetics of Caenorhabditis elegans . Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stiernagle T (2006) Maintenance of C. elegans . WormBook 1: 11101895/wormbook11011 Available: http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html. Accessed 2014 Sep 18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ballestriero F, Thomas T, Burke C, Egan S, Kjelleberg S (2010) Identification of compounds with bioactivity against the nematode Caenorhabditis elegans by a screen based on the functional genomics of the marine bacterium Pseudoalteromonas tunicata D2. Appl Environ Microbiol 76: 5710–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 33. Egan S, James S, Holmström C, Kjelleberg S (2002) Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata . Environ Microbiol 4: 433–442. [DOI] [PubMed] [Google Scholar]
- 34. Herrero M, Delorenzo V, Timmis K (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol 172: 6557–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington D (2005) Linear Rank Tests in Survival Analysis. Encyclopedia of Biostatistics. Hoboken, NJ: John Wiley & Sons, Ltd.
- 36. Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemo Rep Part 1 50: 163–170. [PubMed] [Google Scholar]
- 37. Balibar CJ, Walsh CT (2006) In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum . Biochemistry 45: 15444–15457. [DOI] [PubMed] [Google Scholar]
- 38. Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans . Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- 39. Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, et al. (2002) Inducible antibacterial defense system in C. elegans . Curr Biol 12: 1209–1214. [DOI] [PubMed] [Google Scholar]
- 40. Chavez V, Mohri-Shiomi A, Maadani A, Vega LA, Garsin DA (2007) Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans . Genetics 176: 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alegado RA, Tan MW (2008) Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell Microbiol 10: 1259–1273. [DOI] [PubMed] [Google Scholar]
- 42. August PR, Grossman TH, Minor C, Draper MP, MacNeil IA, et al. (2000) Sequence analysis and functional characterization of the violacein biosynthetic pathway from Chromobacterium violaceum . J Mol Microbiol Biotechnol 2: 513–519. [PubMed] [Google Scholar]
- 43. Zhang X, Enomoto K (2011) Characterization of a gene cluster and its putative promoter region for violacein biosynthesis in Pseudoalteromonas sp. 520P1. Appl Microbiol Biotechnol 90: 1963–1971. [DOI] [PubMed] [Google Scholar]
- 44. Jiang PX, Wang HS, Xiao S, Fang MY, Zhang RP, et al. (2012) Pathway redesign for deoxyviolacein biosynthesis in Citrobacter freundii and characterization of this pigment. Appl Microbiol Biotechnol 94: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 45. Ahmetagic A, Philip DS, Sarovich DS, Kluver DW, Pemberton JM (2011) Plasmid encoded antibiotics inhibit protozoan predation of Escherichia coli K12. Plasmid 66: 152–158. [DOI] [PubMed] [Google Scholar]
- 46. Matz C, Deines P, Boenigk J, Arndt H, Eberl L, et al. (2004) Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl Environ Microbiol 70: 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor CM, Martin J, Rao RU, Powell K, Abubucker S, et al. (2013) Using existing drugs as leads for broad spectrum anthelmintics targeting protein kinases. PLoS Pathog 9: e1003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bromberg N, Dreyfuss JL, Regatieri CV, Palladino MV, Durán N, et al. (2010) Growth inhibition and pro-apoptotic activity of violacein in Ehrlich ascites tumor. Chem-Biol Interact 186: 43–52. [DOI] [PubMed] [Google Scholar]
- 49. Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, et al. (2002) Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 161: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kho MF, Bellier A, Balasubramani V, Hu Y, Hsu W, et al. (2011) The pore-forming protein Cry5B elicits the pathogenicity of Bacillus sp. against Caenorhabditis elegans . PLoS One 6: e29122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gems D, Riddle DL (2000) Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans . Genetics 154: 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, et al. (2003) Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300: 1921. [DOI] [PubMed] [Google Scholar]
- 53. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- 54. Kawli T, He F, Tan MW (2010) It takes nerves to fight infections: insights on neuro-immune interactions from C. elegans . Dis Model Mech 3: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McEwan DL, Kirienko NV, Ausubel FM (2012) Host translational inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an immune response in Caenorhabditis elegans . Cell Host Microbe 11: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Melo JA, Ruvkun G (2012) Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell 149: 452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kleino A, Silverman N (2012) UnZIPping mechanisms of effector-triggered immunity in animals. Cell Host & Microbe 11: 320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Carvalho DD, Costa FT, Duran N, Haun M (2006) Cytotoxic activity of violacein in human colon cancer cells. Toxicol In Vitro 20: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 59. Konzen M, De Marco D, Cordova CA, Vieira TO, Antonio RV, et al. (2006) Antioxidant properties of violacein: possible relation on its biological function. Bioorg Med Chem 14: 8307–8313. [DOI] [PubMed] [Google Scholar]
- 60. Evans EA, Kawli T, Tan MW (2008) Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans . PLoS Pathog 4: e1000175 doi:1000110.1001371/journal.ppat.1000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jousset A (2012) Ecological and evolutive implications of bacterial defences against predators. Environ Microbiol 14: 1830–1843. [DOI] [PubMed] [Google Scholar]
- 62. Penesyan A, Marshall-Jones Z, Holmström C, Kjelleberg S, Egan S (2009) Antimicrobial activity observed among cultured marine epiphytic bacteria reflects their potential as a source of new drugs. FEMS Microbiol Ecol 69: 113–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All sequencing files are available from the National Center for Biotechnology Information (NCBI) public database via accession number JX523957.