Abstract
Background
While omega-6 polyunsaturated fatty acids(n-6 PUFA) have been recommended to reduce CHD, controversy remains about benefits vs. harms, including concerns over theorized pro-inflammatory effects of n-6 PUFA. We investigated associations of circulating n-6 PUFA including linoleic acid(LA, the major dietary PUFA), γ-linolenic acid(GLA), dihomo-γ-linolenic acid(DGLA), and arachidonic acid(AA),with total and cause-specific mortality in the Cardiovascular Health Study, a community-based US cohort.
Methods and Results
Among 2,792 participants(age≥65y) free of CVD at baseline, plasma phospholipid n-6 PUFAwere measured at baseline using standardized methods. All-cause and cause-specific mortality, and total incident CHD and stroke, were assessed and adjudicated centrally. Associations of PUFA with risk were assessed by Cox regression. During 34,291 person-years of follow-up(1992–2010), 1,994 deaths occurred(678 cardiovascular deaths), with 427 fatal and 418 nonfatal CHD, and 154 fatal and 399 nonfatal strokes. In multivariable models, higher LA was associated with lower total mortality, with extreme-quintile HR=0.87(P-trend=0.005). Lower death was largely attributable to CVD causes, especially nonarrhythmic CHD mortality(HR=0.51, 95%CI=0.32–0.82, P-trend=0.001). Circulating GLA, DGLA, and AA were not significantly associated with total or cause-specific mortality; e.g., for AA and CHD death, the extreme-quintile HR was 0.97 (95%CI=0.70–1.34, P-trend=0.87). Evaluated semi-parametrically, LA showed graded inverse associations with total mortality(P=0.005). There was little evidence that associations of n-6 PUFA with total mortality varied by age, sex, race, or plasma n-3 PUFA. Evaluating both n-6 and n-3 PUFA, lowest risk was evident with highest levels of both.
Conclusions
High circulating LA, but not other n-6 PUFA, was inversely associated with total and CHD mortality in older adults.
Keywords: Fatty Acids, Omega-6, Cardiovascular diseases, Epidemiology, Mortality
Current US dietary guidelines recommend higher intake of omega-6 polyunsaturated fatty acids (n-6 PUFA) to reduce the risk of coronary heart disease (CHD).1, 2 However, the influence and potential dose-response relationship of n-6 PUFA on health remain contentious. Whereas clinical studies support blood cholesterol benefits of linoleic acid (LA), the predominant dietary n-6 PUFA;3 and higher dietary PUFA (predominantly LA) is associated with lower CHD risk in prospective cohort studies;4 not all cohort studies observed benefits,5 and older, randomized trials utilizing LA-rich vegetable oils did not consistently reduce risk of CHD.5, 6 As LA is endogenously converted to arachidonic acid (AA, Supplement Figure 1), concern has also been raised over theoretical pro-inflammatory and pro-thrombotic effects of AA metabolites.7, 8 Due to their shared metabolic pathways, another hypothesized potential for harm is competition of LA with n-3 PUFA, and therefore interference with potential cardiovascular benefits of n-3 PUFA.8 Thus, while in 2009 the American Heart Association released a scientific advisory recommending health benefits of dietary LA,2 other academic papers, books, and media reports have recommended that LA consumption be substantially lowered for maximum health benefits.8–12 Because LA is the major dietary PUFA from vegetable oils, its impact on health is of public health importance, and additional studies are needed to improve the scientific basis for dietary recommendations. In addition, the impact of other n-6 PUFA on health, including γ-linolenic acid (GLA), dihomo-γ-linoleinc acid (DGLA), and AA, remains poorly established.
Most prior studies have evaluated potential cardiovascular effects of n-6 PUFA, and much less is known on potential noncardiovascular outcomes such as cancer.13 Meta-analysis of older, largely single-blind randomized trials of LA-rich vegetable oils did not detect an effect on total mortality,14 raising concern for potentially opposing effects on different endpoints. However, most of these trials were conducted in subjects with a history of CHD, and the findings may not be generalizable to primary prevention. Very few prospective cohort studies have assessed the association of n-6 PUFA with total mortality in more general populations. Data are lacking in older individuals, in whom risk is greatest and few interventions can effectively reduce total mortality. In addition, most prior studies have investigated self-reported n-6 PUFA consumption, which may be limited by recall bias and measurement error and also poorly estimates potential effects of GLA, DGLA, and AA, which may differ in their biologic functions.15
To address these gaps in knowledge, we prospectively designed and assessed the association of circulating n-6 PUFA biomarkers with total and cause-specific mortality in the Cardiovascular Health Study (CHS), a community-based cohort of older US adults. Circulating LA are objective biomarkers of LA intake.16 Conversely, circulating level of the other n-6 PUFA tend to show weaker associations with their dietary intake, which suggest endogenous metabolism may play more dominant roles in determining exposure to these fatty acids.16 Because the predominant hypothesized pathway of harm for n-6 PUFA is competition with n-3 PUFA,8 we also assessed whether associations of n-6 PUFA varied in relation to n-3 PUFA levels, and assessed their separate and joint relationships with mortality.
Methods
Design and Population
The Cardiovascular Health Study (CHS) is a multicenter, community-based prospective cohort of older US adults.17 In 1989–1990, 5201 noninstitutionalized older adults (≥65 years) from 4 communities were randomly recruited and enrolled from Medicare eligibility lists. An additional 687 African American participants were similarly selected and enrolled in 1992–1993. Participants attended annual in-clinic evaluations carried out by trained personnel using standardized protocols.17–20 The institutional review committee from each center approved the study, and all participants gave written informed consent.
Study Measures
Plasma phospholipid fatty acids were measured in 3941 study participants with available blood samples collected and stored from the 1992–1993 study visit, which we considered the baseline for this analysis. After exclusion of 1149 participants with prevalent CVD at the time of blood sampling, 2792 participants were included in the present analysis. Additional details of cohort sampling and fatty acid measurements have been published,21 and are described in the Online Data Supplements. Our primary exposures were the individual n-6 PUFA levels, including LA, GLA, DGLA, and AA. We also measured plasma phospholipid long chain n-3 PUFA including eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). Other demographic and clinical risk factors were assessed using standardized protocols at baseline (Online Data Supplements).
Ascertainment of End Points
CHS participants were followed up by means of annual study clinic visits with interim 6-month telephone contact through to 2000, and biannual telephone contacts thereafter. Vital status follow-up was 100% complete; less than 1% of all person-time was otherwise missing and censored early. Based on available data from interviews; next of kin; death certificates; and medical records (including diagnostic tests and consultations), a centralized events committee assessed and adjudicated all-cause and cause-specific mortality, fatal or nonfatal CHD and stroke, and arrhythmic CHD deaths. Methods for follow-up; confirmation; and classification of deaths, CHD, and stroke have been described.22–24 Cardiovascular disease mortality was defined as deaths due to CHD, stroke, other atherosclerotic disease, and other CVD. Non-CVD mortality included deaths due to cancer, infection, dementia, pulmonary diseases, fractures or trauma, and other causes.
Statistical Analysis
Fatty acid levels, expressed as percent of total fatty acids were evaluated in quintiles as indicator variables, and also continuously according to each unit of measurement. To assess linear trends, quintiles were assessed as continuous variables after assigning the participants the median value in each quintile. Potential non-linear associations were assessed semi-parametrically using restricted cubic splines.
Cox proportional-hazards model were used to estimate hazard ratios (HRs), with time-at-risk until first event, other deaths in cause-specific mortality analyses, or the latest adjudicated date of follow-up. Covariates were selected on biologic interest, well-established relations with mortality risk in older adults, or associations with exposures in the final dataset. Further adjustments were also made for fasting HDL-cholesterol, LDL-cholesterol, triglycerides, fibrinogen and C-reactive protein to investigate if n-6 PUFA could be associated with mortality risk via these potential confounders or mediators. The proportional hazards assumption was not violated on the basis of Schoenfeld residuals. Missing covariates were imputed (<2% for most factors, up to 9.7% for dietary factors) by best-subset-regression using demographic/risk variables. Results were similar when subjects with missing values were excluded.
We evaluated the separate and joint effects of LA and n-3 PUFA using jointly stratified analyses. In exploratory analyses, we also assessed effect modification of n-6 PUFA with total mortality using stratified analyses for several subgroups including age (<median, ≥median), gender (men, women), race (white, African Americans), and plasma n-3 PUFA (<median, ≥median), The significance of potential effect modification was tested using the Wald test for a multiplicative interaction term (cross product of the fatty acid exposure and the stratification variable), with a Bonferroni-corrected α-level of 0.003 (4 fatty acids×4 interactions = 16 exploratory comparisons). Given potential for exposure misclassification of fatty acid levels with increasing duration of follow-up, we also performed sensitivity analyses with censoring at the mid-point(9 years) of follow-up, and excluding deaths within the first 2 years to minimize effects of unrecognized subclinical disease on n-6 PUFA levels. All analyses were conducted using Stata (release 12.0, Stata Corp, College Station, Texas), and significance defined as two-tailed-alpha=0.05.
Results
At baseline, the mean age of study participants was 74 years, the majority of whom were women (64%). LA was the most abundant n-6 PUFA (mean±SD, 19.7±2.5% of total plasma phospholipid fatty acid), followed by AA (11.1±2.0%), DGLA (3.1±0.7%), and GLA (0.09±0.05%). Correlations between the individual n-6 PUFA and are shown in Supplement Table 1. LA was inversely correlated with all other n-6 PUFA, in particular with AA (r=−0.65). Intercorrelations between AA, DGLA, and GLA were more modest (r=−0.33 to 0.36). In unadjusted cross-sectional analysis, the individual n-6 PUFA showed different patterns of associations with demographic, clinical, and dietary factors (Supplement Table 2). For example, LA was associated with older age, male gender and Caucasian ethnicity, and lower prevalence of type 2 diabetes; while AA showed opposite directions of association with these factors.
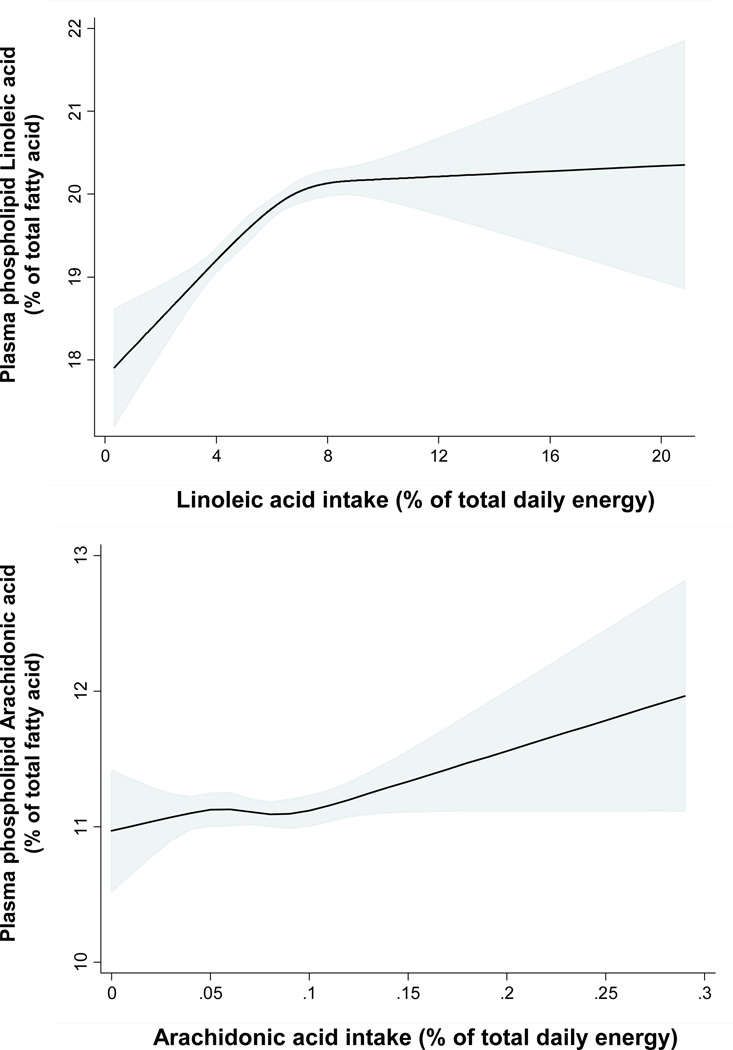
To investigate how dietary n-6 PUFA consumption might be associated with their plasma phospholipid levels, we used semiparametric restricted cubic spline analysis to assess the association of estimated dietary LA and AA intake with their circulating concentrations (Figure 1). The association between dietary circulating LA concentration was nonlinear (P<0.001). Circulating LA showed the greatest dose-response association with intake of LA up to ~8% of total daily energy; with relatively smaller increases at intakes>8%. In contrast, as previously reported by others,16, 25 dietary consumption of AA did not appear to be associated with its circulating levels across the range of intake observed in this study (P=0.24), with little evidence for non-linearity (P=0.40). Estimated dietary consumption data for GLA and DGLA were not available to evaluate potential dose-responses with circulating levels. Estimated dietary LA showed statistically significant but very weak inverse associations with circulating DGLA (per 1SD higher LA; β=−0.04, 95%CI=−0.04, −0.01, p=0.003) and AA (per 1SD higher LA; β=−0.1, 95%CI= −0.17, −0.04, p=0.003), and was not associated with GLA.
Figure 1.
Relationship between estimated dietary consumption of linoleic acid and arachidonic acid and their circulating concentrations in plasma phospholipids, evaluated using restricted cubic splines and adjusted for age, gender, race, BMI, and use of lipid lowering medications. The solid lines and shaded areas represent the central risk estimates and 95% CIs, respectively. Median intakes of linoleic acid and arachidonic acid were 6% of total energy (14.1g/day) and 0.08% of total energy (0.17g/day), respectively. Strong evidence was seen for both overall positive association (P < 0.001) and nonlinearity (P < 0.001) of the relationship between dietary and circulating linoleic acid. In contrast, little evidence of either an overall relationship (P = 0.24) or nonlinearity (P = 0.40) was evident for dietary and circulating arachidonic acid.
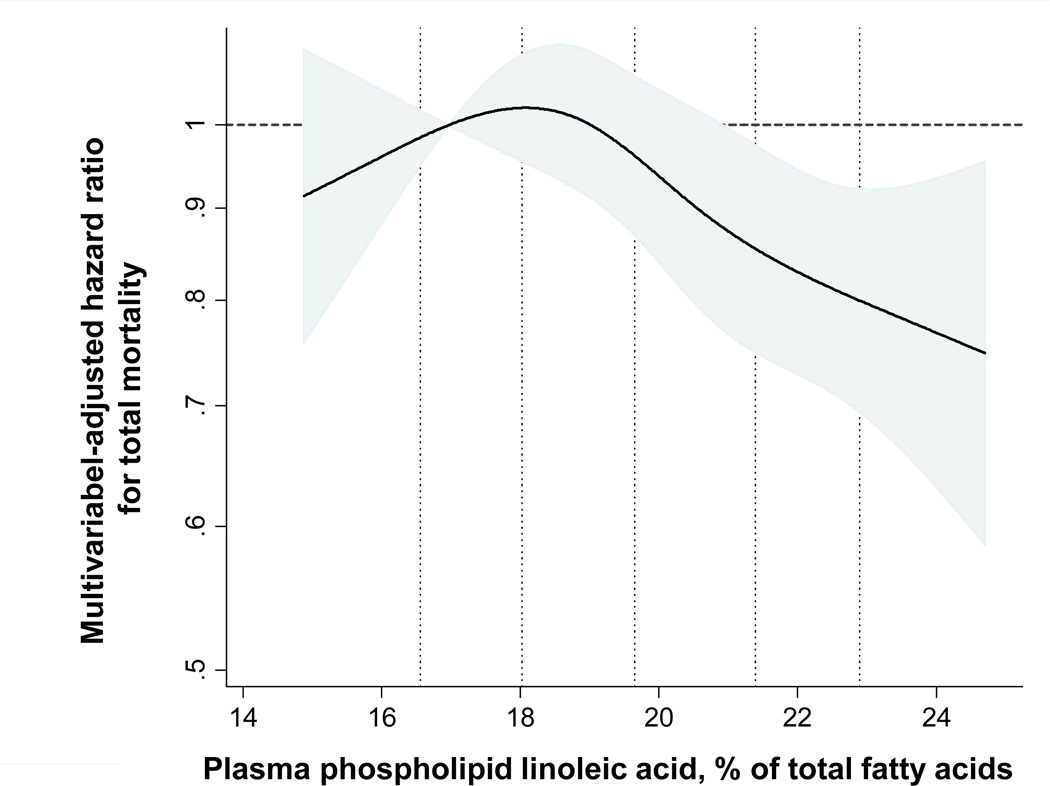
During 34,291 years of follow-up, 1,994 deaths occurred (incidence rate:5.8 per 100 person-years). After adjustment for demographic, lifestyle, cardiovascular, and dietary risk factors, circulating LA was inversely associated with total mortality, with 13% lower risk (HR=0.87; 95% CI=0.74–1.02; P for trend=0.005) among participants in the highest compared to the lowest quintile (Table 1). None of the other n-6 PUFA were significantly associated with total mortality. For example, participants in the highest compared to the lowest quintile of AA had a HR (95% CI)=0.87(0.75–1.01) for death (P for trend=0.25). Evaluated continuously, each 1SD higher LA was associated with 7% lower total mortality (HR=0.93; 95% CI,0.88–0.98); none of the other n-6 PUFA were significantly associated with total mortality (P >0.17 for each). Further adjustment for potential confounders or intermediate risk factors including LDL-C, HDL-C, triglycerides, C-reactive protein, and fibrinogen slightly attenuated the inverse associations of LA with total mortality (HR for top vs. bottom quintile=0.89; 95% CI, 0.76–1.04; P for trend=0.01). Additional adjustment for use of lipid medication, aspirin use, and consumption of fruits, vegetables, dietary fiber, and red meat did not materially alter the results (data not shown). Restricted cubic spline analysis suggested relatively linear inverse associations of LA with total mortality (P for overall association=0.005, Figure 2). There was similarly little evidence for non-linear associations of AA, GLA, or DGLA with total mortality (P non-linearity≥0.21 each).
Table 1.
Risk of Total Mortality According to Plasma Phospholipid n-6 Polyunsaturated Fatty Acids among 2,792 US Adults.
| Quintiles of plasma phospholipid fatty acid levels | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ptrend* | |
| Linoleic acid (% of fatty acids, median) | 16.6 | 18.4 | 19.7 | 21.0 | 22.9 | |
| Person-years of follow-up | 7074 | 6740 | 6668 | 6954 | 6854 | |
| Deaths, n | 384 | 403 | 418 | 393 | 396 | |
| Age and sex adjusted HR (95% CI) | 1.0 (Reference) | 1.08 (0.94, 1.24) | 1.02 (0.89, 1.17) | 0.90 (0.78, 1.04) | 0.91 (0.79, 1.05) | 0.03 |
| Multivariable adjusted HR (95% CI)† | 1.0 (Reference) | 1.11 (0.96, 1.28) | 1.00 (0.86, 1.15) | 0.88 (0.76, 1.03) | 0.87 (0.74, 1.02) | 0.005 |
| Arachidonic acid (% of fatty acids, median) | 8.6 | 10.1 | 11.1 | 12.1 | 13.6 | |
| Person-years of follow-up | 6668 | 6967 | 6699 | 6633 | 7324 | |
| Deaths, n | 421 | 383 | 421 | 404 | 365 | |
| Age and sex adjusted HR (95% CI) | 1.0 (Reference) | 0.88 (0.77, 1.01) | 1.07 (0.93, 1.22) | 1.03 (0.90, 1.18) | 0.88 (0.77, 1.02) | 0.43 |
| Multivariable adjusted HR (95% CI)† | 1.0 (Reference) | 0.90 (0.79, 1.04) | 1.07 (0.93, 1.22) | 1.00 (0.87, 1.15) | 0.87 (0.75, 1.01) | 0.25 |
| γ-Linolenic acid (% of fatty acids, median) | 0.05 | 0.06 | 0.08 | 0.10 | 0.14 | |
| Person-years of follow-up | 6814 | 6816 | 6559 | 7196 | 6904 | |
| Deaths, n | 423 | 409 | 404 | 378 | 380 | |
| Age and sex adjusted HR (95% CI) | 1.0 (Reference) | 1.05 (0.91, 1.20) | 1.10 (0.96, 1.26) | 0.99 (0.86, 1.13) | 1.08 (0.94, 1.25) | 0.49 |
| Multivariable adjusted HR (95% CI)† | 1.0 (Reference) | 1.00 (0.87, 1.15) | 1.00 (0.87, 1.15) | 0.90 (0.78, 1.04) | 0.98 (0.84, 1.13) | 0.46 |
| Di-homo-γ-linolenic acid (% of fatty acids, median) | 2.2 | 2.7 | 3.0 | 3.4 | 4.0 | |
| Person-years of follow-up | 6727 | 6627 | 6938 | 7034 | 6964 | |
| Deaths, n | 401 | 418 | 399 | 378 | 398 | |
| Age and sex adjusted HR (95% CI) | 1.0 (Reference) | 1.13 (0.98, 1.30) | 1.06 (0.92, 1.21) | 1.01 (0.88, 1.17) | 1.10 (0.95, 1.26) | 0.55 |
| Multivariable adjusted HR (95% CI)† | 1.0 (Reference) | 1.03 (0.90, 1.19) | 0.97 (0.84, 1.12) | 0.92 (0.79, 1.06) | 0.96 (0.83, 1.11) | 0.30 |
Linear trend was tested by assigning to participants the median value in each quartile and assessing this as a continuous variable. Findings were similar when fatty acid concentrations were evaluated in their natural units as continuous exposures (data not shown).
Adjusted for age (years), gender (male or female), race (white or non white), enrollment site (4 sites), education (<high school, high school, some college, or college graduate), smoking status (never, former, or current), prevalent diabetes (yes or no), atrial fibrillation (yes or no), and hypertension (yes or no), leisure-time physical activity (mcal/wk), body mass index (kg/m2), waist circumference (cm), alcohol use (6 categories), and plasma phospholipid long-chain n-3 PUFA (sum of EPA+DPA+DHA, % of total fatty acids).
Figure 2.
Multivariable hazard ratios of plasma phospholipid linoleic acid with risk of total mortality, evaluated by restricted cubic splines from Cox models adjusted for age, gender, race, enrollment site, education, smoking status, prevalent diabetes, atrial fibrillation, and hypertension, leisure-time physical activity, body mass index, waist circumference, alcohol use, and plasma phospholipid long-chain n-3 PUFA levels. The solid lines and shaded areas represent the central risk estimates and 95% CIs, respectively, relative to the reference level (12.5th percentile). The dotted vertical lines correspond to the 10th, 25th, 50th, 75th, and 90th percentiles of linoleic acid levels. A significant inverse association was evident (P=0.005), with little evidence for nonlinearity (P nonlinearity=0.16).
When cause-specific mortality was evaluated, LA was associated with lower CVD mortality (Table 2), with 22% lower risk in the top vs. bottom quintile (HR=0.78; 95% CI, 0.60, 1.01; P for trend=0.02). Among CVD subtypes, LA most strongly associated with non-arrhythmic CHD mortality, with 49% lower risk across quintiles (P for trend=0.001). Conversely, LA was not associated with arrhythmic CHD mortality (top vs. bottom quintile, HR=1.22; 95% CI, 0.76, 1.95; P for trend=0.44). There was no evidence that circulating GLA, DGLA or AA were associated with CVD mortality.
Table 2.
Relative Risk of Cardiovascular Mortality and Incident Cardiovascular Disease According to Plasma Phospholipid n-6 Polyunsaturated Fatty Acids among 2792 US Adults.
| Multivariable hazard ratio (95% CI), by quintiles of plasma phospholipid fatty acid levels* | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ptrend† | |
| Total Cardiovascular Mortality (678 cases)‡ | ||||||
| LA | 1.0 (Reference) | 0.88 (0.69, 1.12) | 0.84 (0.66, 1.07) | 0.69 (0.53, 0.90) | 0.78 (0.60, 1.01) | 0.02 |
| AA | 1.0 (Reference) | 0.92 (0.72, 1.18) | 1.20 (0.95, 1.52) | 1.04 (0.81, 1.33) | 1.00 (0.78, 1.30) | 0.70 |
| GLA | 1.0 (Reference) | 0.87 (0.68, 1.10) | 1.02 (0.81, 1.30) | 0.89 (0.70, 1.14) | 0.99 (0.78, 1.27) | 0.89 |
| DGLA | 1.0 (Reference) | 0.85 (0.66, 1.08) | 0.89 (0.70, 1.14) | 0.74 (0.57, 0.96) | 0.94 (0.73, 1.20) | 0.55 |
| Total CHD Mortality (427 cases) | ||||||
| LA | 1.0 (Reference) | 0.98 (0.72, 1.34) | 0.96 (0.71, 1.30) | 0.78 (0.57, 1.08) | 0.80 (0.57, 1.12) | 0.09 |
| AA | 1.0 (Reference) | 0.95 (0.70, 1.30) | 1.09 (0.81, 1.48) | 1.11 (0.82, 1.50) | 0.97 (0.70, 1.34) | 0.87 |
| GLA | 1.0 (Reference) | 0.91 (0.67, 1.24) | 1.11 (0.83, 1.50) | 0.93 (0.68, 1.27) | 1.11 (0.82, 1.51) | 0.46 |
| DGLA | 1.0 (Reference) | 0.90 (0.66, 1.21) | 0.90 (0.67, 1.23) | 0.73 (0.52, 1.02) | 1.02 (0.75, 1.39) | 0.94 |
| Arrhythmic CHD mortality (225 cases)§ | ||||||
| LA | 1.0 (Reference) | 1.27 (0.82, 1.96) | 1.14 (0.73, 1.77) | 1.30 (0.84, 2.02) | 1.22 (0.76, 1.95) | 0.44 |
| AA | 1.0 (Reference) | 1.25 (0.81, 1.92) | 1.29 (0.84, 1.98) | 1.19 (0.77, 1.85) | 0.95 (0.60, 1.52) | 0.78 |
| GLA | 1.0 (Reference) | 0.79 (0.52, 1.19) | 0.91 (0.60, 1.36) | 0.78 (0.51, 1.19) | 0.96 (0.63, 1.46) | 0.99 |
| DGLA | 1.0 (Reference) | 0.82 (0.54, 1.22) | 0.85 (0.57, 1.28) | 0.59 (0.37, 0.93) | 0.85 (0.55, 1.30) | 0.31 |
| Non-arrhythmic CHD mortality (202 cases)§ | ||||||
| LA | 1.0 (Reference) | 0.75 (0.49, 1.16) | 0.79 (0.52, 1.20) | 0.42 (0.26, 0.69) | 0.51 (0.32, 0.82) | 0.001 |
| AA | 1.0 (Reference) | 0.70 (0.44, 1.11) | 0.92 (0.60, 1.41) | 1.04 (0.68, 1.60) | 1.00 (0.64, 1.56) | 0.60 |
| GLA | 1.0 (Reference) | 1.09 (0.69, 1.72) | 1.41 (0.91, 2.20) | 1.17 (0.73, 1.89) | 1.33 (0.83, 2.11) | 0.28 |
| DGLA | 1.0 (Reference) | 1.04 (0.65, 1.65) | 1.00 (0.63, 1.60) | 0.94 (0.58, 1.53) | 1.29 (0.81, 2.05) | 0.31 |
| Congestive Heart Failure mortality§ (84 cases) | ||||||
| LA | 1.0 (Reference) | 0.81 (0.40, 1.65) | 0.88 (0.45, 1.70) | 0.45 (0.21, 0.97) | 0.55 (0.26, 1.16) | 0.05 |
| AA | 1.0 (Reference) | 1.01 (0.51, 1.99) | 1.12 (0.57, 2.20) | 1.27 (0.65, 2.50) | 0.93 (0.44, 1.97) | 0.91 |
| GLA | 1.0 (Reference) | 1.12 (0.54, 2.33) | 1.57 (0.78, 3.15) | 1.48 (0.73, 3.04) | 1.47 (0.70, 3.08) | 0.28 |
| DGLA | 1.0 (Reference) | 1.29 (0.62, 2.69) | 1.81 (0.91, 3.62) | 0.95 (0.43, 2.12) | 1.23 (0.57, 2.66) | 0.95 |
| Other non-arrhythmic CHD mortality§ (118 cases) | ||||||
| LA | 1.0 (Reference) | 0.71 (0.41, 1.24) | 0.75 (0.44, 1.29) | 0.41 (0.22, 0.78) | 0.49 (0.26, 0.91) | 0.009 |
| AA | 1.0 (Reference) | 0.49 (0.26, 0.94) | 0.81 (0.46, 1.42) | 0.88 (0.51, 1.54) | 0.99 (0.57, 1.73) | 0.63 |
| GLA | 1.0 (Reference) | 1.06 (0.59, 1.93) | 1.31 (0.74, 2.32) | 0.96 (0.52, 1.77) | 1.21 (0.66, 2.19) | 0.68 |
| DGLA | 1.0 (Reference) | 0.92 (0.50, 1.68) | 0.60 (0.31, 1.18) | 0.94 (0.51, 1.73) | 1.34 (0.75, 2.39) | 0.19 |
| Stroke Mortality (154 cases) | ||||||
| LA | 1.0 (Reference) | 0.76 (0.45, 1.30) | 0.76 (0.45, 1.28) | 0.55 (0.31, 0.97) | 0.80 (0.47, 1.37) | 0.35 |
| AA | 1.0 (Reference) | 1.00 (0.60, 1.67) | 1.23 (0.76, 2.00) | 1.07 (0.64, 1.81) | 1.05 (0.61, 1.80) | 0.77 |
| GLA | 1.0 (Reference) | 0.74 (0.45, 1.24) | 0.87 (0.53, 1.43) | 0.89 (0.54, 1.46) | 0.90 (0.54, 1.50) | 0.99 |
| DGLA | 1.0 (Reference) | 0.80 (0.48, 1.34) | 1.11 (0.68, 1.81) | 0.93 (0.55, 1.57) | 0.93 (0.55, 1.56) | 0.94 |
| Total fatal and nonfatal CHD (720 cases)‖ | ||||||
| LA | 1.0 (Reference) | 0.99 (0.77, 1.27) | 1.11 (0.88, 1.41) | 1.03 (0.81, 1.32) | 0.98 (0.76, 1.27) | 0.95 |
| AA | 1.0 (Reference) | 0.98 (0.78, 1.24) | 1.00 (0.80, 1.27) | 1.09 (0.86, 1.37) | 0.89 (0.69, 1.14) | 0.59 |
| GLA | 1.0 (Reference) | 1.02 (0.81, 1.29) | 1.19 (0.94, 1.50) | 1.05 (0.83, 1.34) | 1.16 (0.91, 1.47) | 0.28 |
| DGLA | 1.0 (Reference) | 0.91 (0.72, 1.16) | 0.89 (0.70, 1.13) | 0.84 (0.65, 1.08) | 1.07 (0.84, 1.37) | 0.57 |
| Nonfatal myocardial infarction (418 cases) | ||||||
| LA | 1.0 (Reference) | 1.02 (0.73, 1.43) | 1.39 (1.02, 1.90) | 1.22 (0.88, 1.69) | 1.19 (0.84, 1.67) | 0.23 |
| AA | 1.0 (Reference) | 0.96 (0.71, 1.30) | 0.87 (0.64, 1.18) | 1.11 (0.83, 1.50) | 0.87 (0.63, 1.20) | 0.65 |
| GLA | 1.0 (Reference) | 1.13 (0.83, 1.55) | 1.32 (0.96, 1.80) | 1.28 (0.93, 1.75) | 1.18 (0.85, 1.64) | 0.36 |
| DGLA | 1.0 (Reference) | 0.94 (0.68, 1.29) | 0.95 (0.69, 1.30) | 1.01 (0.73, 1.40) | 1.14 (0.83, 1.57) | 0.29 |
| Total fatal and nonfatal stroke (463 cases)# | ||||||
| LA | 1.0 (Reference) | 0.96 (0.71, 1.30) | 1.00 (0.74, 1.36) | 0.87 (0.64, 1.19) | 0.92 (0.67, 1.27) | 0.50 |
| AA | 1.0 (Reference) | 1.08 (0.80, 1.44) | 1.00 (0.74, 1.34) | 1.19 (0.89, 1.60) | 1.06 (0.78, 1.45) | 0.56 |
| GLA | 1.0 (Reference) | 1.09 (0.81, 1.47) | 1.12 (0.83, 1.51) | 1.15 (0.85, 1.55) | 1.03 (0.76, 1.41) | 0.93 |
| DGLA | 1.0 (Reference) | 0.96 (0.71, 1.29) | 1.03 (0.77, 1.38) | 1.02 (0.76, 1.38) | 0.87 (0.63, 1.18) | 0.45 |
| Ischemic stroke (362 cases) | ||||||
| LA | 1.0 (Reference) | 1.00 (0.71, 1.40) | 0.90 (0.64, 1.27) | 0.88 (0.62, 1.26) | 0.88 (0.61, 1.27) | 0.38 |
| AA | 1.0 (Reference) | 1.07 (0.77, 1.49) | 1.05 (0.75, 1.47) | 1.08 (0.77, 1.51) | 1.17 (0.83, 1.66) | 0.39 |
| GLA | 1.0 (Reference) | 1.08 (0.76, 1.53) | 1.17 (0.83, 1.66) | 1.20 (0.85, 1.69) | 1.09 (0.76, 1.55) | 0.67 |
| DGLA | 1.0 (Reference) | 1.01 (0.70, 1.45) | 1.28 (0.91, 1.81) | 1.29 (0.91, 1.82) | 1.04 (0.72, 1.50) | 0.63 |
| Hemorrhagic stroke (65 cases) | ||||||
| LA | 1.0 (Reference) | 0.72 (0.30, 1.74) | 0.98 (0.44, 2.21) | 0.91 (0.39, 2.10) | 1.10 (0.47, 2.57) | 0.65 |
| AA | 1.0 (Reference) | 0.72 (0.34, 1.53) | 0.62 (0.28, 1.37) | 1.39 (0.71, 2.74) | 0.33 (0.12, 0.90) | 0.22 |
| GLA | 1.0 (Reference) | 1.43 (0.69, 2.98) | 1.16 (0.53, 2.54) | 1.04 (0.46, 2.33) | 0.95 (0.40, 2.24) | 0.60 |
| DGLA | 1.0 (Reference) | 0.73 (0.36, 1.45) | 0.46 (0.21, 1.03) | 0.55 (0.25, 1.22) | 0.62 (0.29, 1.35) | 0.17 |
CHD, coronary heart disease, LA, linoleic acid, AA, arachidonic acid, GLA, γ-linolenic acid, DGLA, di-homo-γ-linolenic acid.
See Table 1 for median fatty acid levels in each quintile. Adjusted for age (years), gender (male or female), race (white or non white), enrollment site (4 sites), education (<high school, high school, some college, or college graduate), smoking status (never, former, or current), prevalent diabetes (yes or no), atrial fibrillation (yes or no), and hypertension (yes or no), leisure-time physical activity (mcal/wk), body mass index (kg/m2), waist circumference (cm), alcohol use (6 categories), and plasma phospholipid long-chain n-3 PUFA (sum of EPA+DPA+DHA, % of total fatty acids).
Linear trend was tested by assigning to participants the median value in each quartile and assessing this as a continuous variable. Findings were similar when fatty acid concentrations were evaluated in their natural units as continuous exposures.
Including 427 CHD deaths, 154 stroke deaths, 37 other atherosclerotic deaths (e.g., due to abdominal aortic aneurysm, mesenteric ischemia/infarctions, or peripheral vascular disease), and 60 other cardiovascular deaths (e.g., due to aortic stenosis, nonischemic cardiomyopathy, or venous thromboembolism).
Subsets of CHD mortality, with adjudication as to whether the underlying event was arrhythmic or nonarrhythmic. Non-arrhythmic CHD mortality included Congestive heart failure death (n=84) and other non-arrhythmic CHD death (cardiac procedure, multiple CHD causes of death, and unknown mechanism of cardiac death, n=118).
Includes 418 nonfatal myocardial infarctions and 302 CHD deaths. Analyses of incident CHD deaths included an additional 125 deaths that occurred with additional follow-up after an incident nonfatal myocardial infarction.
Includes 362 ischemic strokes, 65 hemorrhagic strokes, and 36 strokes for which clinical information was insufficient for subtype classification.
n-6 PUFA levels were generally unassociated with non-CVD causes of death, except for inverse associations of LA with respiratory death (top vs. bottom quintile, HR=0.42; 95% CI, 0.20, 0.87; P for trend=0.008), and of AA with death due to dementia (top vs. bottom quintile, HR=0.66; 95% CI, 0.45, 0.95; P for trend=0.05) (Supplement Table 3).
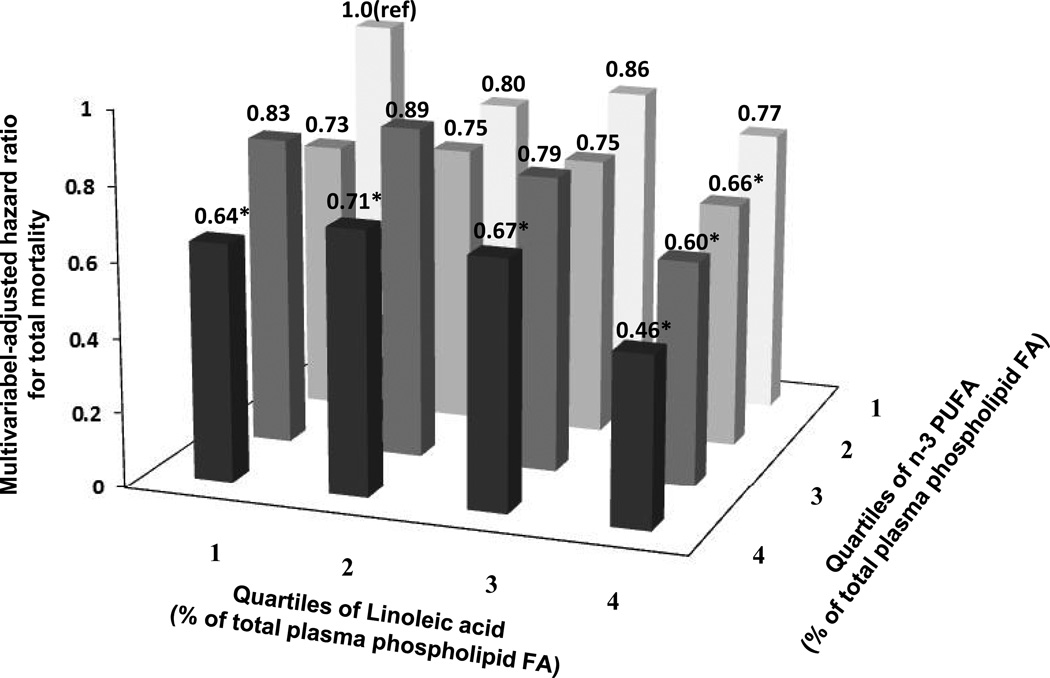
The associations of plasma phospholipid n-6 PUFA with mortality were similar with censoring at the mid-point of follow-up post-blood draw, or excluding deaths within the first 2 years after study baseline (data not shown). The association of each n-6 PUFA biomarker with total mortality was not significantly different by age, gender, race or plasma n-3 PUFA (Bonferroni corrected P > 0.003 for each). When subjects were stratified based on their joint LA and n-3 PUFA concentrations, those with the highest circulating levels of both LA and n-3 PUFA had 54% lower risk of total mortality (HR=0.46; 95% CI, 0.30–0.69) relative to those with lowest levels of both (Figure 3). Similarly, LA and n-3 PUFA biomarkers were each independently associated with lower risk of CVD death (Supplement Figure 2), and those with the highest circulating concentrations of both LA and n-3 PUFA had 64% lower risk of CVD mortality (HR=0.36; 95% CI, 0.17–0.74).
Figure 3.
Multivariable hazard ratios for total mortality by joint levels of plasma phospholipid linoleic acid and long-chain n-3 PUFA, adjusted for age, gender, race, enrollment site, education, smoking status, prevalent diabetes, atrial fibrillation, and hypertension, leisure-time physical activity, body mass index, waist circumference, and alcohol use, *P<0.05 compared with the referent category. Associations appeared independent, with little evidence for significant interaction between linoleic acid and long-chain n-3 PUFA (Wald test for multiplicative interaction: P=0.54).
Discussion
In this prospective cohort study of older US adults, higher circulating LA was associated with lower total mortality. The inverse association appeared relatively linear, with each 1SD increase in LA associated with ~7% lower risk. Among causes of mortality, LA demonstrated stronger inverse association with CVD death, in particular non-arrhythmic CHD death and CHF death, with ~50% lower risk across quintiles. Other n-6 PUFA including GLA, DGLA, and AA, were not significantly associated with total or CVD-specific mortality.
Health effects of LA remain strongly debated, resulting in uncertainties for setting dietary recommendations, and even concerns that current levels of intake could lead to harm. For example, while US guideline target at least 5–10% of energy from LA, the French Food Safety Agency in 2010 recommended limiting LA to <4% of total energy to avoid potential harm.26 Our results utilizing objective circulating biomarkers suggest that higher plasma phospholipid LA are associated with lower risk of total and CVD mortality in generally healthy older adults, without evidence for increased risk. These findings are supported by metabolic studies and animal experiments demonstrating physiologic benefits of LA, including lowering of LDL-C; raising of HDL-C; reductions in triglycerides, post-prandial lipemia, systemic inflammation, and liver fat accumulation; and improved glucose homeostasis.,27–29 In the present analysis, the inverse association of circulating LA with total mortality was slightly attenuated following adjustment for lipid and inflammatory biomarkers, suggesting these factors may partly mediate the association. The strong inverse association of LA with non-arrhythmic CHD mortality, including CHF death, is another novel and striking finding. In animal models of congestive heart failure, higher dietary LA protects against development of cardiac hypertrophy and systolic dysfunction, and improves total mortality.30, 31 Interestingly, whereas circulating n-3 PUFA was strongly associated with lower risk of arrhythmic CHD mortality in the CHS,21 LA was not associated with this outcome. In animal-experimental and in vitro studies, n-3 PUFA directly reduce myocyte excitability and susceptibility to triggered arrhythmia, possibly via altered function of membrane ion channels.32 In such experimental systems LA has been observed to possess much less potent effects than n-3 PUFA,33, 34 which may partly account for the lack of association with arrhythmic CHD death. Overall, our results are consistent with cardiac benefits of LA, support present dietary recommendations for intake of LA-rich vegetable oils, and provide little evidence for theorized harms. Our findings also highlight the need for additional mechanistic studies to elucidate physiologic actions of LA including potential benefits for heart failure and respiratory conditions.
The clear association between estimated dietary and plasma phospholipid LA confirms the role of the latter as a biomarker of consumption. However, our data indicated a non-linear relationship, with greatest dose-response up to ~8% energy from LA, with relatively smaller increases in circulating LA thereafter. Nonlinear associations between dietary consumption and circulating biomarker concentrations have also been observed for n-3 PUFA21 and micronutrients35, 36, consistent with relative saturation of carrying capacity or endogenous regulation to prevent excess accumulation. Overall, our findings suggest that targeting at least ~8–10% of energy from LA – rather than up to 10% of energy from LA as currently recommended by the US dietary guidelines1, 2 – may be associated with a lower risk of mortality.
Concern for harm of n-6 PUFA most often relates to theorized competition with n-3 PUFA, which may have important health benefits.37 n-3 and n-6 PUFA do share common enzymatic pathways that regulate their conversion to downstream bioactive metabolites, raising concerns for displacement of beneficial n-3 PUFA metabolites by LA.8, 9, 12 However, our findings provide no evidence for interaction between LA and n-3 PUFA for risk of mortality in older adults; and indeed support independent benefits of each for total and CVD mortality, with lowest mortality risk among people having highest biomarker levels of each. These findings utilizing objective biomarkers and evaluating total mortality are consistent with prior studies evaluating estimated dietary intakes and risk of CHD and inflammatory biomarkers.38, 39
Potential pro-inflammatory effects of dietary n-6 PUFA, and of AA in particular, are an additional putative danger for health.7, 8 Our results do not support harmful associations of circulating AA with total or cause-specific mortality in older adults. These findings are consistent with short-term randomized trials in which dietary supplementation with LA and AA had no appreciable effects on inflammatory markers, platelet function, and immune activation.40–43. Importantly, consistent with prior reports,16, 25 our findings also demonstrate lack of relationship between dietary consumption and phospolipid levels of AA. This indicates that tissue levels of AA is highly regulated endogenously. In animal experiments, GLA and DGLA modulate lipid metabolism, vasodilation, and inflammation.44 AA is also converted to a range of active metabolites, including potent resolvers of inflammation such as lipoxins and epoxy fatty acids (EETs).45, 46 However, our findings do not support substantial influence of circulating AA, GLA, or DGLA on total mortality in older adults. The inverse association of AA and death from dementia deserves further investigation as prior observational studies provide mixed evidence for associations of AA with dementia and cognitive function.47–49
In two prior prospective cohorts in Finland and Sweden, circulating LA was inversely associated with total mortality among middle-aged males, with reported relative risks of 0.66 (top vs. bottom tertile, 95% CI: 0.43–0.97),50 and 0.87 (per 1SD higher LA, 95% CI, 0.81, 0.93).51 One of these studies also reported no significant associations of serum GLA, DGLA, or AA with total mortality.51 Our findings build upon and substantially extend these prior results by including both men and women, evaluating phospholipids which are more closely correlated with membrane and tissue levels, assessing older adults in whom the risk of mortality is greatest, and evaluating cause-specific deaths and incident total (fatal+nonfatal) CVD events in addition to total mortality. Our investigation also had substantially larger number of deaths (1,994 vs. 1,237 in both prior studies combined), increasing statistical power. We additionally evaluated joint associations of circulating n-3 and n-6 PUFA levels, providing evidence for independent benefits of each. The consistency of beneficial associations for LA and mortality across these distinct populations with different ages, dietary habits, medical history, and lifestyle practices suggest that residual confounding is unlikely to entirely explain our observations.
Our study has several strengths. Measurement of objective biomarkers allowed investigation of individual n-6 PUFA while also avoiding potential errors and biases associated with self-reported dietary consumption. The investigation focused on older adults, for whom mortality risk is greatest. The prospective cohort design minimized selection and recall bias. Low loss to follow-up and central application of validated methods to determine mortality and incident CVD events reduced the possibility of missed or misclassified outcomes. Large number of incident events provided statistical power. Detailed and standardized assessment of demographic, clinical, and lifestyle variables reduced the influence of confounding. Recruitment of men and women from multiple communities across the US increased generalizability.
Potential limitations should be considered. n-6 PUFA biomarkers were measured once at baseline, and expected variation in circulating levels over time would cause misclassification over follow-up, causing underestimation of true associations. The 13-year within person correlation for circulating n-6 PUFAs were comparable to such correlations for other major CVD risk factors such as blood pressure.52 These data suggest the single baseline fatty acid measurement provides an adequate, but not perfect approach to estimate long term n-6 PUFA concentrations. Residual confounding due to imprecisely measured or unmeasured factors cannot be excluded. The association with specific CVD outcomes should be regarded as descriptive because a competing risk model was not used. Our cohort consisted of older men and women, and results may not be generalizable to younger populations.
In summary, our findings suggest that circulating levels of LA, the major dietary n-6 PUFA, is related to lower total mortality and especially subtypes of CVD mortality in older adults. Other circulating n-6 PUFA, including AA, were not significantly associated with total or CVD mortality.
Supplementary Material
Acknowledgements
The authors thank all CHS participants, CHS investigators, and institutions (see www.chs-nhlbi.org).
Funding Sources: The research reported in this article was supported by The National Heart, Lung, and Blood Institute (NHLBI) with co-funding from the National Institutes of Health Office of Dietary Supplements (R01 HL 085710-01). A subset of additional fatty acid measurements were supported by a Searle Scholar Award (to DM). CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Footnotes
Author Contributions: Study concept and design: Wu, Mozaffarian, Acquisition of data: Psaty, Siscovick, Mozaffarian, King, Song. Analysis and interpretation of data: Wu, Lemaitre, King, Song, Psaty, Siscovick, Mozaffarian. Drafting of the manuscript: Wu. Critical revision of the manuscript for important intellectual content: Wu, Lemaitre, King, Song, Psaty, Siscovick, Mozaffarian. Final approval of the manuscript: Wu, Lemaitre, King, Song, Psaty, Siscovick, Mozaffarian. Statistical analysis: Wu. Obtained funding: Lemaitre, King, Psaty, Siscovick, Mozaffarian. Administrative, technical, or material support: Song.
Conflict of Interest Disclosures: Dr. Mozaffarian reports ad hoc travel reimbursement or honoraria from Bunge, Pollock Institute, Quaker Oats, and Life Sciences Research Organization; ad hoc consulting fees from McKinsey Health Systems Institute, Foodminds, Nutrition Impact, Amarin, Omthera, and Winston and Strawn LLP; membership, Unilever North America Scientific Advisory Board; royalties from UpToDate; and research grants from GlaxoSmithKline, Sigma Tau, Pronova, the Gates Foundation, the Sackler Institute of Nutrition, and the National Institutes of Health. The other authors report no conflicts.
References
- 1.Dietary Guidelines for Americans. 7th ed. Washington DC: UC Government Printing Office; 2010. US Department of Agriculture, US Department of Health and Human Services. [Google Scholar]
- 2.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 3.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 4.Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw K, Mozaffarian D, Danesh J, Di Angelantonio E. Association of Dietary, Circulating, and Supplement Fatty Acids With Coronary Risk: A Systematic Review and Meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 6.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder PC. Dietary arachidonic acid: harmful, harmless or helpful? Br J Nutr. 2007;98:451–453. doi: 10.1017/S0007114507761779. [DOI] [PubMed] [Google Scholar]
- 8.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 9.Ramsden CE, Hibbeln JR, Lands WE. Letter to the Editor re: Linoleic acid and coronary heart disease. Prostaglandins Leukot. Essent. Fatty Acids (2008) In: Harris WS, editor. Prostaglandins Leukot Essent Fatty Acids. Vol. 80. 2009. p. 77. [DOI] [PubMed] [Google Scholar]
- 10.Huffington Post. Have We Been Misled? [Accessed March 5th, 2014];What You Should Know About Dietary Fats. http://www.huffingtonpost.com/leonard-smith/omega-6-omega-3_b_2971859.html Huffington Post. [Google Scholar]
- 11.Time Magazine. [Accessed March 5th 2014];Omega-6 Fats Linked to Increased Risk of Heart Disease. http://healthland.time.com/2013/02/06/omega-6-fats-linked-to-increased-risk-of-heart-disease/ [Google Scholar]
- 12.Allport S. The Queen of Fats. Why Omega-3s Were Removed from the Western Diet and What We Can Do to Replace Them. University of California Press; 2008. [Google Scholar]
- 13.Azrad M, Turgeon C, Demark-Wahnefried W. Current Evidence Linking Polyunsaturated Fatty Acids with Cancer Risk and Progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104:1586–1600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Sjogren P, Cederholm T, Arnlov J, Lindholm B, Riserus U, Carrero JJ. Serum and adipose tissue fatty acid composition as biomarkers of habitual dietary fat intake in elderly men with chronic kidney disease. Nephrol Dial Transplant. 2014;29:128–136. doi: 10.1093/ndt/gfs478. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 19.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 20.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Plasma Phospholipid Long-Chain omega-3 Fatty Acids and Total and Cause-Specific Mortality in Older Adults: A Cohort Study. Ann Intern Med. 2013;158:515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, Siscovick DS. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–206. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkkinen ES, Agren JJ, Ahola I, Ovaskainen ML, Uusitupa MI. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long-term adherence to fat-modified diets. Am J Clin Nutr. 1994;59:364–370. doi: 10.1093/ajcn/59.2.364. [DOI] [PubMed] [Google Scholar]
- 26.French Food Safety Agency. French population reference intakes (ANCs) for fatty acids. [Accessed March 5th, 2014]; http://www.anses.fr/sites/default/files/documents/NUT2006sa0359EN.pdf. [Google Scholar]
- 27.Asp ML, Collene AL, Norris LE, Cole RM, Stout MB, Tang SY, Hsu JC, Belury MA. Time-dependent effects of safflower oil to improve glycemia, inflammation and blood lipids in obese, post-menopausal women with type 2 diabetes: a randomized, double-masked, crossover study. Clin Nutr. 2011;30:443–449. doi: 10.1016/j.clnu.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlstrom H, Riserus U. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 29.Masson CJ, Mensink RP. Exchanging saturated fatty acids for (n-6) polyunsaturated fatty acids in a mixed meal may decrease postprandial lipemia and markers of inflammation and endothelial activity in overweight men. J Nutr. 2011;141:816–821. doi: 10.3945/jn.110.136432. [DOI] [PubMed] [Google Scholar]
- 30.Chicco AJ, Sparagna GC, McCune SA, Johnson CA, Murphy RC, Bolden DA, Rees ML, Gardner RT, Moore RL. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension. 2008;52:549–555. doi: 10.1161/HYPERTENSIONAHA.108.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulligan CM, Sparagna GC, Le CH, De Mooy AB, Routh MA, Holmes MG, Hickson-Bick DL, Zarini S, Murphy RC, Xu FY, Hatch GM, McCune SA, Moore RL, Chicco AJ. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc Res. 2012;94:460–468. doi: 10.1093/cvr/cvs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Wu JH. Omega-3 Fatty Acids and Cardiovascular Disease – Effects on Risk Factors, Molecular Pathways, and Clinical Events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 33.Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel DP, Maddaford TG, Pierce GN. Effects of omega-3 polyunsaturated fatty acids on cardiac sarcolemmal Na(+)/H(+) exchange. Am J Physiol Heart Circ Physiol. 2002;283:H1688–H1694. doi: 10.1152/ajpheart.00664.2001. [DOI] [PubMed] [Google Scholar]
- 35.Johnston CS, Cox SK. Plasma-Saturating intakes of vitamin C confer maximal antioxidant protection to plasma. J Am Coll Nutr. 2001;20:623–627. doi: 10.1080/07315724.2001.10719159. [DOI] [PubMed] [Google Scholar]
- 36.Roberts LJ, 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JH, Mozaffarian D. omega-3 Fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: new pieces in a complex puzzle. Heart. 201(100):530–533. doi: 10.1136/heartjnl-2013-305257. Epub 2014 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 40.Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Mackey BE, Kyle D. Effects of dietary arachidonic acid on human immune response. Lipids. 1997;32:449–456. doi: 10.1007/s11745-997-0059-3. [DOI] [PubMed] [Google Scholar]
- 41.Kusumoto A, Ishikura Y, Kawashima H, Kiso Y, Takai S, Miyazaki M. Effects of arachidonate-enriched triacylglycerol supplementation on serum fatty acids and platelet aggregation in healthy male subjects with a fish diet. Br J Nutr. 2007;98:626–635. doi: 10.1017/S0007114507734566. [DOI] [PubMed] [Google Scholar]
- 42.Nelson GJ, Schmidt PC, Bartolini G, Kelley DS, Phinney SD, Kyle D, Silbermann S, Schaefer EJ. The effect of dietary arachidonic acid on plasma lipoprotein distributions, apoproteins, blood lipid levels, and tissue fatty acid composition in humans. Lipids. 1997;32:427–433. doi: 10.1007/s11745-997-0056-6. [DOI] [PubMed] [Google Scholar]
- 43.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–1041. doi: 10.1016/j.jand.2012.03.029. 41 e1–15. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Lin H, Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, Hwang SH, Lee KS, Rogawski MA, Morisseau C, Hammock BD. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS One. 2013;8:e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, Yao SL, Shang Y. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J Mol Neurosci. 2010;42:226–234. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 47.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 48.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 49.Lin PY, Chiu CC, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J Clin Psychiatry. 2012;73:1245–1254. doi: 10.4088/JCP.11r07546. [DOI] [PubMed] [Google Scholar]
- 50.Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 51.Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 52.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.