Abstract
Purpose of review
Variations in extracellular calcium level have a large impact on kidney function. Most of the effects seen are attributed to the calcium-sensing receptor (CaSR), a widely expressed G-protein-coupled cell surface protein with important function in bone mineral homeostasis. The purpose of this review is to recapitulate novel functional aspects of CaSR.
Recent findings
Results from mouse models surmise important functions for CaSR in various tissues. In the kidney, the main role of CaSR is the regulation of calcium reabsorption in the thick ascending limb, independently of its role on parathyroid hormone secretion. CaSR modulates claudin 14, the gatekeeper of paracellular ion transport in the thick ascending limb that is associated with urinary calcium excretion. One intracellular signaling pathway by which CaSR alters tight junction permeability is the calcineurin-NFAT1c-microRNA-claudin14 axis.
Summary
The main function of CaSR in the kidney is the regulation of calcium excretion in the thick ascending limb, independently of parathyroid hormone. CaSR modulates paracellular cation transport by altering expression of the tight junction protein claudin 14. Still more work is needed to fully understand all functions of CaSR in the kidney. Alternative pathways of calcium “sensing” in the kidney need to be investigated.
Keywords: calcium-sensing receptor, paracellular transport, claudin 14, thick ascending limb
Introduction
Calcium affects diverse biological processes in the body ranging from bone formation to blood pressure regulation. The tight control of serum calcium levels is crucial for the normal function of various organs, including the kidney. Changes in serum calcium levels directly affect renal tubular physiology. Acute hypercalcemia increases urinary calcium, magnesium and salt excretion, while urinary phosphate excretion and pH level are typically decreased in this setting (1). The exact mechanisms for these effects are not entirely understood. Chronic hypercalcemia has been associated with decreased urinary concentrating ability and decreased water reabsorption (2). Variations in extracellular calcium level also affect 1-α-hydroxylation of 25-hydroxy-vitaminD and thereby its activity (3). Several studies have demonstrated that these effects occur in part directly through calcium, acting as a “first messenger” in the kidney, independent of changes in hormone levels that regulate calcium homeostasis (4).
A key step towards understanding calcium actions as “first messenger” was the cloning of the extracellular calcium-sensing receptor (CaSR) from bovine parathyroid gland tissue (5). CaSR is a dimer-forming G-protein-coupled receptor (GPCR) consisting of three protein domains. The extracellular domain (ECD) binds the principal physiological ligand calcium and other polyvalent molecules (6). The seven-transmembrane domain (7TMD) couples the receptor to activating or inhibitory G-proteins, which transduce intracellular signals (7). The C-terminal tail can bind filamin-A, contribute to CaSR localization in caveolae, and link it to the actin-based cytoskeleton (8).
CaSR is highly expressed in the parathyroid gland where it maintains extracellular calcium levels by regulating parathyroid hormone (PTH) release. Numerous animal and in vitro studies have investigated CaSR function in several tissues, including cell types without direct roles in calcium homeostasis. Recent data suggest critical functions of CaSR also in the bone (9), intestine (10), developing lung (11), colon (12), epidermis (13) and mammary gland (14). However, individuals with inherited CaSR dysfunction do not typically display clinical findings consistent with these findings and they live a “relatively healthy” life when their abnormal PTH release is addressed (15). Explanations for this discrepancy include a) only “partial” CaSR dysfunction in reported inherited human cases, b) genetic redundancy for calcium-sensing, c) physiological differences in CaSR function among species, d) non-specific effects of allosteric CaSR modulators, and e) use of limited model systems to study CaSR functions (16).
The Role of CaSR in Human Disease
Inherited CASR alterations cause three distinct disorders of calcium homeostasis. Heterozygosity for inactivating mutations is responsible for familial hypocalciuric hypercalcemia (FHH), while bi-allelic loss-of-function mutations cause neonatal severe hyperparathyroidism (NSHPT) (17). Individuals with FHH have typically a life-long mild increase in serum calcium level, along with increased magnesium reabsorption (18). Interestingly, in contrast to individuals with primary hyperparathyroidism, FHH patients may have preserved urinary concentrating ability (19).
Not all FHH cases are caused by CASR mutations. Autoantibodies to the ECD can impair activation of CaSR, mimicking the FHH phenotype (20). Recently, loss-of-function mutations in GNA11, encoding for the G-protein Gα11, have been identified as cause for FHH type 2 (21)■. In vitro data suggested that GNA11 mutations decrease the sensitivity of CaSR-expressing cells to extracellular calcium, presumably due to decreased signal transduction by altered Gα11 protein. Missense mutations in AP2S1, encoding for the σ-subunit of the adaptor protein-2 (AP2), have also been reported as cause for FHH cases negative for CASR mutations (FHH type 3) (22)■. AP2 is a ubiquitously expressed protein with a central role in clathrin-mediated endocytosis and internalization of GPRCs in general. All documented mutations altered the amino acid arginine at position 15 (Arg15) of the AP2-σ-subunit. The investigators speculated that the Arg15 residue of AP2-σ-subunit is specific for recognizing the C-terminal dileucine motif of CaSR for its internalization, thereby “only” causing a FHH phenotype (22)■. They hypothesized mutations in other AP2S1 codons could affect different tissues and result in different diseases.
By contrast, activating CASR mutations cause autosomal-dominant hypocalcemia (ADH type 1) with hypercalciuria and, in some cases, renal salt wasting, resembling Bartter’s syndrome (23). Gain-of-function mutations in GNA11 were recently reported as cause for ADH type 2 (21)■. If individuals with ADH type 2 are affected by salt wasting is unclear. Notably, a role for CaSR in renal salt handling was also suggested by a small study in parathyroidectomized individuals with CaSR loss-of-function mutations. These individuals showed a markedly reduced natriuretic response to calcium infusion (24).
Recent genetic population studies investigated the association of allelic CASR variants with various common diseases, including kidney stones (25), hypertension (26), coronary heart disease (27), diabetes mellitus (27), bone mineral density (28), Alzheimer disease (29), epilepsy (30), pancreatitis (31) and various cancers (27, 32). These studies showed either no association with allelic CASR variants (27, 28, 33), minor effects on the tested outcomes (25, 27, 34), or non-replicable results, which may be related to genetic heterogeneity of the tested populations (26, 27, 32, 35). More recently, we tested the association of rare allelic variants in 40 genes associated with urinary calcium excretion in 960 well-characterized individuals, including CASR (36). We found no association with allelic CASR variation, instead our data suggested association of urinary calcium excretion with claudin 14, which had been associated with nephrolithiasis and bone mineral density in a large genome-wide association study (37).
Mouse Models of CaSR
To study the importance of CaSR in tissues outside the parathyroid gland, various CaSR-deficient mouse models were generated, which are summarized in Table (9, 10, 13, 14, 38–42). Mice lacking both copies of Casr in all tissues, generated by “targeting” Casr exon 5, recapitulated human NSHPT (38). Crossing these ΔCasrE5 mice with animals lacking PTH expression markedly improved the NSHPT phenotype (43). However, the ability to increase calcium excretion in response to calcium loading remained decreased (44). Further studies in ΔCasrE5 mice revealed the expression of functional Casr splice variants lacking Casr exon 5 in some tissues (45, 46), rendering this germline CaSR-deficient mouse model incomplete. This discovery prompted the development of another CaSR-deficient mouse model (ΔCasrE7), which allows to “knockout” Casr in selected cell types by Cre/loxP recombination. This model was used to study CaSR in numerous tissues including chondrocytes (utilizing the collagen 2 alpha 1 promoter, Col2α1). Surprisingly Col2α1ΔCasrE7 mice developed complete embryonic lethality (41). This finding could be related to the intact intracellular expression of a truncated “Δexon7-CaSR” protein, resembling the ECD (encoded by Casr exons 2–6). Although the Δexon7-CaSR expression was not toxic to HEK293 cells (41), truncated or mutated proteins have led to dominant-negative phenotypes in other transgenic animal models (47, 48). Additionally, the Δexon7-CaSR is likely secreted in considerable amounts. The ECD is known to get into the secretory pathway in the absence of the endoplasmic reticulum retention signal of full-length CaSR (49). Therefore Δexon7-CaSR could presumably be at high concentrations in restricted intercellular spaces that are hard to mimic by adding exogenous ECD. Therefore, both secreted as well as intracellular Δexon7-CaSR could have unwanted effects in target tissues of conditional ΔCasrE7 mice (Table).
Table.
Phenotype of germline (ΔCasrE5, CasrLeu723Gln) and conditional (ΔCasrE3, ΔCasrE7) CaSR mouse models
| Target tissue | ΔCasrE5 (extracellular domain, ECD) |
CasrLeu723Gln (transmembrane domain, 7TMD) |
ΔCasrE3 (extracellular domain, ECD) |
ΔCasrE7 (7TMD & intracellular domain) |
|---|---|---|---|---|
| Germline | hyperparathyroidism, hypercalcemia, osteomalacia, short lifespan (38) [Pgk] | hypoparathyroidism, hypocalcemia, hyperphosphatemia, hypercalciuria, ectopic calcifications, cataracts (39) IPMS mutagenesis | hyperparathyroidism, hypercalcemia, osteomalacia, short lifespan, “partial” embryonic lethality (40) [Prm1] | |
| Parathyroid gland [Pth] | hyperparathyroidism, hypercalcemia, hypercalciuria, growth retardation, short lifespan (41) | |||
| Chondrocyte [Col2α1]* | hyperparathyroidism, # hypercalcemia, hypercalciuria, hypophosphatemia, hyper-phosphaturia, short lifespan (42) | embryonically lethal (41) | ||
| Osteoblast | temporary growth delay [Osx] # | severely retarded bone development [Osx, 2.3Col, 3.6Col]; severe osteoporosis [Ocn, Dmp1] (9) | ||
| Renal tubules [Six2] | Hypocalciuria (40) | |||
| Mammary gland epithelia [Blg] | hypoparathyroidism, hypercalcemia, hypercalciuria, decreased bone formation (14) | |||
| Keratinocyte [K41] | impaired keratinocyte differentiation and permeability barrier homeostasis (13) | |||
| Intestine[Vil1] | hyperproliferation of colon epithelia (10) |
Not chondrocyte-specific (also expressed in parathyroid gland).
Unpublished data. Promoters used to generate germline / tissue-specific mice are shown in brackets:Pgk = 3-phosphoglycerate kinase, Prm1 = Protamine 1, Pth = Parathyroid hormone, Col2α1 = Collagen 2 α 1, Osx = Osterix, 2.3Col = Collagen 1, 3.6Co l= Collagen 1,Ocn = Osteocalcin, Dmp1 = Dentin Protein Matrix 1, Six2 = Sine oculis 2, Blg = Betalacto-globulin, K14 = type 1 Keratin, Vil1 = Villin 1; IMPS = isopropyl methanesulfonate
We developed a conditional ΔCasrE3 mouse model, targeting the second translated Casr exon, that featured complete CaSR deficiency on both the RNA and protein levels, thereby avoiding possible limitations of the previous mouse models (40)■. Homozygous ΔCasrE3 null mice recapitulated human NSHPT. Increased perinatal lethality as previously reported in ΔCasrE5 was also present, probably due to severe hypercalcemia (38). Notably, “partial” embryonic lethality was observed in ΔCasrE3 probably occurring about E13. The expected Mendelian proportion of the null genotype (~25%) was significantly lower about E18 (~11%) (unpublished data). We also tested our model under the expression of the chrondrocyte-specific Col2α1 promoter that had been used in ΔCasrE7 mice (41). Our Col2α1ΔCasrE3 mice were born at expected Mendelian ratio with life expectancy of 3–5 weeks. Their phenotype was consistent with severe hyperparathyroidism (Table). Further studies, utilizing a Cre reporter mouse (mT/mG) to test the tissue specificity of Col2α1 expression (unpublished data), showed that Col2α1 is expressed not only in chondrocytes but also in the parathyroid gland and other tissues (42).
A mouse model with an activating Casr mutation (Leu723Gln) in the 7TMD featured hypoparathyroidism associated with hypocalcemia and hyperphosphatemia (Table) (39). This mutant mice displayed no evidence of salt wasting, in contrast to human CASR gain-of-function mutations (23). Species differences with regard to CaSR and electrolyte transport in the TAL could explain this discrepancy. Notably, rats treated with a CaSR antagonist can develop hypertension (50); however, the mechanism of blood pressure rise in these animals is unclear (51, 52).
Expression of CaSR in the Kidney
Several studies showed CaSR expression throughout the kidney with segment-specific polarization (53, 54). Expression was abundant on the basolateral membrane in the cortical (CTAL) and medullary TAL (MTAL). Distinct apical CaSR staining was detected in H+-ATPase-positive intercalated cells in the connecting tubule (CNT) and cortical collecting duct (CCD) as well as in cells of the inner medullary collecting duct (IMCD). In the proximal tubule (PT), CaSR was found in the apical brush border. Co-localization of CaSR expression with the TRPV5 calcium channel in the distal convoluted tubule (DCT) and CNT was also reported (55). In the developing kidney, CaSR expression was described in the TAL and distal tubule (56).
These reports prompted many in vitro and in vivo studies that suggested roles for CaSR in many tubular functions, including calcium reabsorption, phosphate homeostasis, urinary acidification and concentration, and renin release (57). Recently, however, Houillier and colleagues applied quantitative PCR experiments to microdissected tubular segments from rodents kidneys (58). They found CaSR expression to be highest in MTAL and CTAL. Only faint expression was found in the CCD, while expression was undetectable in other tubular segments. Immunohistochemistry studies localized CaSR protein exclusively on the basolateral membrane of TAL cells with no detectable expression in the PT, DCT, CNT or CD. Our expression data showed that CaSR is heavily expressed in the TAL and absent in the distal nephron. Significantly increased CYP27B1 (1-α-hydroxylase) mRNA levels from kidney tissue of renal tubule-specific ΔCasrE3 mice suggested that CaSR is expressed in the PT (40)■. Expression of CaSR in the glomerulus was also reported (59), but this finding could not be reproduced (58).
Based on the literature, there is consensus on CaSR expression on the basolateral membrane of the TAL, but the degree of expression on the apical aspects of the nephron and the glomerulus remain debated among different research groups (57).
Function of CaSR in the Thick Ascending Limb
Previous data suggested that CaSR activation in the TAL reduces the activity of apical potassium channels (60), leading to a decreased lumen-positive potential difference and thereby reducing (passive) paracellular cation transport (61). In contrast, Houillier and colleagues showed that parathyroidectomized rodents on stable PTH infusion develop hypocalciuria and increased serum calcium level when exposed to CaSR antagonist, suggesting PTH-independent functions of renal CaSR (58). In microperfusion studies of isolated TAL tubules, they found no alteration in sodium flux or transepithelial voltage despite increased paracellular calcium transport. This finding suggested that the increase in paracellular ion permeability is not due to altered transepithelial electrical gradient.
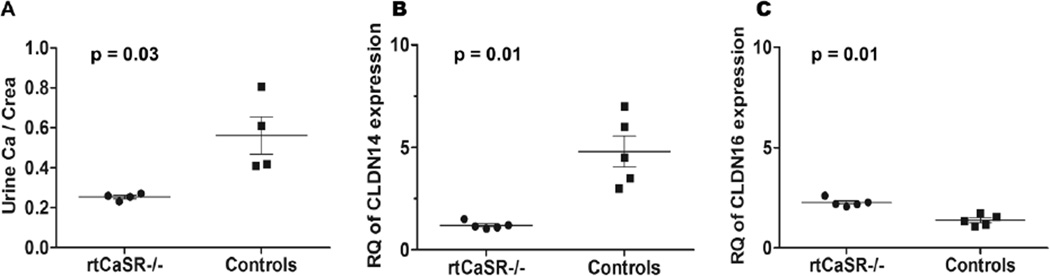
Renal tubule-specific ΔCasrE3 mice (rtCasr−/−) displayed normal serum concentrations of calcium, magnesium and PTH in the presence of relative hypocalciuria (40)■, which was more pronounced under high calcium diet (Figure 1A). RNA expression studies from renal tissue in these mice revealed ~80% decreased claudin 14 expression (CLDN14; Figure 1B) and a slight but significant increase in claudin 16 expression (CLDN16; Figure 1C). In vitro data demonstrated that CLDN14 inhibits paracellular ion permeability in the TAL by negative regulation of the tight junction CLDN16/CLDN19 heterodimer complex presumably via direct interaction with CLDN16 (62). This finding is supported by CLDN14-deficient mice, which feature hypocalciuria despite high calcium diet (62). These recent data suggested that CaSR controls paracellular calcium transport in the TAL by regulating CLDN14, the “watchdog” of paracellular ion permeability. The main role of CaSR in the kidney is the inhibition of calcium reabsorption (40)■.
Figure 1.
Three-month-old renal tubule-specific CaSR-deficient mice (rtCasr−/−) compared to littermate controls. A. Urinary calcium to creatinine ratio (Uca/crea) in rtCasr−/− mice demonstrate relative hypocalciuria under high calcium diet (1.5% calcium chloride drinking water); 0.25±0.02 vs. 0.56±0.2. B. Relative quantitation (RQ) of claudin14 (CLDN14), a negative regulator of paracellular ion transport in the thick ascending limb (TAL), displays ~80% decreased expression levels in rtCasr−/− from whole kidney RNA; 1.2±0.18 vs. 4.8±1.7. C. On the contrary, expression level of claudin16 (CLDN16), which facilitates paracellular cation transport in the TAL (together with claudin19), is slightly but significantly increased; 2.3±0.2 vs. 1.4±0.8. Data were analyzed with non-parametric test.
CaSR activation was previously shown to regulate calcineurin activity, which is necessary for prostaglandin production in the TAL (63). The calcineurin-NFAT (Nuclear Factor of Activated T-cells) pathway is a widely studied canonical calcium-signaling pathway, regulating gene transcription. Gong et al. investigated the mechanism by which CaSR regulates CLDN14 in vivo, utilizing CLDN14-deficient mice and transgenic animals overexpressing CLDN14 (64)■. By using pharmacological agents, they demonstrated that CaSR regulates CLDN14 via calcineurin-NFATc1-microRNA signaling pathway, thereby altering tight junction (TJ) permeability for calcium and magnesium in the TAL (Figure 2). Their data showed that CaSR inhibition can activate calcineurin, which then dephosphorylates NFATc1 (NFAT, cytoplasmic 1). Nuclear translocation of activated NFATc1 increases expression of the microRNAs miR-9-1 and miR-374, which decrease CLDN14 mRNA level (62). The resultant reduction in CLDN14 protein level leads to activation of the paracellular ion channel formed by the CLDN16/CLDN19 complex, increasing calcium reabsorption (62). The calcineurin inhibitor cyclosporine abolishes the effects of CaSR inhibition in vitro, potentially explaining how cyclosporine induces hypercalciuria (65). The potential importance of CaSR in regulating the TJ assembly was further demonstrated by recent in vitro studies in MDCK cells. CaSR activation induced plasmalemmal deposition of TJ components, such as ZO-1 (66)■, altering transepithelial electrical resistance and presumably TJ barrier function.
Figure 2.
Simplified schema of a renal tubular epithelial cell in the TAL illustrating the CaSR-NFATc1-microRNA-CLDN14 pathway. CaSR activity on the basolateral membrane determines intracellular calcium-signaling and calcineurin activity, thereby regulating NFATc1 via (de)phosphorylation. When NFATc1 is activated, it translocates to the nucleus and increases transcription of CLDN14-regulating microRNAs miR-9-1 and miR-374. These microRNAs decrease CLDN14 expression and thereby alter the cation permeability of the paracellular ion channel formed by the CLDN16/CLDN19 heterodimer complex. CaSR could regulate CLDN14 by additional mechanisms as well. Abbreviations: CaSR = calcium-sensing receptor, NFATc1 = Nuclear Factor of Activated T-cells, cytoplasmic 1, CLDN = claudin, TAL = thick ascending limb
Renal effects of hypercalcemia are not fully reproduced by activating renal CaSR in the TAL (58, 67). This was shown by studying the effects of CaSR agonists in comparison to high calcium diet in parathyroidectomized and PTH-supplemented rats. CaSR agonist decreased calcium reabsorption, inducing a negative calcium balance and lowering serum calcium concentration. However, CaSR activation did not change urinary excretion of sodium, phosphate, magnesium, or urine volume. By contrast, high calcium diet raised blood calcium concentrations and elicited renal salt wasting and water losses. These findings suggest that extracellular calcium can act on the renal tubule through ‘sensors’ other than CaSR (67).
One of these calcium sensors in the kidney could be GPRC6A, which shares ~34% amino acid sequence identity with CaSR (also known as GPRC2A) (68). Interestingly, GPRC6A can be modulated by CaSR ligands, and even “responded” to CaSR modulators, albeit not in a specific fashion (69). Germline GPRC6A-deficient mice featured complex metabolic changes, including hypercalciuria (70). Similar to CaSR, GPRC6A is ubiquitously expressed (71). Its exact localization in the kidney remains uncertain, but it appeared to be heavily expressed in the CD (67). Recent data showed that GPRC6A, as previously reported for CaSR, can modulate acid secretion in the distal nephron segment (67). These data implied that GPRC6A could serve as another calcium sensor in the kidney, functioning independently from CaSR, and perhaps subserving distinct functions. Further investigations on other renal GPCRs that can bind calcium and could form heterodimers with CaSR or GPRC6A are necessary.
Function of CaSR in the Proximal Tubule
The PT plays a critical role in maintaining extracellular fluid volume, calcium excretion, citrate and phosphate transport, 1-α-hydroxylation of vitaminD, and urinary acidification. Despite the “controversial” CaSR expression data, there are several lines of functional data that suggest biological roles for CaSR in this tubule segment (72). CaSR has been shown to regulate PTH-suppressible phosphate reabsorption in the PT (73), modulate 1-α-hydroxylation of 25-hydroxy-vitaminD (3), and dampen the response to 1,25-dihydroxy-vitaminD independently of PTH action (74). Nevertheless, the potential functions of CaSR in this nephron segment remain overall ill-defined. One contributing factor is that CaSR expression may not only be regulated by calcium, but also by PTH, 1,25-dihydroxy-vitaminD, dietary phosphate, and fibroblast growth factor 23 (FGF23), making CaSR function in the PT quite complex (75).
Recent data highlight a role for CaSR in fluid reabsorption and acid secretion in the PT (76)■. Geibel and colleagues utilized both in vivo micropuncture in rats and in vitro perfused mouse PT segments. In wildtype rodents, raising luminal calcium or adding a CaSR agonist caused increased proton secretion and fluid absorption, while no effect was seen in ΔCasrE5 mice. The investigators postulated that CaSR might regulate the luminal sodium-proton-exchanger (NHE), thereby increasing acid secretion. This could lead to increase ionization of luminal calcium, potentially enhancing its reabsorption in later tubule segments. In their studies, they also demonstrated that CaSR function might be required for transepithelial fluid flux in the PT in response to apical changes of divalent ion concentration (76)■.
Although the studies on CaSR expression in the PT are controversial, in vitro data support an intertwined role for CaSR in PT physiology with the sodium-phosphate cotransporter 2a (NaPi-2a), PTH and its receptor PHT1R, 1-α-hydroxylase, vitaminD, and FGF23. However, the relatively “mild” phenotype of rtCasr−/− mice (hypocalciuria) with normal PT anatomy and absence of significant changes in serum calcium, PTH, 1,25-dihydroxy-vitaminD, phosphate and FGF23 level suggest that CaSR may not have a major biological role in this tubular segment (40)■. Genetic redundancy of calcium-sensing in the whole organism may blunt tissue or cell-specific roles for CaSR, which could be a possible cause for inconsistent results.
Conclusion
Recent studies establish that CaSR in the kidney modifies paracellular calcium reabsorption in the TAL by regulating the tight junction protein CLDN14. The role of renal CaSR in net sodium chloride and water transport remains unclear due to inconsistent data. It seems likely that additional calcium-sensing mechanisms exist along the renal tubule responding to and regulating changes in blood and urine calcium levels. Further investigations examining all renal calcium-sensing mechanisms, including CaSR, will help elucidate the role of extracellular calcium-sensing in renal physiology.
Key Points.
Inherited syndromes caused by mutations in CASR can be mimicked by DNA sequence variations in at least two other genes, GNA11 and AP2S1, which are involved in CaSR signal transduction and trafficking.
Renal CaSR regulates the paracellular ion permeability in the TAL, independently of PTH release. The role of CaSR in the kidney is the inhibition of calcium reabsorption.
CaSR alters the expression of the tight junction protein claudin14 in the TAL in response to changes in serum calcium levels. One of its intracellular signaling pathways involves NFATc1 and microRNAs.
The existence of other extracellular calcium sensors is likely.
Acknowledgement
I thank Salvatore Dibartolo III and Victoria Charoonratana for excellent technical support. I want to express my sincere gratitude to Drs. Seth L. Alper, Edward M. Brown and Ali Hariri for their critical comments in preparing this manuscript. I am indebted to Dr. Martin Pollak for his mentorship.
Footnotes
Conflicts of interest
There are none.
References and Recommended Reading
- 1.Edwards BR, Sutton RA, Dirks JH. Effect of calcium infusion on renal tubular reabsorption in the dog. The American journal of physiology. 1974 Jul;227(1):13–18. doi: 10.1152/ajplegacy.1974.227.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Gill JR, Jr, Bartter FC. On the impairment of renal concentrating ability in prolonged hypercalcemia and hypercalciuria in man. The Journal of clinical investigation. 1961 Apr;40:716–722. doi: 10.1172/JCI104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiti A, Beckman MJ. Extracellular calcium is a direct effecter of VDR levels in proximal tubule epithelial cells that counter-balances effects of PTH on renal Vitamin D metabolism. The Journal of steroid biochemistry and molecular biology. 2007 Mar;103(3–5):504–508. doi: 10.1016/j.jsbmb.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Hulter HN, Sebastian A, Toto RD, Bonner EL, Jr, Ilnicki LP. Renal and systemic acid-base effects of the chronic administration of hypercalcemia-producing agents: calcitriol, PTH, and intravenous calcium. Kidney international. 1982 Mar;21(3):445–458. doi: 10.1038/ki.1982.45. [DOI] [PubMed] [Google Scholar]
- 5.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993 Dec 9;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 6.Wellendorph P, Johansen LD, Brauner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Molecular pharmacology. 2009 Sep;76(3):453–465. doi: 10.1124/mol.109.055244. [DOI] [PubMed] [Google Scholar]
- 7.Hendy GN, Canaff L, Cole DE. The CASR gene: alternative splicing and transcriptional control, and calcium-sensing receptor (CaSR) protein: structure and ligand binding sites. Best practice & research Clinical endocrinology & metabolism. 2013 Jun;27(3):285–301. doi: 10.1016/j.beem.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. The Journal of biological chemistry. 2001 Sep 14;276(37):34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- 9.Dvorak-Ewell MM, Chen TH, Liang N, et al. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 Dec;26(12):2935–2947. doi: 10.1002/jbmr.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Liu G, Chakrabarty S. Cellular responses to TGFbeta and TGFbeta receptor expression in human colonic epithelial cells require CaSR expression and function. Cell calcium. 2013 May-Jun;53(5–6):366–371. doi: 10.1016/j.ceca.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Riccardi D, Brennan SC, Chang W. The extracellular calcium-sensing receptor, CaSR, in fetal development. Best practice & research Clinical endocrinology & metabolism. 2013 Jun;27(3):443–453. doi: 10.1016/j.beem.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative cross-talk between calcium-sensing receptor and beta-catenin signaling systems in colonic epithelium. The Journal of biological chemistry. 2012 Jan 6;287(2):1158–1167. doi: 10.1074/jbc.M111.274589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu CL, Crumrine DA, Man MQ, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. The Journal of investigative dermatology. 2012 Oct;132(10):2350–2359. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamillapalli R, VanHouten J, Dann P, et al. Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual. Endocrinology. 2013 Sep;154(9):3031–3042. doi: 10.1210/en.2012-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx SJ, Attie MF, Levine MA, Spiegel AM, Downs RW, Jr, Lasker RD. The hypocalciuric or benign variant of familial hypercalcemia: clinical and biochemical features in fifteen kindreds. Medicine. 1981 Nov;60(6):397–412. doi: 10.1097/00005792-198111000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Riccardi D. Parathyroid hormone-independent role for the calcium-sensing receptor in the control of urinary calcium excretion. Journal of the American Society of Nephrology : JASN. 2012 Nov;23(11):1766–1768. doi: 10.1681/ASN.2012090955. [DOI] [PubMed] [Google Scholar]
- 17.Pollak MR, Brown EM, Chou YH, et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993 Dec 31;75(7):1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 18.Pearce SH, Trump D, Wooding C, et al. Calcium-sensing receptor mutations in familial benign hypercalcemia and neonatal hyperparathyroidism. The Journal of clinical investigation. 1995 Dec;96(6):2683–2692. doi: 10.1172/JCI118335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx SJ, Attie MF, Stock JL, Spiegel AM, Levine MA. Maximal urine-concentrating ability: familial hypocalciuric hypercalcemia versus typical primary hyperparathyroidism. The Journal of clinical endocrinology and metabolism. 1981 Apr;52(4):736–740. doi: 10.1210/jcem-52-4-736. [DOI] [PubMed] [Google Scholar]
- 20.Kifor O, Moore FD, Jr, Delaney M, et al. A syndrome of hypocalciuric hypercalcemia caused by autoantibodies directed at the calcium-sensing receptor. The Journal of clinical endocrinology and metabolism. 2003 Jan;88(1):60–72. doi: 10.1210/jc.2002-020249. [DOI] [PubMed] [Google Scholar]
- 21. Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit alpha11 in hypercalcemia and hypocalcemia. The New England journal of medicine. 2013 Jun;368(27)(26):2476–2486. doi: 10.1056/NEJMoa1300253. * This recent work demonstrates genetic heterogeneity for human syndromes that can be caused by CASR mutations. Mutations in the G-protein gene GNA11 can lead to similar abnormalities in bone mineral homeostasis resembling CaSR dysfunction.
- 22. Nesbit MA, Hannan FM, Howles SA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nature genetics. 2013 Jan;45(1):93–97. doi: 10.1038/ng.2492. * This report demonstrates further evidence for genetic heterogeneity for syndromes caused by inherited CaSR dysfunction. Mutations in AP2S1 can alter CaSR endocytosis and trafficking, thereby resembling CASR loss-of-function.
- 23.Toka HR, Koshy JM, Hariri A. The molecular basis of blood pressure variation. Pediatr Nephrol. 2013 Mar;28(3):387–399. doi: 10.1007/s00467-012-2206-9. [DOI] [PubMed] [Google Scholar]
- 24.Attie MF, Gill JR, Jr, Stock JL, et al. Urinary calcium excretion in familial hypocalciuric hypercalcemia. Persistence of relative hypocalciuria after induction of hypoparathyroidism. The Journal of clinical investigation. 1983 Aug;72(2):667–676. doi: 10.1172/JCI111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vezzoli G, Terranegra A, Aloia A, et al. Decreased transcriptional activity of calcium-sensing receptor gene promoter 1 is associated with calcium nephrolithiasis. The Journal of clinical endocrinology and metabolism. 2013 Sep;98(9):3839–3847. doi: 10.1210/jc.2013-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung J, Foroud TM, Eckert GJ, et al. Association of the calcium-sensing receptor gene with blood pressure and urinary calcium in African-Americans. The Journal of clinical endocrinology and metabolism. 2009 Mar;94(3):1042–1048. doi: 10.1210/jc.2008-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorde R, Schirmer H, Njolstad I, et al. Serum calcium and the calcium-sensing receptor polymorphism rs17251221 in relation to coronary heart disease, type 2 diabetes, cancer and mortality: the Tromso Study. European journal of epidemiology. 2013 Jul;28(7):569–578. doi: 10.1007/s10654-013-9822-y. [DOI] [PubMed] [Google Scholar]
- 28.Harding B, Curley AJ, Hannan FM, et al. Functional characterization of calcium sensing receptor polymorphisms and absence of association with indices of calcium homeostasis and bone mineral density. Clinical endocrinology. 2006 Nov;65(5):598–605. doi: 10.1111/j.1365-2265.2006.02634.x. [DOI] [PubMed] [Google Scholar]
- 29.Conley YP, Mukherjee A, Kammerer C, et al. Evidence supporting a role for the calcium-sensing receptor in Alzheimer disease. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009 Jul 5;150B(5):703–709. doi: 10.1002/ajmg.b.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapoor A, Satishchandra P, Ratnapriya R, et al. An idiopathic epilepsy syndrome linked to 3q13.3–q21 and missense mutations in the extracellular calcium sensing receptor gene. Annals of neurology. 2008 Aug;64(2):158–167. doi: 10.1002/ana.21428. [DOI] [PubMed] [Google Scholar]
- 31.Muddana V, Lamb J, Greer JB, et al. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: role of serine protease inhibitor Kazal 1type and alcohol. World journal of gastroenterology : WJG. 2008 Jul 28;14(28):4486–4491. doi: 10.3748/wjg.14.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shui IM, Mucci LA, Wilson KM, et al. Common genetic variation of the calcium-sensing receptor and lethal prostate cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 Jan;22(1):118–126. doi: 10.1158/1055-9965.EPI-12-0670-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenab M, McKay J, Bueno-de-Mesquita HB, et al. Vitamin D receptor and calcium sensing receptor polymorphisms and the risk of colorectal cancer in European populations. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Sep;18(9):2485–2491. doi: 10.1158/1055-9965.EPI-09-0319. [DOI] [PubMed] [Google Scholar]
- 34.Kapur K, Johnson T, Beckmann ND, et al. Genome-wide meta-analysis for serum calcium identifies significantly associated SNPs near the calcium-sensing receptor (CASR) gene. PLoS genetics. 2010 Jul;6(7):e1001035. doi: 10.1371/journal.pgen.1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly C, Gunn IR, Gaffney D, Devgun MS. Serum calcium, urine calcium and polymorphisms of the calcium sensing receptor gene. Annals of clinical biochemistry. 2006 Nov;43(Pt 6):503–506. doi: 10.1258/000456306778904632. [DOI] [PubMed] [Google Scholar]
- 36.Toka HR, Genovese G, Mount DB, Pollak MR, Curhan GC. Frequency of rare allelic variation in candidate genes among individuals with low and high urinary calcium excretion. PloS one. 2013;8(8):e71885. doi: 10.1371/journal.pone.0071885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorleifsson G, Holm H, Edvardsson V, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nature genetics. 2009 Aug;41(8):926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 38.Ho C, Conner DA, Pollak MR, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nature genetics. 1995 Dec;11(4):389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 39.Hough TA, Bogani D, Cheeseman MT, et al. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proceedings of the National Academy of Sciences of the United States of America. 2004 Sep 14;101(37):13566–13571. doi: 10.1073/pnas.0405516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toka HR, Al-Romaih K, Koshy JM, et al. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. Journal of the American Society of Nephrology : JASN. 2012 Nov;23(11):1879–1890. doi: 10.1681/ASN.2012030323. * This article demonstrates that renal tubule-specific CaSR disruption increases calcium reabsorption in vivo by altering expression of paracellular tight junction proteins (claudins) in the TAL.
- 41.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Science signaling. 2008;1(35):ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toka HR, DiBartolo S, 3rd, Lanske B, Brown EM, Pollak MR. Chondrocyte-specific calcium-sensing receptor-deficient mice driven by the collagen type 2 alpha 1 promoter display hyperparathyroidism. Nephrology Dialysis Transplantation. 2013 May;28:314. [Google Scholar]
- 43.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. The Journal of clinical investigation. 2003 Apr;111(7):1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantham L, Quinn SJ, Egbuna OI, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. American journal of physiology Endocrinology and metabolism. 2009 Oct;297(4):E915–E923. doi: 10.1152/ajpendo.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oda Y, Tu CL, Chang W, et al. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. The Journal of biological chemistry. 2000 Jan 14;275(2):1183–1190. doi: 10.1074/jbc.275.2.1183. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez L, Tu C, Cheng Z, et al. Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology. 2005 Dec;146(12):5294–5303. doi: 10.1210/en.2005-0256. [DOI] [PubMed] [Google Scholar]
- 47.Leoyklang P, Suphapeetiporn K, Srichomthong C, et al. Disorders with similar clinical phenotypes reveal underlying genetic interaction: SATB2 acts as an activator of the UPF3B gene. Human genetics. 2013 Dec;132(12):1383–1393. doi: 10.1007/s00439-013-1345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbach N, Goeke B, Schneider M, Hermanns W, Wolf E, Wanke R. Overexpression of a dominant negative GIP receptor in transgenic mice results in disturbed postnatal pancreatic islet and beta-cell development. Regulatory peptides. 2005 Feb;15125(1–3):103–117. doi: 10.1016/j.regpep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Goldsmith PK, Fan GF, Ray K, et al. Expression, purification, and biochemical characterization of the amino-terminal extracellular domain of the human calcium receptor. The Journal of biological chemistry. 1999 Apr;274(16)(16):11303–11309. doi: 10.1074/jbc.274.16.11303. [DOI] [PubMed] [Google Scholar]
- 50.Rybczynska A, Lehmann A, Jurska-Jasko A, et al. Hypertensive effect of calcilytic NPS 2143 administration in rats. The Journal of endocrinology. 2006 Oct;191(1):189–195. doi: 10.1677/joe.1.06924. [DOI] [PubMed] [Google Scholar]
- 51.Ziegelstein RC, Xiong Y, He C, Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochemical and biophysical research communications. 2006 Mar;342(31)(1):153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 52.Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension. 2007 Oct;50(4):737–743. doi: 10.1161/HYPERTENSIONAHA.107.095158. [DOI] [PubMed] [Google Scholar]
- 53.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca(2+)-sensing receptor and PTH/PTHrP receptor in rat kidney. The American journal of physiology. 1996 Oct;271(4 Pt 2):F951–F956. doi: 10.1152/ajprenal.1996.271.4.F951. [DOI] [PubMed] [Google Scholar]
- 54.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. The American journal of physiology. 1998 Mar;274(3 Pt 2):F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 55.Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell calcium. 2009 Apr;45(4):331–339. doi: 10.1016/j.ceca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Crisi GM, Rockwell GF, Braden GL, Campfield TJ. Immunolocalization of the calcium-sensing receptor in developing human kidney. Pediatric research. 2013 Aug;74(2):133–140. doi: 10.1038/pr.2013.72. [DOI] [PubMed] [Google Scholar]
- 57.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. American journal of physiology Renal physiology. 2010 Mar;298(3):F485–F499. doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loupy A, Ramakrishnan SK, Wootla B, et al. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. The Journal of clinical investigation. 2012 Sep 4;122(9):3355–3367. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh J, Beckmann J, Bloch J, et al. Stimulation of the calcium-sensing receptor stabilizes the podocyte cytoskeleton, improves cell survival, and reduces toxin-induced glomerulosclerosis. Kidney international. 2011 Sep;80(5):483–492. doi: 10.1038/ki.2011.105. [DOI] [PubMed] [Google Scholar]
- 60.Wang W, Lu M, Balazy M, Hebert SC. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. The American journal of physiology. 1997 Sep;273(3 Pt 2):F421–F429. doi: 10.1152/ajprenal.1997.273.3.F421. [DOI] [PubMed] [Google Scholar]
- 61.Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. The American journal of physiology. 1996 Jul;271(1 Pt 1):C103–C111. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
- 62.Gong Y, Renigunta V, Himmerkus N, et al. Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signalling via a novel microRNA pathway. The EMBO journal. 2012 Apr 18;31(8):1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdullah HI, Pedraza PL, McGiff JC, Ferreri NR. Calcium-sensing receptor signaling pathways in medullary thick ascending limb cells mediate COX-2-derived PGE2 production: functional significance. American journal of physiology Renal physiology. 2008 Oct;295(4):F1082–F1089. doi: 10.1152/ajprenal.90316.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gong Y, Hou J. Claudin-14 Underlies Ca++-Sensing Receptor-Mediated Ca++ Metabolism via NFAT-microRNA-Based Mechanisms. Journal of the American Society of Nephrology : JASN. 2013 Dec 12; doi: 10.1681/ASN.2013050553. * This latest work identified an intracellular signaling cascade for CaSR-regulated paracellular ion transport in the TAL.
- 65.Chang CT, Hung CC, Tian YC, Yang CW, Wu MS. Ciclosporin reduces paracellin-1 expression and magnesium transport in thick ascending limb cells. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007 Apr;22(4):1033–1040. doi: 10.1093/ndt/gfl817. [DOI] [PubMed] [Google Scholar]
- 66. Jouret F, Wu J, Hull M, et al. Activation of the Ca(2)+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. Journal of cell science. 2013 Nov 15;126(Pt 22):5132–5142. doi: 10.1242/jcs.127555. * This recent article showed that CaSR activation can induce deposition of tight junction (TJ) components in vitro, suggesting that CaSR participates in transepithelial electrical resistance and TJ barrier function.
- 67.Houillier P. Calcium-sensing in the kidney. Current opinion in nephrology and hypertension. 2013 Sep;22(5):566–571. doi: 10.1097/MNH.0b013e328363ff5f. [DOI] [PubMed] [Google Scholar]
- 68.Wellendorph P, Brauner-Osborne H. Molecular basis for amino acid sensing by family C G-protein-coupled receptors. British journal of pharmacology. 2009 Mar;156(6):869–884. doi: 10.1111/j.1476-5381.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faure H, Gorojankina T, Rice N, et al. Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell calcium. 2009 Nov-Dec;46(5–6):323–332. doi: 10.1016/j.ceca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Pi M, Chen L, Huang MZ, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PloS one. 2008;3(12):e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wellendorph P, Burhenne N, Christiansen B, Walter B, Schmale H, Brauner-Osborne H. The rat GPRC6A: cloning and characterization. Gene. 2007 Jul 15;396(2):257–267. doi: 10.1016/j.gene.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Riccardi D, Traebert M, Ward DT, et al. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+-dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflugers Archiv : European journal of physiology. 2000 Dec;441(2–3):379–387. doi: 10.1007/s004240000436. [DOI] [PubMed] [Google Scholar]
- 73.Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. American journal of physiology Renal physiology. 2003 Dec;285(6):F1233–F1243. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- 74.Egbuna O, Quinn S, Kantham L, et al. The full-length calcium-sensing receptor dampens the calcemic response to 1alpha,25(OH)2 vitamin D3 in vivo independently of parathyroid hormone. American journal of physiology Renal physiology. 2009 Sep;297(3):F720–F728. doi: 10.1152/ajprenal.00164.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. The Journal of clinical investigation. 2004 Feb;113(4):561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Capasso G, Geibel PJ, Damiano S, Jaeger P, Richards WG, Geibel JP. The calcium sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney international. 2013 Aug;84(2):277–284. doi: 10.1038/ki.2013.137. * This recent work suggests functions for CaSR in fluid reabsorption and acid secretion in the proximal tubule, thereby further suggesting a complex role for CaSR in this tubule segment.