SUMMARY
The normal functions and pathologic facets of the small presynaptic protein α-synuclein (α-syn) are of exceptional interest. In previous studies, we found that α-syn attenuates synaptic exo/endocytosis [1, 2]; however underlying mechanisms remain unknown. More recent evidence suggests that α-syn exists as metastable multimers and not solely as a natively-unfolded monomer [11-16]. However conformations of α-syn at synapses – its physiologic locale – are unclear; and potential implications of such higher-order conformations to synaptic function is unknown. Exploring α-syn conformations and synaptic function in neurons, we found that α-syn promptly organizes into physiological multimers at synapses. Furthermore, our experiments indicate that α-syn multimers cluster synaptic-vesicles and restrict their motility – suggesting a novel role for these higher-order structures. Supporting this, α-syn mutations that disrupt multimerization also fail to restrict synaptic-vesicle motility or attenuate exo/endocytosis. We propose a model where α-syn multimers cluster synaptic-vesicles, restricting their trafficking and recycling – consequently attenuating neurotransmitter release.
Keywords: α-synuclein, synapse, vesicle-trafficking, pHluorin, tetramers
RESULTS AND DISCUSSION
Contemporary insights into α-synuclein biology
The presynaptic bouton is a central communicating hub, where a sequence of well-orchestrated events leads to exocytosis of neurotransmitter-loaded vesicles into the presynaptic cleft. A variety of presynaptic proteins participate, mainly assisting in the organization and trafficking of synaptic-vesicles. One such protein is α-syn; of singular interest due to its involvement in Parkinson's disease and related movement-disorders/dementias. In previous studies we found that small increments in α-syn-levels lead to suppression of exo/endocytosis [1, 2]; and that α-syn restricts the lateral mobility of synaptic-vesicles between en-passant boutons [2], called “superpool” trafficking [3]. Along with other studies [4-9], available data advocate the concept that α-syn physiologically attenuates neurotransmitter release; however underlying mechanisms are unclear. α-syn also binds to VAMP2 and promotes SNARE-assembly [10], but the consequence of these interactions on synaptic physiology is uncertain [7, 10]. Regardless, a clear picture of the physiologic role of α-syn has not emerged yet.
As function often follows form in biology, understanding physiologic α-syn conformations is important. Recent studies offer surprising insights, suggesting that α-syn exists as metastable helical multimers, with predominant tetramers [11]. Though this view has been challenged [12, 13], available data from purified brain α-syn show higher-order multimers and mixed helical conformations [13, 14], consistent with the idea that α-syn exists as metastable conformers, exchanging between a monomeric and multimeric state. Even so, key questions remain unresolved. What is the conformation of α-syn at synapses, its normal locale? Do α-syn assemblies influence its function? If so how? Here we couple fluorescence-complementation assays – that selectively stabilize putative α-syn assemblies – with various cell-biological paradigms to evaluate vesicle-trafficking and synaptic function.
Multimeric α-syn conformations at presynaptic boutons
Though recent studies have demonstrated α-syn multimers [11, 15, 16], most experiments used biochemical or biophysical methods that do not provide spatial information; thus α-syn conformations at the presynapse are not entirely clear. We first evaluated the organization of α-syn at synapses of cultured neurons using bimolecular fluorescence complementation (BiFC) – an established method to visualize protein-protein interactions [17]. In this assay, one partner of an interacting-pair is tagged to the N-terminus fragment of the Venus fluorescent protein (VN), while the other partner to the complimentary C-terminus (VC). If and when the two interacting partners associate, the Venus fragments are reconstituted and become fluorescent [see schematic in fig. 1A (i)]. Reconstitution is irreversible, thus even transient interactions can be “captured” by these methods [18].
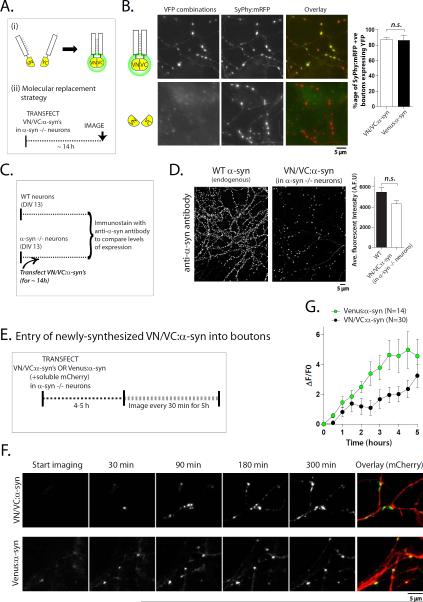
Figure 1. Multimeric α-syn conformations at presynaptic boutons.
(A) Schematic of complementation assay (i) and molecular replacement strategy (ii). Cultured hippocampal neurons from α-syn −/− mice were transiently transfected with various VN/VC-tagged α-syn's (see “results”) and visualized after ~ 14 hours.
(B) Top: Representative images of reconstituted Venus fluorescence in neurons expressing VN/VC:α-syn's (also see Supp. fig. 1A). Note these neurons are co-transfected with synaptophysin:mRFP (SyPhy:mRFP) to label boutons. Bottom: No fluorescence was seen in boutons expressing un-tagged VN + VC alone. Right: The vast majority (~85%) of SyPhy:mRFP-positive boutons also expressed VN/VC:α-syn; comparable to boutons expressing Venus:α-syn and SyPhy:mRFP (N~700 boutons for each group from two separate batches of cultures, p=0.90).
(C) Overall design to compare expression-levels of transfected VN/VC:α-syn to endogenous mouse α-syn. Un-transfected cultured neurons from WT mice and VN/VC:α-syn-transfected cultured neurons from α-syn −/− mice were fixed and immunostained with an anti-α-syn antibody (guinea-pig α-syn antibody). Cell culture and immunostaining of both groups were processed in parallel. Note that while the antibody would recognize mouse α-syn in WT neurons, it would only label transfected α-syn in the VN/VC:α-syn transfected group.
(D) Representative images from the two groups in (C) (left) and quantification of overall average fluorescence intensities (right; N~10 visual fields containing ~ 3000-10,000 boutons; p=0.06). Note that the number of VN/VC:α-syn transfected boutons is much lower than immunostained WT boutons (as expected with transient transfections), but the fluorescence-intensities are similar.
(E) Overall design. Cultured α-syn −/− neurons were co-transfected with VN/VC:α-syn's (or Venus:α-syn) + soluble mCherry, and kinetics of initial α-syn entry and synaptic accumulation was evaluated by long-term imaging (see “results” and [21] for more details).
(F) Representative frames from two time-lapse movies showing pre-synaptic accumulation of VN/VC:α-syn (top) and Venus:α-syn (bottom) over 5 hrs of imaging.
(G) Quantification of average VFP intensities of boutons over 5 hrs. Note that though the kinetics of VN/VC:α-syn accumulation (black dots) is slower than Venus:α-syn (green dots) as expected, the difference is modest, suggesting that complementation is a relatively early event.
To mitigate concerns related to over-expression, we used a ‘molecular replacement strategy’, where the exogenous protein is expressed in a knockout background, achieving near-physiologic expression-levels [19]. Specifically, we expressed VN/VC tagged human wild-type α-syn's (VN/VC:α-syn's) in cultured hippocampal neurons (days in vitro-DIV 14) from α-syn null mice and visualized fluorescence after ~14h expression [strategy in fig. 1A(ii)]. Expression of human α-syn in cultured α-syn null neurons was similar to the expression of native mouse α-syn in parallel-processed cultures (see below). Three different VN/VC-tagged α-syn combinations were used (see methods). In all cases, robust fluorescence was seen at boutons (fig. 1B-top and Supp. fig. 1A, B). Co-transfection of VN and VC alone did not show any synaptic fluorescence (fig. 1B-bottom and data not shown). Complementation was also seen in nonneuronal cells as reported previously [20], and excess un-tagged α-syn diminished VN/VC:α-syn complementation in HEK cells – presumably by competition – suggesting that complementation was specific for α-syn (Supp. fig. 1C). Synaptic fluorescence due to VN/VC:α-syn complementation was widespread, seen in virtually all transfected boutons; overall similar to neurons transfected with Venus:α-syn (fig. 1B, graph on right). It is unlikely that the complementation in our experiments is an artifact of over-expression, as fluorescence intensities of transfected VN/VC:α-syn boutons in α-syn -/- neurons is similar to endogenous mouse α-syn fluorescence in WT neurons (fig. 1C, D and also see next).
Next we visualized the time-course of accumulation of newly-synthesized VN/VC:α-syn at boutons. We transfected α-syn null neurons with VN/VC:α-syn (or Venus:α-syn) and visualized the entry of newly-synthesized (somatically-derived) fluorescent molecules into boutons (4-5 hours after transfection), adopting an imaging strategy that we recently developed ([21], see schematic in fig. 1E). Figure 1F shows representative images from one such experiment. Note that the kinetics of VN/VC:α-syn entry into boutons is only slightly slower than Venus:α-syn, quantified in figure 1G. These data indicate that multimerization of α-syn is an early event, and likely not a consequence of abnormal long-term intra-molecular associations. Also note that the experimental paradigm (visualizing entry of newly-synthesized proteins into α-syn −/− boutons) further argues that the complementation is unlikely to be a result of over-expression.
α-synuclein multimers cluster synaptic-vesicles
Do α-syn multimers have a physiologic role? While qualitatively comparing the synaptic VN/VC:α-syn fluorescence to the fluorescence of endogenous or Venus:α-syn, we noticed that the reconstituted VN/VC:α-syn fluorescence seemingly occupied only a subset of the total bouton-area. To verify this, we used a previously-described protocol to label the entire bouton-profile (using fluorescent actin, see [22]) and visualized VN/VC:α-syn fluorescence in these boutons. Indeed reconstituted VN/VC:α-syn occupied only a fraction of the bouton-area, as shown in representative bouton-crops (fig. 2A). This distribution is unusual, distinct from Venus:α-syn that typically occupies the entire bouton (see Supp. fig. 2A). We also developed custom algorithms to quantify these data. Briefly, as bouton shapes vary, we measured the cross-sectional area of each bouton along 20 circumferential angles and then calculated its mean “synapse-width” (see fig. 2B for general concept; Supp. fig. 2B and methods for details). Compiled data from these analyses are shown in fig. 2C, D. Note that the VN/VC:α-syn fluorescence only occupies a subset of the bouton cross-sectional area and is indistinguishable from the area occupied by the synaptic-vesicle cluster (labeled with synaptophysin:GFP, fig. 2C). Furthermore, in neurons co-transfected with VN/VC:α-syn and synaptophysin:mRFP (SyPhy:mRFP), there is a significant overlap of fluorescence (also reflected in correlations between their “synapse-widths”, see fig. 2D).
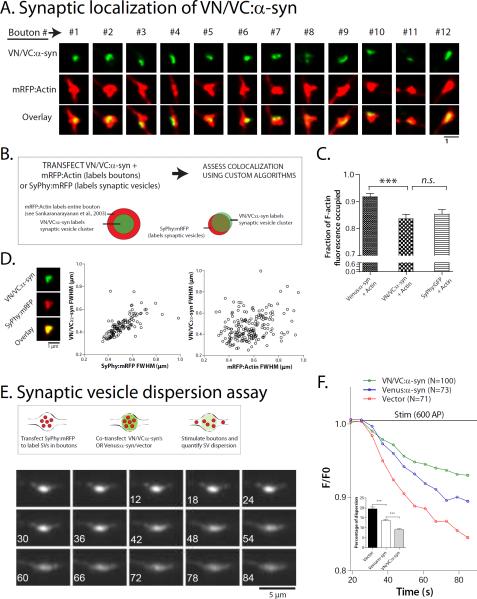
Figure 2. α-syn multimers cluster synaptic-vesicles.
(A) Bouton-crops from neurons co-transfected with VN/VC:α-syn and mRFP:Actin (to label entire bouton-profile, see “results”). Note that reconstituted VN/VC:α-syn's only occupy a fraction of the bouton cross-sectional area.
(B) Experimental design: Neurons were co-transfected with VN/VC:α-syn and markers to label the entire bouton-profile (mRFP:Actin) or synaptic-vesicles (SyPhy:mRFP); and extent of overlap was determined by custom algorithms (see “results” and “methods” for details).
(C, D) Both reconstituted VN/VC:α-syn and SyPhy:GFP occupied a smaller fraction of the bouton than Venus:α-syn (~ 200 boutons analyzed for each group from two separate batches of cultures, ***p < 0.001). (D) Bouton-widths (FWHM, see methods) of VN/VC:α-syn and SyPhy:mRFP were correlated (left; r=0.36, p<0.0001), unlike VN/VC:α-syn and mRFP:Actin, further indicating associations of complemented VN/VC:α-syn's with synaptic-vesicles (N=120 boutons from two separate batches of cultures).
(E) Top: Schematic of “synaptic-vesicle dispersion assay”. Synaptic-vesicles are labeled by SyPhy:mRFP and neurons are stimulated to disperse synaptic-vesicles (see “results”). Bottom: A time-series showing dispersion of synaptic-vesicles from a bouton (elapsed time in seconds on lower left, asterisk marks the start of stimulation).
(F) Quantification of synaptic-vesicle dispersion using above assay. While Venus:α-syn diminishes dispersion-kinetics (compared to vector), the dispersion is further attenuated by VN/VC:α-syn (note that error bars are too small to be seen). Extent of dispersion quantified in inset (19.5%, 13.6% and 9% of total synaptic-vesicles were dispersed in vector, Venus:α-syn and VN/VC:α-syn groups respectively; ***p < 0.001, unpaired t test).
Previous studies expressing WT α-syn in yeast revealed a dramatic clustering of vesicles by α-syn [23, 24]. Previously, we found that α-syn restricts synaptic-vesicle motility between enpassant boutons [2], suggesting that α-syn might cluster vesicles within synaptic boutons and restrict vesicle-motility. Since α-syn multimers associate with synaptic-vesicles (above), we asked whether stabilized VN/VC:α-syn multimers facilitate clustering of synaptic-vesicles and inhibit vesicle-motility even further. To test this we designed an assay to directly visualize synaptic-vesicle dispersion; based on previous observations that neuronal activity disperses synaptic-vesicles from boutons into flanking axons [25, 26]. Specifically, we asked if stabilized α-syn multimers (reconstituted VN/VC:α-syn's) would attenuate the activity-dependent dispersion of synaptic-vesicles (labeled with SyPhy:mRFP, see fig. 2E). Indeed while Venus:α-syn alone inhibited this dispersion (as expected), VN/VC:α-syn's attenuated this dispersion even further (fig. 2F and also see fig. 4C later). Collectively, these experiments suggest that α-syn multimers associate with synaptic-vesicle clusters and restrict their trafficking.
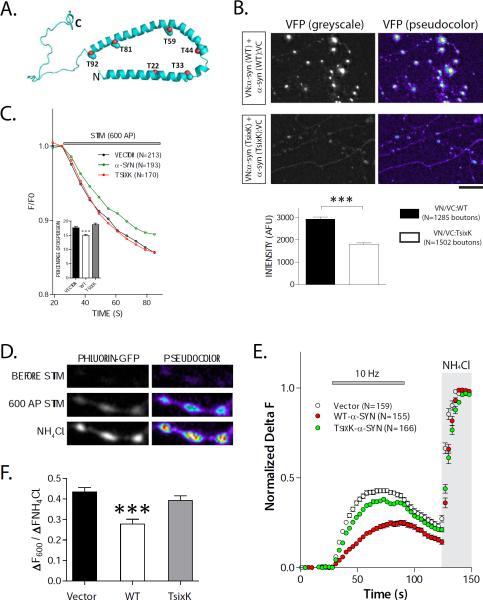
Figure 4. Mechanistic links between α-syn multimerization and synaptic function.
(A) Schematic of the α-syn helices (shaded) and position of the six mutations.
(B) Neurons from α-syn −/− mice were transfected with VN/VC:WT or VN/VC:TsixK α-syn's and fluorescence was quantified in boutons. There were clear diminutions in the TsixK datasets as shown in the representative images and quantification below.
(C) “Synaptic-vesicle dispersion” assay: Neurons were co-transfected with SyPhy:mRFP (to label synaptic-vesicles) and untagged WT or TsixK α-syn (or vector alone). Boutons were stimulated and decay of RFP fluorescence from boutons was quantified (see “results”). Note that while WT α-syn attenuates activity-induced synaptic-vesicle dispersion, the TsixK mutant has no effect on vesicle-trafficking (N=number of boutons).
(D) Synaptic recycling evaluated by vGlut-pHluorin assays. Cultured neurons were co transfected with vGlut-pHluorin and either untagged WT α-syn or TsixK α-syn. Fluorescence-change of the pH-sensitive vGlut-pHluorin probe reflects synaptic-vesicle recycling in this assay (see “results” and “methods”). Representative panels show fluorescence intensity change of vGlut-pHluorin upon 600 AP stimulation and NH4Cl perfusion. Note that NH4Cl alkalinizes all vesicles, revealing the total (recycling + resting) pool in these neurons.
(E, F) Representative ensemble average of vGlut-pHluorin traces from empty vector, WT α-syn or TsixK α-syn transfected neurons (N=number of boutons). Note that while WT α-syn nattenuates neurotransmitter release and decreases mean recycling-pools compared to vector-controls, TsixK α-syn fails to show this effect; quantified in (F) (all data normalized to total pools). Recycling/total pool for vector=43±2.17 %; WT α-syn =28±2.38%; TsixK α-syn =39±2.29% (~ 160 boutons on 7-9 coverslips were analyzed for each group from three separate batches of cultures; ***p < 0.001 compared to vector by one-way ANOVA followed by Dunnet's post hoc test). Total (alkalinized) pools of vector, WT-α-syn and TsixK-α-syn groups were 317.1 ± 16 AFU, 317.5 ± 11 AFU and 376 ± 18 AFU (mean ± SEM) respectively).
Biochemical analysis of α-synuclein multimers
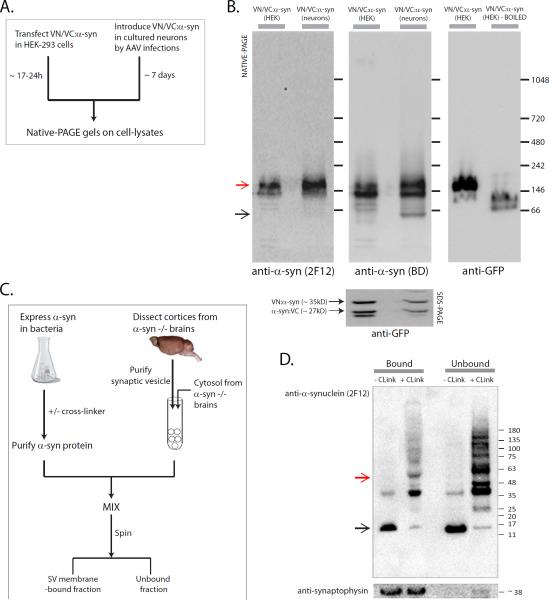
To biochemically evaluate α-syn multimers, we transiently introduced VN/VC:α-syn's into HEK-293 cells or cultured neurons (by adenoviral infections) and analyzed cell-lysates by Native/SDS-PAGE gels (fig. 3A). As shown in the native gels (fig. 3B), only a few higher-order α-syn bands were typically seen. Though precise molecular weights cannot be determined by these methods, these bands run at ~ 146 kD (α-syn tetramers would be expected to run at ~ 114kD in our system – 4 α-syn's + 2 VFP's). These experiments were repeated several times with similar results, and notably, all three VN/VC combinations showed similar biochemical profiles (Supp. fig. 3A).
Figure 3. Biochemical analyses of α-syn multimers.
(A) VN/VC:α-syn's were introduced into HEK293T cells or neurons (by viruses), expressed for the times indicated, and cell-lysates were analyzed by Native/SDS-PAGE.
(B) Native-PAGE show α-syn higher-order multimers immunoblotted with two α-syn antibodies and an anti-GFP antibody that also recognizes YFP (note disruption upon boiling). The red arrow marks the position where bands are typically seen, black arrow marks putative monomeric α-syn in neurons. An SDS-PAGE immunoblotted with anti-GFP marks the VFP-fragments. Each experiment was repeated 3-5 times with similar results.
(C) In-vitro reconstitution assay. Purified synaptic-vesicles and cytosol from α-syn −/− mouse brains were mixed with WT-α-syn purified from bacteria with/without a chemical cross linker (DSG). Vesicle membrane bound and unbound fractions were separated by centrifugation and analyzed by SDS-PAGE.
(D) Both monomeric and cross-linked α-syn multimers bound to synaptic-vesicles (a synaptophysin stain confirms that all synaptic-vesicles are in the bound fraction). Red and black arrows mark positions of putative tetramers and monomers. Experiment was repeated twice with similar results.
Though our above data (fig. 2) suggest that α-syn multimers associate with synaptic-vesicles, they do not directly show vesicle-binding. To address this, we evaluated the association of monomeric and multimeric α-syn with purified synaptic-vesicles. Based on previously published protocols [27, 28], we incubated α-syn-free synaptic-vesicles and cytosol with purified monomeric or multimeric α-syn (chemically cross-linked, see [15]; fig. 3C and Supp. fig. 3B). The main advantage of this assay is that brain cytosolic factors known to affect α-syn binding to membranes are available, recapitulating the in-vivo situation [27]. As shown in figure 3D, α-syn multimers are indeed capable of binding to synaptic-vesicles, though multimers are also present in cytosolic fractions. Note that these data are in general agreement with Dettmer et al., 2013.
A mechanistic link between α-syn multimerization and synaptic function
The above data suggest that α-syn multimers associate with and cluster synaptic-vesicles. As α-syn suppresses exo/endocytosis [1, 2], one can imagine a scenario where α-syn multimers cluster synaptic-vesicles, thus restricting vesicle-recycling and consequently, neurotransmitter release. If correct, this model predicts that disrupting α-syn multimers would also diminish α-syn-induced vesicle clustering and abrogate α-syn-induced synaptic attenuation. Though molecular determinants of α-syn multimerization are unknown, we reasoned that the most striking feature of the α-syn molecule – N-terminal repeats – might play a role. The N-terminus of α-syn has seven 11-residue repeats that are predicted to fold into amphipathic alpha-helices, highly conserved among species [29-31]. Recent simulation-models also implicate these repeats in α-syn tetramerization [32]. We used a rationally-designed synthetic mutant, where six threonines (T) – centrally lying along the helical face of the N-terminus – are mutated to lysines (K; known as TsixK, see fig. 4A and [33]. These mutations are expected to disrupt significant portions of the extended hydrophobic face of the helix, and greatly diminish the helical conformation of α-syn [33]. Notably, this reduction in helicity occurs despite robust association with vesicles [33], and indeed both WT and TsixK protein bind synaptic-vesicles with equal affinity in our in-vitro assay (Supp. fig. 3C).
Accordingly, we tested the predictions of our model by comparing the ability of WT and TsixK mutants to: 1) organize into multimers, 2) cluster synaptic-vesicles, and 3) influence synaptic-vesicle recycling. First we transfected VN/VC pairs of WT α-syn's (VN/VC:WT) or TsixK α-syn's (VN/VC:TsixK) in cultured neurons from α-syn −/− mice as described previously. As shown in fig. 4B, fluorescence complementation in the VN/VC:TsixK α-syn's was markedly attenuated. Diminution of higher-order conformers was also seen biochemically (Supp. fig. 3D). Next we tested the ability of the TsixK mutant to suppress activity-induced synaptic-vesicle dispersion. As shown in fig. 4C, while WT α-syn attenuated synaptic-vesicle dispersion as expected, the TsixK mutant failed to do so. Finally, we asked if TsixK mutations also abrogated the ability of α-syn to attenuate synaptic-vesicle recycling. Towards this we used a pHluorin-based assay that directly reports synaptic-vesicle recycling ([34, 35]; see fig. 4D). While WT-α-syn attenuated recycling as reported previously [5], TsixK-α-syn only had a mild (non-significant) effect (fig. 4E, F).
Dynamic α-synuclein multimers at synapses
Collectively, the data support a model where synaptic α-syn is organized into metastable conformers that bind to and cluster synaptic-vesicles, restricting their trafficking. We posit that by influencing synaptic-vesicle trafficking, multimeric α-syn conformers restrict recycling, consequently attenuating neurotransmitter release. Using complementation-assays that stabilize putative protein-protein interactions, we found that near-physiologic levels of α-syn result in robust and widespread complementation at synapses (fig. 1). Given the transient transfection of proteins into an α-syn null background; the resultant low expression-levels (comparable to endogenous-levels, see fig. 1D); and the paradigms used to visualize initial entry of newly-synthesized α-syn into boutons (fig. 1E-G), it is unlikely that the complementation seen in our experiments is a result of over-expression. Moreover, data from three different combinations of VN/VC-fragments (tagged to α-syn's) are similar, untagged α-syn appears to compete with fluorescence complementation in HEK cells, and the TsixK α-syn mutant also fails to complement; collectively arguing that complementation is not due to vagaries of the Venus-fragments, but reflect bona-fide α-syn interactions.
Clustering synaptic-vesicles and regulating recycling/neurotransmitter release – a potential function of α-syn multimers
Do α-syn multimers have a physiologic role? Stabilized VN/VC:α-syn multimers were associated with synaptic-vesicles (fig. 2A-D), and also inhibited the trafficking of synaptic-vesicles (fig. 2E, F; and also fig. 4C). Our biochemical data also show that α-syn multimers can associate with synaptic vesicles (fig. 3D). Notably, the data do not rule out a role for cytosolic α-syn multimers. A recent study showed that purified α-syn protein clusters synthetic vesicles in an in-vitro lipid-binding assay [36]. Though in isolation, the relevance of these in-vitro findings to neurons and synapses is uncertain; in light of data shown here, the collective evidence advocate the concept that α-syn plays a physiologic role in clustering synaptic-vesicles. Supporting the idea that helical folding of α-syn is important for multimerization, Varkey et al. recently showed that incubation of α-syn with lipid-nanoparticles – known to induce helicity – increases intra-molecular FRET of α-syn [37]. However, another recent paper suggests that α-syn is exclusively involved in attenuating endocytosis [38]. Nevertheless, many studies indicate that α-syn influences the exocytic-cycle and SNARE-assemblies, and a more complete dissection of exo- v/s endo-cytosis is warranted. The exact mechanisms by which α-syn multimers restrict vesicle mobility are still unclear. One possibility is that α-syn multimers between adjacent vesicles associate with each other (an “interlocking model”), with perhaps α-syn/VAMP2 interactions also playing a role as suggested by Diao et al. [36] – an open question for future studies.
Supplementary Material
Acknowledgements
We thank Julia George (Univ. of London) for the original TsixK plasmid; Shelley Halpain/Barbara Calabrese (UCSD) for the mRFP:Actin construct; Timothy Ryan (Weill Cornell) for pHluorin constructs and technical advice; and Tim Bartels/Dennis Selkoe (Harvard) for the 2F12 antibody. We also thank Virginia Lee (Univ. of Penn), Leon Laganado (MRC, Cambridge) and Christoph Kaether for the guinea-pig synuclein antibody, synaptophysin:mRFP, and synaptophysin:GFP constructs respectively. This work was supported by grants to SR (P50AG005131 – project 2, NIH) and PJM (RO1 NS073740, NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTARY INFORMATION
Supplemental Information includes three figures and Supplemental Experimental Procedures can be found with this article online at xxxxxxx
References
- 1.Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott D, Roy S. alpha-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012;32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staras K, Branco T, Burden JJ, Pozo K, Darcy K, Marra V, Ratnayaka A, Goda Y. A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron. 2010;66:37–44. doi: 10.1016/j.neuron.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci U S A. 2012;109:3213–3219. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, et al. alphabetagamma-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci U S A. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 10.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burre J, Vivona S, Diao J, Sharma M, Brunger AT, Sudhof TC. Properties of native brain alpha-synuclein. Nature. 2013;498:E4–6. doi: 10.1038/nature12125. discussion E6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould N, Mor D, Lightfoot R, Malkus K, Giasson B, Ischiropoulos H. Evidence of Native alpha-Synuclein Conformers in the Human Brain. J Biol Chem. 2014 doi: 10.1074/jbc.C113.538249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. In vivo cross-linking reveals principally oligomeric forms of alpha-synuclein and beta-synuclein in neurons and non-neural cells. J Biol Chem. 2013;288:6371–6385. doi: 10.1074/jbc.M112.403311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, et al. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerppola TK. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat Protoc. 2006;1:1278–1286. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodama Y, Hu CD. Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. Biotechniques. 2012;53:285–298. doi: 10.2144/000113943. [DOI] [PubMed] [Google Scholar]
- 19.Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One. 2008;3:e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Scott D, Das U, Gitler D, Ganguly A, Roy S. Fast vesicle transport is required for the slow axonal transport of synapsin. J Neurosci. 2013;33:15362–15375. doi: 10.1523/JNEUROSCI.1148-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat.Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- 23.Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, Burd CG, Lee VM. {alpha}-Synuclein-induced Aggregation of Cytoplasmic Vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1093–1103. doi: 10.1091/mbc.E07-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc.Natl.Acad.Sci.U.S.A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J.Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- 27.Wislet-Gendebien S, D'Souza C, Kawarai T, St George-Hyslop P, Westaway D, Fraser P, Tandon A. Cytosolic proteins regulate alpha-synuclein dissociation from presynaptic membranes. J Biol Chem. 2006;281:32148–32155. doi: 10.1074/jbc.M605965200. [DOI] [PubMed] [Google Scholar]
- 28.Wislet-Gendebien S, Visanji NP, Whitehead SN, Marsilio D, Hou W, Figeys D, Fraser PE, Bennett SA, Tandon A. Differential regulation of wild-type and mutant alpha-synuclein binding to synaptic membranes by cytosolic factors. BMC Neurosci. 2008;9:92. doi: 10.1186/1471-2202-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J.Biol.Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 30.Jo E, McLaurin J, Yip CM, George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J.Biol.Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 31.Ulmer TS, Bax A. Comparison of structure and dynamics of micelle-bound human alpha-synuclein and Parkinson disease variants. J.Biol.Chem. 2005;280:43179–43187. doi: 10.1074/jbc.M507624200. [DOI] [PubMed] [Google Scholar]
- 32.Kara E, Lewis PA, Ling H, Proukakis C, Houlden H, Hardy J. alpha-Synuclein mutations cluster around a putative protein loop. Neurosci Lett. 2013;546:67–70. doi: 10.1016/j.neulet.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J.Biol.Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 34.Burrone J, Li Z, Murthy VN. Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nat Protoc. 2006;1:2970–2978. doi: 10.1038/nprot.2006.449. [DOI] [PubMed] [Google Scholar]
- 35.Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diao J, Burre J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT. Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varkey J, Mizuno N, Hegde BG, Cheng N, Steven AC, Langen R. alpha-Synuclein oligomers with broken helical conformation form lipoprotein nanoparticles. J Biol Chem. 2013;288:17620–17630. doi: 10.1074/jbc.M113.476697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas KJ, Makani S, Davis T, Westphal CH, Castillo PE, Chandra SS. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci. 2014;34:9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.