SUMMARY
Majewski Osteodysplastic Primordial Dwarfism type II (MOPDII) is caused by mutations in the centrosome gene pericentrin (PCNT) which lead to severe pre- and post-natal growth retardation[1]. As in MOPDII patients, disruption of pericentrin (Pcnt) in mice caused a number of abnormalities including microcephaly, aberrant hemodynamics analyzed by in utero echocardiography and cardiovascular anomalies; the latter being associated with mortality, as in the human condition[1]. To identify the mechanisms underlying these defects, we tested for changes in cell and molecular function. All Pcnt−/− mouse tissues and cells examined showed spindle misorientation. This mouse phenotype was associated with misdirected ventricular septal growth in the heart, decreased proliferative symmetric divisions in brain neural progenitors and increased misoriented divisions in fibroblasts; the same phenotype was seen in fibroblasts from three MOPDII individuals. Misoriented spindles were associated with disrupted astral microtubules and near complete loss of a unique set of centrosome proteins from spindle poles (ninein, Cep215, centriolin). All these proteins appear to be crucial for microtubule anchoring and all interacted with Pcnt, suggesting that Pcnt serves as a molecular scaffold for this functionally-linked set of spindle pole proteins. Importantly, Pcnt disruption had no detectable effect on localization of proteins involved in the cortical polarity pathway (NuMA, p150glued, aPKC). Not only do these data reveal a spindle-pole-localized complex for spindle orientation, but they identify key spindle symmetry proteins involved in the pathogenesis of MOPDII.
Keywords: pericentrin−/− mouse, centrosome, spindle orientation, cardiovascular defect, primordial dwarfism, pericentrin, centriolin, ninein, Cep215, MOPDII
Results and Discussion
The pathogenic mechanisms underlying the complex clinical features of the primordial dwarfism, Majewski Osteodysplastic Primordial Dwarfism (MOPDII), are unclear. The cause of MOPDII is biallelic loss-of-function mutations in the centrosome gene, pericentrin (PCNT, [2]), which lead to a loss of functional protein. MOPDII is characterized by severe intrauterine growth retardation progressing to microcephaly, bony dysplasia, and unusual facial features [3, 4]. MOPDII patients develop vascular abnormalities including vascular overgrowth and cardiovascular defects such as atrioventricular septal defects (AVSD). These vascular defects contribute to high morbidity and mortality secondary to cerebral aneurysms, stroke and myocardial infarction [5]. Despite identification of the causative genetic mutation, a common mechanism for the multi-organ defects in MOPDII has not been elucidated.
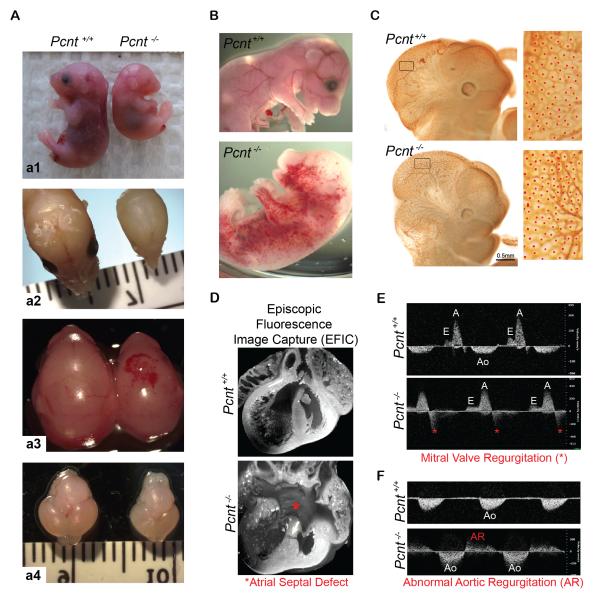
To understand the cellular and molecular basis of MOPDII phenotypes, we engineered Pcnt−/− mice by insertional mutagenesis (Figure S1A). Mouse tissues, mouse embryonic fibroblasts (MEFs), and immortalized MEF lines confirmed loss of protein (Figure S1B-D and S3F) and mRNA (data not shown). Strikingly, Pcnt−/− mice exhibited many MOPDII features [2, 4–6] including small body size, microcephaly, craniofacial developmental anomalies (abnormal head shape, eye defects, and cleft palate, Figures 1A, S2A-C), and structural kidney defects (Figures S2I-J). As in MOPDII, Pcnt−/− mice also developed vascular anomalies. These included structural and hemodynamic cardiovascular defects from E11.5-17.5, including head and whole-body hemorrhaging (Figures 1A-B), increased vascular density in head and abdomen (Figure 1C, and data not shown), atrioventricular septal defects (Figure 1D), aberrant valve formation, and aortic blood collection from both ventricles (overriding aorta; summary of related cardiac defects and other anomalies, see Table S1). These defects were associated with severe cardiac dysfunction. In utero ultrasound and spectral Doppler imaging of each individual embryo revealed mitral valve and aortic regurgitation (Figures 1E-F; n=5/7 in 1E and n=2/5 in 1F), and disrupted hemodynamics, leading to heart failure and prenatal lethality at E15.5 and E17.5. Similar cardiovascular anomalies are manifested in MOPDII, and appear to be major contributors to death in both organisms.
Figure 1. Pcnt−/− mice exhibit growth retardation, microcephaly, vascular anomalies and structural heart defects.
(A) Pcnt−/− mice exhibit intrauterine growth retardation (a1, 35% smaller, p<0.01; >50 mice in 7 liters); defects in cranial development (a2), intracranial hemorrhaging (a3); and microcephaly (a4).
(B) Representative images showing whole-body hemorrhaging in Pcnt−/− mouse (bottom) compared to the wild-type littermate (top).
(C) PECAM stain of vascular network in head of E11.5 Pcnt+/+ (top row) and Pcnt−/− (bottom row) embryos. Boxed regions (left column) are enlarged on the right, showing increased density of vascular plexuses (red dots) in Pcnt−/− head versus region-matched Pcnt+/+ (p<0.0001).
(D) 3-dimensional episcopic fluorescence image capture (EFIC) obtained from heart of E17.5 Pcnt+/+ (top) and Pcnt−/− (bottom) mice reveal defective septum formation (asterisk) in Pcnt−/− neonates in contrast to the Pcnt+/+ littermate.
(E-F) Doppler echocardiography reveals structural heart defects and congestive heart failure in Pcnt−/− embryos (bottom panels), but not in Pcnt+/+ littermates (top panels), as indicated by abnormal mitral valve regurgitation (asterisks, E) and aortic regurgitation (AR, F) at E15.5 and E17.5, respectively. Aortic flow (Ao); A wave, ventricular filling during atrial contraction (A); E wave, passive ventricular filling (E).
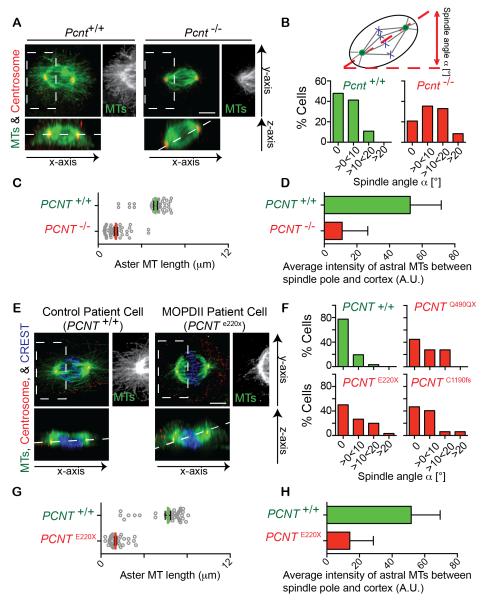
To identify potential disease mechanisms, we first examined cellular and molecular functions associated with Pcnt during mitosis using primary Pcnt−/− MEFs. The most prominent feature of mitotic spindles in Pcnt−/− MEFs was a dramatic reduction in astral microtubules (MTs; Figure 2A, inset). We identified a ~4-fold decrease in both astral MT immunofluorescence intensity and MT length (Figure 2C-D). This phenotype is known to disrupt MT contacts with the cell cortex and thus, retention of spindle symmetry [7, 8]. Further inspection of mitotic spindles revealed spindle misorientation. The spindle angle relative to the cell-substrate adhesion plane in ~80% of Pcnt−/− MEFs was >10°, whereas spindles in Pcnt+/+ MEFs were largely (~50%) parallel to the substratum (0°; Figures 2AB, S3A). Importantly, spindle misorientation (Figures 2E-F, S3B) and diminished astral MTs (Figure 2G-H) were also identified in all three MOPDII skin fibroblast lines examined, strengthening the idea that spindle misorientation is a conserved phenotype of Pcnt deficiency in both organisms, and is likely a contributing factor to the pathogenic mechanisms of MOPDII.
Figure 2. Spindle misorientation in both primary Pcnt−/− MEFs and cultured Pcnt-deficient MOPDII patient cells.
(A) Spindle angle, angles between dashed lines (lower panels; x/z-axis projections) and the substratum. Top panels, maximal projections of z-axis; MTs, green (α-tubulin); centrosomes, red (γ-tubulin). Insets show the lack of astral MTs in Pcnt−/− MEFs compared to control.
(B) Quantification of (A) showing a significant increase (>0°) of spindle angle in Pcnt−/− MEFs (n>45 cells for Pcnt+/+ and Pcnt−/−; p<0.0002; individual scatter plots for each angle shown in Figure S3A).
(C-D) Quantification of astral MT length and average intensity of astral MTs between spindle pole and cortex (n > 50 asters per treatment, p < 0.001).
(E) x/y and x/z-axis projections of control and Pcnt-deficient MOPDII patient fibroblasts (PCNTe220x) are shown. MTs, green (α-tubulin); centrosomes, red (5051); kinetochores, blue (CREST). Insets show diminished astral MTs in Pcnt-deficient cells compared to control.
(F) A significant increase in misoriented spindles is observed in Pcnt-deficient MOPDII fibroblasts (red; PCNTQ490X, PCNTE220X, and PCNTC1190fs) versus control patients (green; PCNT+/+). 29-32 spindles were analyzed for each group; p values compared to control: 0.0034(PCNTQ490X), 0.011(PCNTE220X), 0.0166(PCNTC1190fs). Individual scatter plot for each angle is shown in Figure S3B.
(G-H) Astral MT length and average intensity of astrial MTs quantified between spindle pole and cortex (n > 50 asters per treatment, p < 0.001).
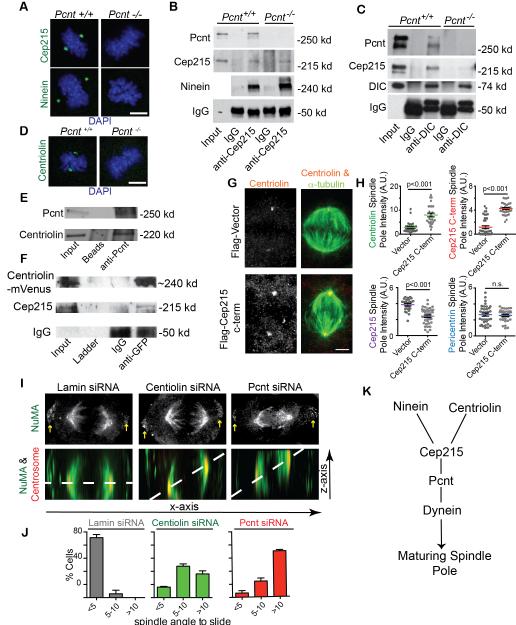
To explore the molecular mechanism of spindle misorientation in Pcnt-deficient cells, we screened known centrosomal proteins for changes in localization by immunofluorescence (~30 proteins tested). We unexpectedly identified a subset of proteins (Cep215/Cdk5rap2/hCnn, ninein, centriolin) that showed near-complete (up to 99.35%) loss from spindle poles in the absence of Pcnt (Figures 3A, D, and S3C-E). Total levels of Cep215 and ninein in cell lysates were unaffected (Figures S1C and S3F), suggesting a model in which Pcnt is required to localize ninein and Cep215 to spindle poles. Indeed, ninein and Cep215 localized to mitotic spindle poles (Figure 3A) as well as motile pericentriolar satellites (Figure S3C). Similar to Pcnt, these two proteins have roles in MT organization and anchoring at centrosomes/spindle poles [9–11]. Moreover, mutation of Cep215 or its fly homolog (Cnn) cause spindle misorientation [12, 13], due to disruption of astral MTs [14].
Figure 3. Pcnt interacts with Centriolin, Cep215 and ninein, is required for their localization to the mitotic spindle poles, and contributes to spindle orientation.
(A) Ninein and Cep215 (green) are significantly displaced from mitotic spindle poles in Pcnt−/− MEFs compared to Pcnt+/+ MEFs. DNA, blue. Bar, 5 μm. Quantification in Figure S3D.
(B-C) Immunoprecipitation from Pcnt+/+ MEFs compared to Pcnt−/− MEFs reveals protein complexes: Pcnt and Cep215 (also see in Figure S3G-H); Cep215 and ninein; Dynein, Cep215, and Pcnt.
(D) Pcnt−/− MEFs show significant displacement of centriolin (green) from mitotic spindle poles compared to Pcnt+/+ MEFs. DNA, blue. Bar, 5 μm.
(E-F) Immunoprecipitation from human epithelial cells demonstrates an interaction between Pcnt and centriolin (E, reciprocal immunoprecipitation in Figure S4B). An interaction was observed in cells stabily expressing Centriolin-mVenus [21] and Cep215.
(G-H) Cells expressing Cep215 c-term (c-term; aa1201-1822; reported in [22]) have an increase in Centriolin recruitment to spindle poles that was comparable to the increased recruitment of the c-terminus of Cep215 (p<0.001 as marked, n.s. is non-significant, representative of three experiments). A loss of endogenous Cep215 at the spindle pole was observed using an antibody targeting the Cep215 n-terminal domain. No effect was observed on Pcnt localization when Cep215 c-term was expressed.
(I) Centriolin or Pcnt-depleted cells show increased spindle misorientation. Shown are maximum projections of z-axis; centrosome (5051, red), NuMA (green). Note that NuMA localization is unaffected at both spindle poles and cell cortex (yellow arrows, x/y-axis maximum projection).
(J) Quantification showing a significant increase (>5°) of spindle angle in cells depleted of either centriolin or Pcnt (~3-fold, n=3 experiments, p<0.001 comparing spindle angles >5° across treatments. n>20 cells counted/experiment).
(K) Model represents a Pcnt-mediated interaction between Cep215 and dynein (as demonstrated by immunoprecipitation in 3C), and Ninein interacting peripherally with Cep215 (shown both by immunoprecipitation in 3B, S3G, and genetically in S3I). Pcnt-anchored Cep215 further interacts with Centriolin (3E-F) through the c-terminal domain of Cep215 (4G-H).
The requirement for Pcnt in localizing Cep215 and ninein to centrosomes/spindle poles suggested that they might form protein complexes. Immunoprecipitation from cell lysates demonstrated novel interactions between Pcnt and ninein, as well as ninein and Cep215, and confirmed a previously identified interaction between Pcnt and Cep215 (Figures 3B, S3G-H, [10, 15]). Intriguingly, our results also showed that in the absence of Pcnt, ninein and Cep215 still co-precipitated (Figures 3B, S3G), suggesting that they formed a subcomplex. Using a previously identified Cep215-binding domain of Pcnt (PcntB7, aa1801-2100; [20]), we found that when expressed in Pcnt−/− MEFs, spindle misorientation was diminished (Figures S3J-K). Furthermore, this Pcnt-fragment rescued localization of Cep215 to spindle poles in Pcnt−/− MEFs compared to mock-transfected Pcnt−/− MEFs (Figures S3L-M), suggesting that the spindle-association of Cep215 through Pcnt is crucial for spindle orientation. Together, these studies reveal a novel multi-molecular Pcnt complex(es) and implicate these associated components in spindle orientation.
Pcnt is known to interact with dynein [16, 17], thus, we tested if the Pcnt interacting proteins, Cep215 and ninein, also interacted with dynein. We found that dynein and Cep215 co-precipitated in a Pcnt-dependent manner (Figure 3C). Using a siRNA-targeted-depletion-based approach, we characterized the nature of the interaction between ninein, Cep215, and Pcnt at spindle poles. Strikingly, neither Cep215 nor Pcnt required ninein for spindle pole localization (Figure S3I) suggesting Pcnt and Cep215 anchor ninein at the mitotic spindle pole (Figures 3A-B, S3G-I) and likely get there through dynein-mediated transport (Figure 3C, modeled in Figure 3K). This result, together with the Pcnt-independent interaction between Cep215 and ninein (Figures 3B, S3G-H), suggested that a complex of pericentrin, ninein, and Cep215 interacted with dynein through Pcnt. We propose that the association with dynein is required for this complex to target to spindle poles, as shown for Pcnt, Cep215, and γ-tubulin ([17, 18], modeled in Figure 3K). Consistent with this idea was the localization of Pcnt-interacting proteins to pericentriolar satellites (Figure S3C), assemblies of centrosome proteins carried by dynein to centrosomes [17–19].
The third protein identified in our screen, centriolin [20, 21], was essentially undetectable at spindle poles upon Pcnt loss (Figures 3D, S3E) suggesting Pcnt anchoring. Centriolin levels were slightly diminished in Pcnt−/− cells (unlike Cep215 and ninein) but this minimal depletion could not account for the significant centriolin loss from poles (Figure S4A). We demonstrated that centriolin coimmunoprecipitated with Pcnt (Figures 3E and S4B) and Cep215 (Figure 3F). We took advantage of the previously identified Pcnt-interacting domain of Cep215 (Cep215 c-term, aa1201-1822; [22]), and tested whether this domain affected the spindle pole localization of centriolin (Figures 3G-H). Cep215 c-term localized robustly to mitotic spindle poles (Figures 3H), uncoupled pole localization of endogenous Cep215 (Figure 3H), and was accompanied by a 2-fold increase in the spindle pole localization of centriolin (Figures 3G-H). Pcnt-intensity at mitotic spindle poles was unchanged with Cep215 c-term expression suggesting that Pcnt is upstream of Cep215 recruitment at poles (Figure 3H, modeled in Figure 3K).
Since centriolin has never been implicated in spindle orientation, we compared human epithelial cells depleted of centriolin or Pcnt to controls (lamin siRNA) using previously published siRNAs ([21, 23]; Figure S4C). We found that centriolin-depletion increased spindle misorientation to a level similar to Pcnt-depleted cells (Figures 3I-J). Also similar to Pcnt-deficient cells (Figure 2), centriolin-depleted cells showed a decrease in overall astral MT length and number (Figure S4D), strengthening the idea that the novel mitotic roles of centriolin are functionally linked with Pcnt.
To clarify the potential interplay between the centrosome-localized Pcnt-associated complex for spindle orientation, we compared it to the classical spindle orientation complex located at the cell cortex (NuMA, aPKC, p150glued). We found that it remained intact at the cell cortex (Figures 3I and S4E-F), suggesting that the cortical NuMA pathway for spindle symmetry/orientation [24] was not responsible for the observed phenotypes with Pcnt or Centriolin loss. Our observations suggest a spindle pole-anchored complex where Pcnt recruits Cep215, which, in turn, recruits centriolin and/or ninein (Figure 3K). This centrosome-based complex would be required for modulating orientation of the spindle and thus, the cell division plane.
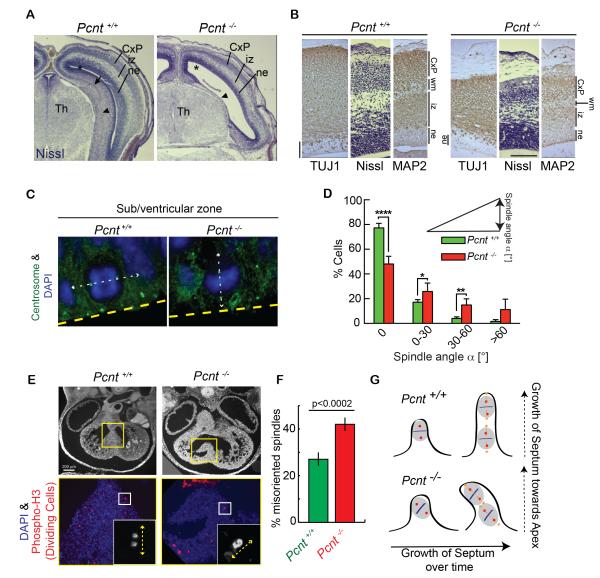
To test whether spindle misorientation occurred in vivo and potentially contributed to MOPDII pathogenesis, we focused on the organs exhibiting the most dramatic phenotypes; namely, the brain, heart, and kidney (data not shown). The small brains of Pcnt−/− mice showed enlarged cerebral ventricles, and thinning of the cerebral cortex, particularly the cortical plate, which harbors young neurons at this stage (Figures 4A-B; n=5/5). The phenotypic marker for differentiated neurons (MAP2) was expressed at low levels in Pcnt−/− mouse cortex (Figure 4B; n=3/3) and hippocampus (data not shown), suggesting a delay in neuronal differentiation and/or defects in neurogenesis. Indeed, neuron-specific TUJ1 staining was reduced in the neuroepithelium (ne) where nascent neurons arise (Figure 4B; n=3/3, E17), demonstrating that neurogenesis and the earliest phases of neuronal differentiation were compromised, a condition known to lead to microcephaly [25]. Further examination of the brain ventricular zone (E13.5) showed a significant increase in asymmetric divisions (Figures 4C-D) compared to wild-type controls. Increased asymmetric divisions or aberrant proliferative/symmetric divisions [26] in this area of the brain are associated with microcephaly and can be caused by mutations in other centrosome genes [25, 27–29].
Figure 4. Asymmetric spindles and misoriented divisions are significantly increased in microcephalic brains and developing hearts of Pcnt−/− mice.
(A) Coronal forebrain sections reveal defective brain development and enlarged ventricles (*) in Pcnt−/− mice from E17 littermates (Nissl staining). Note severe dysplasia of hippocampus compared to control (arrow), and medial temporal cortex (arrowheads) in Pcnt−/− mice.
(B) An enlargement from a portion in (A) demonstrates Nissl staining, and staining for MAP2 (phenotypic marker for differentiated neurons), and TUJ1 (neuron-specific class III β-tubulin) to determine neuronal differentiation. In Pcnt−/− section note thinning of the cortical plate (CxP); a significant decrease in the development of interstitial zones (iz), neuroepithelium (ne), and future white matter (wm); and reduced levels of MAP2 and TUJ1.
(C) Shown are single mitotic cells localized at the ventricular zone (VZ) from E13.5 Pcnt+/+ and Pcnt−/− littermates. Sections were stained for γ-tubulin to label mitotic spindle poles. White dashed lines depict the cell division axis and a yellow dashed line identifies the ventricular lining. DAPI, (DNA, blue).
(D) Compared to age-matched Pcnt+/+ embryos (n=4), Pcnt−/− embryos showed a significant increase in asymmetric divisions at the ventricular zone (E13.5, n=5; **** p<0.0001, **p<0.01, *p<0.05; n>250 cells).
(E-F) Cell division axis within the developing atrial septum of Pcnt−/− embryos is significantly misdirected compared to Pcnt+/+ embryos (E12.5; n=4 to 5 embryos for each genotype, quantification in F). (E) Top, in utero ultrasound image demonstrating misdirected septal development in Pcnt−/− embryo compared to Pcnt+/+ embryo. Bottom, enlarged view of boxed region in ultrasound was stained for phospho-H3 (red) to specifically label dividing cells. Inset shows a dividing cell with dashed lines depicting the cell division axis.
(G) Shown is a schematic for measuring spindle misorientation in a developing atrial septum. Spindle angles (orange dotted line) were measured in relation to the axis perpendicular to the septum base (black dotted line). The septum grows toward the apex of the heart through symmetric divisions over time. When division axis becomes predominantly asymmetric, we propose that the septum is more likely to grow in an unpolarized way (as seen in E).
Similar to microcephalic brains, mouse hearts in Pcnt−/− mice showed significantly more misoriented divisions compared to normal littermates. This was particularly striking in the developing cardiac septum, and occurred concomitantly with misdirected growth of the septum (Figures 4E-F, modeled in Figure 4G). These results indicate that orientated division through the spindle pole component Pcnt in vivo is crucial for proper organogenesis. The aberrant orientation of spindles and plane of cell division in the absence of Pcnt in mice is likely to contribute to anatomic developmental abnormalities in the same and perhaps other organs in MOPDII.
Our molecular model for spindle misorientation in Pcnt−/− mice involves a novel Pcnt protein complex comprised of key centrosome/spindle pole components that assemble onto spindle poles in a dynein-dependent manner to control astral MT organization at this site. In the absence of Pcnt, these proteins are lost from spindle poles, diminishing the efficacy of astral MT assembly, which is required for proper spindle orientation. Spindle misorientation in Pcnt−/− mice and MOPDII cells provides plausible mechanisms for: (1) cardiac septation defects, through misdirected growth of septa; (2) microcephaly, through decreased proliferative symmetric divisions, which are required to populate neural stem cells for brain growth and 3) cystic kidneys (not shown) through division in the plane of the lumen, increasing its diameter. At a global level, aberrant divisions could affect stem cells and create more differentiated cells at the expense of self-renewing divisions [30] decreasing cell numbers in many, if not all, organs. This could account for small brain, body and organ size of Pcnt−/− mice and MOPDII individuals, as seen in microcephalies caused by mutations in other centrosome genes [12, 28, 29].
Taken together, the unique subset of Pcnt-interacting proteins may collectively define a spindle-pole-based complex for spindle orientation. The genetic association of mutant ninein (NIN) and Cep215 (CEP215) with microcephaly [27, 31] supports the idea that these proteins contribute to the phenotypes in MOPDII and the Pcnt−/− mouse through their loss from spindle poles in the absence of Pcnt. These results suggest that Pcnt may act as the core scaffold for such a complex during embryonic development. It is unclear why mutation of some centrosome genes disrupts a single organ, whereas Pcnt mutations affect many organs.
This work shows for the first time that Pcnt−/− mice exhibit pathology similar to and beyond those of MOPDII. We believe the prenatal lethality of the Pcnt−/− mouse is an excellent model for the human condition. Like Pcnt−/− mice, MOPDII is an extremely rare human disorder. This is likely due to the fact that many human embryos and fetuses do not progress to birth. In addition, many of those that are born weigh only 1-2 pounds at birth and often do not develop beyond this stage. Inbred Pcnt−/− mice avoid confounding effects of genetic heterogeneity in patients, and show that Pcnt deficiency alone can cause a phenotype continuum spanning MOPDII. In addition, variable phenotypes occur between Pcnt−/− mice from the same litter as seen in MOPDII patients. These may result from genetic modifier effects, which would allow long-term survival of some MOPDII patients. Severe cardiovascular defects in Pcnt−/− embryos are not expected in MOPDII patients, as such defects would be prenatal lethal. However, it is interesting that both Pcnt−/− mice and MOPDII individuals die of cardiovascular issues, albeit at different stages of development. These defects are informative regarding developmental processes that may be impacted by Pcnt deficiency. The Pcnt−/− mouse model also reveals unique phenotypes not reported thus far in the human conditions (polydactyly, anopthalmia; Figure S2), which could be species-specific, reflect different gene-environment interactions, or result from the role of Pcnt in cilia integrity/signaling [23, 32], although this was not clearly identified in Pcnt−/− mice. Alternatively, these additional phenotypes may suggest higher risks for such developmental anomalies in MOPDII patients and even their families. Thus, our Pcnt mouse model provides insights into the etiology and broad defects in MOPDII.
Supplementary Material
HIGHLIGHTS.
Pcnt−/− mice recapitulate many features of primordial dwarfisms.
Spindles are misoriented and tissue growth misdirected in Pcnt−/− mice.
Pcnt−/− mice dramatically displace spindle orientation proteins from spindle poles.
Identification of a spindle pole localized spindle orientation complex.
ACKNOWLEDGEMENTS
The article is dedicated to the memory of Chun-Ting Chen, a thoughtful, innovative, and energetic scientist, and most importantly our dear friend. Ting spearheaded a major part of the work presented in this contribution and he will be truly missed amongst his peers. SD is supported by NIH RO1-GM051994-14, CL is supported by NIH U01-HL098180-05, and HH is supported by NIH K99-GM107355. We thank Stephen Jones and the UMMS Transgenic Animal Modeling Core, Paul Furcinitti of the UMMS Digital Light Microscopy, Yu Liu of the UMMS DERC Morphology Core, and James Fitzpatrick of the Waitt Advanced Biophotonics Center at Salk institutes.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental information includes four figures, one table, and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Delaval B, Doxsey SJ. Pericentrin in cellular function and disease. J. Cell Biol. 2010;188:181–90. doi: 10.1083/jcb.200908114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008;319:816–9. doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- 3.Hall JG, Flora C, Scott CI, Pauli RM, Tanaka KI. Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am. J. Med. Genet. A. 2004;130A:55–72. doi: 10.1002/ajmg.a.30203. [DOI] [PubMed] [Google Scholar]
- 4.Majewski F, Ranke M, Schinzel A. Studies of microcephalic primordial dwarfism II: the osteodysplastic type II of primordial dwarfism. Am. J. Med. Genet. 1982;12:23–35. doi: 10.1002/ajmg.1320120104. [DOI] [PubMed] [Google Scholar]
- 5.Bober MB, Khan N, Kaplan J, Lewis K, Feinstein JA, Scott CI, Steinberg GK. Majewski osteodysplastic primordial dwarfism type II (MOPD II): expanding the vascular phenotype. Am. J. Med. Genet. A. 2010;152A:960–5. doi: 10.1002/ajmg.a.33252. [DOI] [PubMed] [Google Scholar]
- 6.Ucar B, Kilic Z, Dinleyici EC, Yakut A, Dogruel N. Seckel syndrome associated with atrioventricular canal defect: a case report. Clin. Dysmorphol. 2004;13:53–5. doi: 10.1097/00019605-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 2011;13:461–8. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hehnly H, Doxsey S. Rab11 Endosomes Contribute to Mitotic Spindle Organization and Orientation. Dev. Cell. 2014:1–11. doi: 10.1016/j.devcel.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Rhee K. Importance of the CEP215-Pericentrin Interaction for Centrosome Maturation during Mitosis. PLoS One. 2014;9:e87016. doi: 10.1371/journal.pone.0087016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haren L, Stearns T, Lüders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One. 2009;4:e5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logarinho E, Maffini S, Barisic M, Marques A, Toso A, Meraldi P, Maiato H. CLASPs prevent irreversible multipolarity by ensuring spindle-pole resistance to traction forces during chromosome alignment. Nat. Cell Biol. 2012;14:295–303. doi: 10.1038/ncb2423. [DOI] [PubMed] [Google Scholar]
- 12.Lizarraga SB, Margossian SP, Harris MH, Campagna DR, Han A-P, Blevins S, Mudbhary R, Barker JE, Walsh CA, Fleming MD. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–17. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megraw TL, Kao LR, Kaufman TC. Zygotic development without functional mitotic centrosomes. Curr. Biol. 2001;11:116–20. doi: 10.1016/s0960-9822(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 15.Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, Hyman A, Mann M. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J. Cell Biol. 2010;189:739–54. doi: 10.1083/jcb.200911091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purohit A, Tynan SH, Vallee R, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 1999;147:481–92. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young a, Dictenberg JB, Purohit a, Tuft R, Doxsey SJ. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell. 2000;11:2047–56. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Y, Fong K-W, Choi Y-K, See S-S, Qi RZ. Dynamic recruitment of CDK5RAP2 to centrosomes requires its association with dynein. PLoS One. 2013;8:e68523. doi: 10.1371/journal.pone.0068523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo A, Sasaki H, Yuba-Kubo A, Tsukita S, Shiina N. Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J. Cell Biol. 1999;147:969–80. doi: 10.1083/jcb.147.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gromley A, Jurczyk A, Sillibourne J, Halilovic E, Mogensen M, Groisman I, Blomberg M, Doxsey S. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 2003;161:535–45. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hehnly H, Chen C-T, Powers CM, Liu H-L, Doxsey S. The centrosome regulates the rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr. Biol. 2012;22:1944–50. doi: 10.1016/j.cub.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchman JJ, Tseng H-C, Zhou Y, Frank CL, Xie Z, Tsai L-H. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron. 2010;66:386–402. doi: 10.1016/j.neuron.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat. Cell Biol. 2007;9:160–70. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- 24.Mapelli M, Gonzalez C. On the inscrutable role of Inscuteable: structural basis and functional implications for the competitive binding of NuMA and Inscuteable to LGN. Open Biol. 2012;2:120102. doi: 10.1098/rsob.120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton GK, Woods CG. Primary microcephaly: do all roads lead to Rome? Trends Genet. 2009;25:501–10. doi: 10.1016/j.tig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillies TE, Cabernard C. Cell division orientation in animals. Curr. Biol. 2011;21:R599–609. doi: 10.1016/j.cub.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 27.Tan CA, Topper S, Ward Melver C, Stein J, Reeder A, Arndt K, Das S. The first case of CDK5RAP2-related primary microcephaly in a non-consanguineous patient identified by next generation sequencing. Brain Dev. 2013 doi: 10.1016/j.braindev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Fish JL, Kosodo Y, Enard W, Pääbo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10438–43. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber R, Zhou Z, Sukchev M, Joerss T, Frappart P-O, Wang Z-Q. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat. Cell Biol. 2011;13:1325–34. doi: 10.1038/ncb2342. [DOI] [PubMed] [Google Scholar]
- 30.Neumüller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauber A, Lafranchi SH, Maliga Z, Lui JC, Moon JE, McDeed C, Henke K, Zonana J, Kingman GA, Pers TH, et al. Novel microcephalic primordial dwarfism disorder associated with variants in the centrosomal protein ninein. J. Clin. Endocrinol. Metab. 2012;97:E2140–51. doi: 10.1210/jc.2012-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Curr. Top. Dev. Biol. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.