Abstract
With the advent of neuroimaging techniques, especially functional MRI (fMRI), studies have mapped brain regions that are associated with good and poor reading, most centrally a region within the left occipito-temporal/fusiform region (L-OT/F) often referred to as the visual word form area (VWFA). Despite an abundance of fMRI studies of the VWFA, research about its structural connectivity has just started. Provided that the VWFA may be connected to distributed regions in the brain, it remains unclear how this network is engaged in constituting a well-tuned reading circuitry in the brain. Here we used diffusion MRI to study the structural connectivity patterns of the putative VWFA and surrounding areas within the L-OT/F in children with typically developing (TD) reading ability and with word recognition deficits (WRD; sometimes referred to as dyslexia). We found that L-OT/F connectivity varied along a posterior- anterior gradient, with specific structural connectivity patterns related to reading ability in the ROIs centered upon the putative VWFA. Findings suggest that the architecture of the VWFA connectivity is fundamentally different between TD and WRD, with TD showing greater connectivity to linguistic regions than WRD, and WRD showing greater connectivity to visual and parahippocampal regions than TD. Findings thus reveal clear structural abnormalities underlying the functional abnormalities in the VWFA in WRD.
Keywords: visual word form area, reading, brain connectivity, diffusion MRI, tractography, children
1. Introduction
A significant number (5–17%) of individuals experience difficulty with word recognition, which is sometimes referred to as dyslexia (see (Elliott and Grigorenko, 2014) for a discussion of the complexity of the dyslexia definition). These types of reading difficulties are characterized by inaccurate and slow recognition and decoding of words, or difficulty with phonological to orthographic conversions, despite adequate instruction and intelligence (Lyon et al., 2003). While the behavioral deficits of word recognition deficits (Word Recognition Deficits; WRD) are comparatively well characterized, the underlying neural mechanisms are still being investigated.
With the advent of neuroimaging techniques, especially functional MRI (fMRI), brain areas associated with word reading have been proposed. These areas comprise two distinct routes, corresponding to distinct approaches to word reading. The dorsal route, which comprises the left parieto-temporal region, has been proposed to be important for orthographic-to- phonological conversions (Pugh et al., 2000), and is activated during fMRI tasks with novice readers and during phonological decoding tasks. The ventral route, which is in the left occipitotemporal/fusiform region (L-OT/F), is associated with the visual orthographic conversion crucial to word identification skill, and is thought to be important for fast and efficient word recognition (McCandliss et al., 2003). Within this route, there is a smaller area of particular interest which is known by some as the putative visual word form area (VWFA (Cohen et al., 2000; Petersen et al., 1988)). Anomalous activation of this region is seen in individuals who struggle with word-level reading. Evidence from lesion (Epelbaum et al., 2008; Mandonnet et al., 2009), positron emission tomography (PET) (Petersen et al., 1990; Price et al., 1996), and fMRI and event-related potential (ERP) studies (Braet et al., 2012; Brem et al., 2010; Brem et al., 2006; Brem et al., 2009; Cohen et al., 2002; Nestor et al., 2012; Rauschecker et al., 2011) indicates that the VWFA is selectively responsive to written letter strings relative to other visual categories such as line drawings. (Note, however, that some have challenged this degree of specificity of the VWFA, as it also activates during other non-linguistic tasks [see (Price and Devlin, 2003; Vogel et al., 2012)]).
Despite an abundance of evidence on how the VWFA is functionally associated with word reading performance, discovering the underpinning structural connectivity of the VWFA is of no less importance. Fluent reading requires efficient and well-tuned left hemisphere circuitry, and therefore difficulties with reading have long been hypothesized to be reflective of inefficiencies in cortical connections; such suppositions date as far back as the initial studies of pure alexia, the original “disconnection syndrome” (Dejerine, 1892). Diffusion weighted MR imaging (Lebihan and Breton, 1985; LeBihan et al., 1986) is a non-invasive technique that can detect the preferential diffusion direction of water molecules in anisotropic tissues, such as muscular and axonal tissues. By fitting the diffusion MR (dMRI) signal to a tensor model, diffusion tensor imaging (Basser et al., 1994), or DTI, has already become a widely used method to characterize the diffusion anisotropy in neuronal tissues. It has been used to reveal differences in white matter microstructure between typical readers (TD) and those with WRD in both adults (Klingberg et al., 2000) and children (Beaulieu et al., 2005; Deutsch et al., 2005). In addition to the voxel based morphometry (VBM) analysis, dMRI has also been utilized in numerous neuroimaging studies to track white matter fiber pathways and to relate structural brain connectivity to specific brain functions (Behrens et al., 2003a; Johansen-Berg et al., 2004; Saygin et al., 2012), including reading abilities (Beaulieu et al., 2005; Rimrodt et al., 2010; Vandermosten et al., 2012b).
Compared to the wide use of dMRI in other cognitive studies, research that specifically explores the structural aspects of the VWFA has just started, but is of nontrivial significance. In the first modern study examining the in vivo anatomical connections of the VWFA, Epelbaum and colleagues used DT-tractography to map the connectivity of the VWFA in an adult epilepsy patient before and after surgery (Epelbaum et al., 2008). Before surgery, the patient was a proficient reader, and tractography results showed that the VWFA (identified by fMRI) was linked to the occipital lobe and to supramarginal gyrus through the inferior longitudinal fasciculus (ILF) and arcuate fasciculi (AF), respectively. Then, a small part of the cortex in the neighborhood of the VWFA (including part of the VWFA) was resected. Post-surgery, preservation of the AF was observed, but degeneration of the ILF was revealed; in conjunction with these findings, pure alexia with letter-by-letter reading developed in the patient. This case study provided direct evidence that the connection between the VWFA and occipital cortex plays a key role in visual word form conversion, and that disruption to these white matter connections can result in reading difficulty. However, the ILF does not seem to be the only pathway through which the VWFA communicates with the rest of the brain. A limitation of the study that Epelbaum et al. (2008) acknowledged was the limited number of diffusion sensitizing directions in the dataset (Epelbaum et al., 2008), which may have underestimated connections via the inferior frontal occipital fasciculus. This postulation was indeed supported by a more recent elegant DTI study in healthy children English speakers (Yeatman et al., 2012), where VWFA were identified with localizer and used as seed region for diffusion tractography. This study (Yeatman et al., 2012) also identified other plausible white matter tracts including the vertical occipital fasciculus. Of note, the VWFA connections via the inferior frontal occipital fasciculus have also been reported in healthy adult Dutch speakers (Vandermosten et al., 2012a).
These studies provided an anatomical description of the white matter in the neighborhood that the VWFA sits in, but it remains unclear how this neighboring wiring system is engaged in constituting a well-tuned reading network in the brain. For example, provided that the VWFA circuitry could be used to support various tasks (Ben-Shachar et al., 2007), what precise pattern of engagement is associated with reading performance? As fMRI becomes widely adopted to measure gray matter engagements in a particular cognitive process, it has been newly suggested that the fMRI activations in the brain can be precisely predicted from how this region is wired with the rest of the brain (Saygin et al., 2012). In other words, a map of structural connectivity pattern of a particular region with the rest of the whole brain can be used to estimate its functions and ultimately its role in mediating cognitive behaviors. This study (Saygin et al., 2012) is particularly informative for researchers investigating the VWFA circuitry, since it was carried out in the right fusiform gyrus while the VWFA sits in the left fusiform region. Overall, the combined VWFA and right fusiform dMRI literature suggest that the VWFA connectivity profiles of individuals may carry information about how the circuitry is organized to support reading, and therefore may be able to differentiate between TD and WRD.
The current study aimed to investigate the L-OT/F (inclusive of the putative VWFA) connectivity patterns along a posterior-anterior gradient that included the VWFA, and compare these connectivity patterns between children with WRD, as defined by difficulty with word-level decoding, and TD children. Previous studies have demonstrated that more anterior regions in the L-OT/F are engaged in increasingly multimodal and semantic computations, whereas posterior regions are responsible for perception of the more visual aspects of print, including false-fonts (Cohen et al., 2004; Cohen et al., 2002). In order to obtain a fine grained understanding of L-OT/F connectivity, five consecutive spherical regions of interest (ROI) were defined within the L-OT/F region, which was based upon the functional neuroimaging literature (Brem et al., 2009). These ROIs progress along a posterior to anterior gradient, corresponding to an increased specialized response to print in children (Brem et al., 2009; van der Mark et al., 2009) and in adolescents and adults (Brem et al., 2006; Brem et al., 2009; Vinckier et al., 2007). While various studies have reported a number of regions in the brain related to visual word reading (see meta-analyses (Maisog et al., 2008; Richlan et al., 2009)), the results of meta-analyses (Maisog et al., 2008; Richlan et al., 2009), along with a more recent study using the VWFA localizer method (Rauschecker et al., 2011), overlap within the L-OT/F region, specifically ROIs 2, 3, and 4 (Fig. 1) in the current study. Distinct functioning within these ROIs may imply that the neuronal networks comprising these sub-systems link to distinct cortical areas, and thus suggest that the white matter connectivity patterns specific to each ROI would be differentiable from each other.
Figure 1.
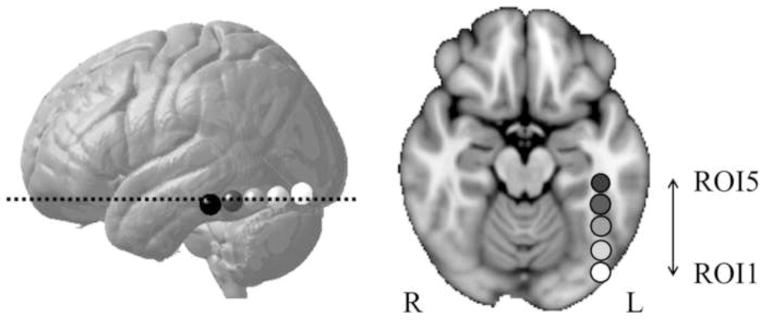
Illustation of the five L-OT/F ROIs. A lateral view of the five L-OT/F ROIs is shown in (a), where the ROIs progress along a posterior to anterior gradient, each with a radius of 6 mm. The brightest ROI was numbered 1st, and the 3rd ROI represents center of the putative VWFA. An axial view of the five L-OT/F ROIs was shown in (b), corresponding to the slice labeled with a dotted line in (a).
Based upon the previous research, we aim to examine the patterns of connectivity between each L-OT/F ROI and various cortical regions. We hypothesized that we would find: (1) overall different cortical connectivity patterns across the five L-OT/F ROIs, with more posterior regions showing greater connectivity to occipital cortex, and more anterior ROIs showing greater connectivity to language related left hemisphere regions and (2) different cortical connectivity patterns for WRD versus TD in ROIs2, 3, and 4 that would be associated with differences in relative emphasis of connectivity to cortical regions engaged in visual word form recognition versus those potentially involved in compensatory mechanisms.
2. Results
2.1. Comparison across five ROIs
To examine whether there are differences in cortical connectivity patterns across the five L-OT/F ROIs, the TD and WRD groups were combined to compute the distance matrix (Supplementary Fig. 1). Results of the permutation test (see method session for more details) showed that statistically significant differences existed between most of the ROIs, except between ROI4 and ROI5 (Supplementary Table 2). To further explore the cortical contributions to these differences, the connectivity profiles of each pair of the consecutive ROIs were contrasted, i.e., 1 for the anterior ROI and 0 for the posterior ROI, in the multiple linear regression analysis. Findings demonstrated that connections with lateral occipital cortex in the L-OT/F progressively decreased along a posterior to anterior gradient. Similar to lateral occipital cortex, the ratio of connections with lingual cortex decreased continuously from ROI1 (the most posterior ROI) to ROI4 and increase from RO4 to ROI5. Opposite to the lingual cortex, connections to inferior temporal were found to increase from ROI1 to ROI4 and decrease from ROI4 to ROI5. Connections to fusiform (excluding the seed region) were found to increase from ROI1 to ROI2 and decrease from ROI2 to ROI5 (Supplementary Fig. 2, Supplementary Table 3). Similar findings were observed in the right hemisphere (Supplementary Table 4, Supplementary Table 5).
2.2. Comparison between groups within ROI
2.2.1. Group differences in connectivity patterns
To compare groups, we performed a distance-based permutation test (Reiss et al., 2010) to explore whether the connectivity pattern differed between the TD and WRD groups across the five ROIs, with a specific focus on the VWFA region. Statistically significant differences in connectivity patterns, after correcting for multiple comparisons within the VWFA ROIs, were found between TD and WRD in ROI2 (Table 1), (pseudo-F[1,34] = 4.694, p = 0.0296, FDR corrected) but not for ROI3 (pseudo-F[1,34] = 1.647, raw p = 0.252) or ROI4 (pseudo-F[1,34] = 0.260, raw p = 0.609). As expected, no statistically significant differences were found in either those L-OT/F ROIs external to the putative VWFA ROIs (ROI 1: raw p = 0. 627; ROI 5: raw p = 0.416), or for the right hemisphere homologous ROIs (including those ROIs homologous to the putative VWFA), all raw ps > 0.066.
Table 1.
Results of the permutation tests in the VWFA (ROIs 2, 3, and 4) for connectivity pattern differences between the TD and WRD groups
A total number of 15,000 permutations were computed, and p was calculated as percentage of random permutations (pr) which yielded a pseudo-F statistic greater than the real group membership did.
p < 0.05, corrected for multiple comparisons using the FDR method.
2.2.2. Characterization of the group differences in connectivity patterns
To identify the cortical contributions to the connectivity pattern that best differentiated the TD and WRD groups for left hemisphere ROI2, multiple linear regression analyses of group membership (i.e., 1 for TD, 0 for WRD) on connectivity profiles were performed. The results are listed in Fig. 2 and Table 2, where 26.14% variation in group membership was explained by the model. Specifically, the WRD group was found to have lower connectivity with middle and inferior temporal regions, and more connections to lateral occipital cortex and parahippocampal regions compared to the TD group.
Figure 2.
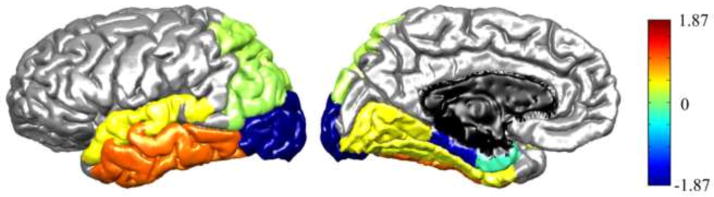
Cortical contributions to the group differences in the connectivity pattern of L-OT/F ROI2. A linear regression of group membership (1 for TD, 0 for WRD) onto connectivity profiles was performed, and regression coefficients, β, were displayed in colors to show contributions from each cortical parcel that best described the group difference, i.e., cortical parcels that had a higher connection ratio in TD than WRD were labeled with hot colors, vice versa.
Table 2.
Cortical contributions to the connectivity pattern characteristic of the group difference found in left hemisphere ROI2
| Cortical parcels | coefficient | Cortical parcels | coefficient |
|---|---|---|---|
| TD > WRD | WRD > TD | ||
| L middletemporal | 1.056555 | L lateraloccipital | −1.870934 |
| L inferiortemporal | 1.018094 | L parahippocampal | −1.707080 |
| L superiortemporal | 0.455544 | L entorhinal | −0.200536 |
| L lingual | 0.435474 | ||
| L fusiform | 0.378173 | ||
| L superiorparietal | 0.169229 | ||
| L inferiorparietal | 0.128544 | ||
Predictors positively contributing to TD group membership are listed on the left. Coefficients whose absolute values are greater than 5 percent of the maximum absolute value of coefficients are listed.
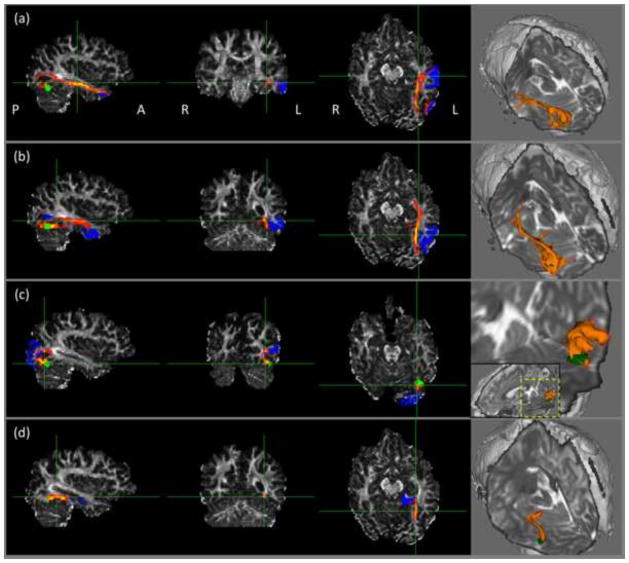
To further examine which fiber tracts the reconstructed fibers belong to, we performed probabilistic fiber tracking in FSL from left hemisphere ROI2, and used a) middle temporal, b) inferior temporal, c) lateral occipital, and d) parahippocampal regions as target ROIs respectively. The fiber tracking results in one TD subject were shown in Figure 3. By comparing our fiber tracking results visually with the results by (Yeatman et al., 2012), the tracts from ROI2 to middle temporal, inferior temporal and parahippocampal regions in the left hemisphere seemed to be part of the inferior longitudinal fasciculus (ILF), which runs anterior-posteriorly from the occipital lobe till the temporal pole. The tract between ROI2 and lateral occipital cortex on the left was found to be (part of) the vertical occipital fasciculus (VOF).
Figure 3.
Illustration of fiber tracts from left hemisphere ROI2. The three-orthogonal views were shown on the left panel and 3D renderings were on the right. Target regions include: (a) middle temporal, (b) inferior temporal, (c) lateral occipital, and (d) parahippocampal regions. Green: seed; blue: target ROI; orange: fiber tracts.
3. Discussion
The current study examined the connectivity patterns of the L-OT/F region, including the putative VWFA, in children with normal to poor word reading ability. We hypothesized that across all children, we would find varying connectivity patterns between the five L-OT/F ROIs, which is consistent with what would be expected based on previous fMRI L-OT/F findings. Indeed, we demonstrated that progressing along a posterior to anterior gradient in L-OT/F, the connectivity pattern varied significantly, with decreasing connectivity to visual areas (lateral occipital lobe, and increasing connectivity to regions classically associated with reading (including regions local to the VWFA, e.g., left fusiform gyrus and inferior temporal gyrus, as well as distal regions, such as supramarginal and inferior parietal areas). Our second hypothesis was that we would find evidence of a specific relationship between structural connectivity patterns and children’s reading abilities and that these differences would be observed in the ROIs corresponding to the putative VWFA. Indeed, results also supported our second hypothesis, showing differences in connectivity patterns for the L-OT/F ROI2, such that TD participants showed a higher connection ratio to the left hemisphere reading-related regions (e.g., left temporal and parietal regions) than WRD, whereas WRD had a higher connection ratio to visual and parahippocampal areas than TD, and the fiber tracts found by tractography were plausible compared with previous research by (Yeatman et al., 2012).
The finding across all participants that the connectivity pattern in the consecutive ROIs progressively changed from heavily connected to visual cortex to favoring regions thought to be responsible for word recognition in the left inferior-temporal region (McCandliss et al., 2003) was largely anticipated. However, we also found a somewhat unexpected finding, which was that towards the anterior tip of L-OT/F, ROI5 (the most anterior ROI), differed from ROI3 by favoring entorhinal and parahippocampal regions instead of the classical reading-related regions. Nevertheless, there are some findings that corroborate with ours. Epelbaum and colleagues (Epelbaum et al., 2008) have noted that macaque brain studies have shown connections between the equivalent of the VWFA and hippocampal regions (specifically, the hippocampal commissures), suggesting a potential link between the VWFA and hippocampal regions. Additionally, the left hippocampus has also been found to be co-activated with the VWFA selectively by word stimuli (Reinke et al., 2008). Because the entorhinal cortex and parahippocampal areas are an important part of neural circuitry for establishing long-term memory and are related to recognition memory (Murray and Richmond, 2001; Squire and Zola-Morgan, 1991), the finding of a higher connection ratio to these regions in the anterior portion of the L-OT/F may reflect the mechanism that readers use for memory-based retrieval of word meaning.
Our findings showing differences between TD and WRD in structural connectivity patterns in regions crucial for reading are quite consistent with the long-standing hypothesis that fluent reading requires efficient and well-tuned left hemisphere circuitry. Specifically, in an ROI central to the putative VWFA, the connectivity patterns yielding group differences involved cortical contributions from left lateral occipital, fusiform and other classical reading related regions. More specifically, connectivity with classical reading-related regions, such as left inferior temporal gyrus and fusiform, was found to be positive predictors of reading ability. Among these regions, L-OT/Fusiform region has long been hypothesized to play an important role in modulating visual stimuli of words and/or letters (Altarelli et al., 2013; McCandliss et al., 2003; Mechelli et al., 2000; van der Mark et al., 2011). The finding of lower connectivity with lingual and fusiform regions, coupled with left occipital cortex as a negative indicator, points to a link between WRD with the VWF recognition functionality, such that TD had a stronger tendency to connect more proximally to L-OT/fusiform region as well as more distally to temporal (and potentially parietal) regions, while for WRD, a larger portion of white matter tracts were dedicated for communication with brain regions that support visual perception.
The finding of left parahippocampal being a negative predictor was unexpected. However, it is intriguing given that children with WRD have been found to show superior recognition memory as compared to TD children (Hedenius et al., 2013). This may reflect a mechanism that WRD children employ when learning to read -- to involve declarative memory to compensate for deficits in lexical retrieval for example. As also suggested by Hedenius et al., this could be a temporary state that incurs during the process of learning to read, so that when the WRD finally reach a comparable level of reading, the advantage in recognition memory in WRD will finally disappear.
It is notable that no differences were observed in ROI1, which is reportedly mainly responsible for lower level processing. The findings that WRD do not show anomalous connectivity patterns in regions (ROI1) that involve connections to basic visual perceptual areas are consistent with many other reports that WRD is not associated with global abnormalities, including basic visual perceptual abnormalities, and are highly consistent with previous studies of WRD indicating the specificity of dysfunction in WRD (Tarkiainen et al., 2002; Tarkiainen et al., 2003). Similarly, the findings that anomalous connectivity patterns were not found in ROI 5, which is associated with amodal semantic processing (Lau et al., 2008; Vigneau et al., 2006), are also consistent with the thought that the abnormalities in WRD have some specificity to print, versus reflecting a general weakness in language altogether (Joanisse et al., 2000). Last but not least, none of the right hemisphere homologous ROIs yielded significant group difference, which further confirms the specificity of the VWFA abnormality found in the L-OT/F region.
Overall, our results showed that L-OT/F for both TD and WRD were comprised of connectivity to regions important for visual word form recognition, for example, occipital cortex and fusiform. Nevertheless, the relative load on cortical regions for children with WRD was different from TD, with WRD showing greater connectivity to more basic visual processing areas and memory-related regions than TD, while TD showed greater connectivity to classical reading-related areas as well as L-OT/fusiform region that putatively plays a role in integrating meaning and form to relate visual stimuli to the linguistic-specific network (van der Mark et al., 2011). It is worth mentioning, though, that the connectivity was quantified as connection ratio. Therefore, a larger portion of fiber tracking streamlines connecting to lateral occipital cortex could result from less amount/dense axonal connections with L-OT/fusiform and other regions, so that the portion of streamlines connecting to left occipital cortex was therefore “increased” due to normalization; such abnormalities therefore may be indicative of a relatively lower number of connections in WRD supporting the visual processing specific to word forms, not necessarily greater absolute connections to visual cortices. Thus, while differences were found between TD and WRD, these differences are relative differences, reflecting the propensity of the L-OT/F ROIs, including the putative VWFA, to connect to various cortical regions differentially in a relative manner. Overall, however, across both groups, connections were evident in fusiform regions, suggesting that while the circuitry in WRD may be different, it may not be completely aberrant. Indeed, it has been suggested that the linguistic system in WRD is poorly tuned, but not completely disrupted (Pugh et al., 2008).
Despite the contribution our study makes to the current literature, there are several limitations. The ROIs were defined anatomically instead of functionally. Due to the variability of the location of the VWFA as studied by fMRI experiments (Altarelli et al., 2013; Rauschecker et al., 2011), the same ROI might not correspond to exactly the same brain functionality across all subjects even in the same group, thus reducing the effect size of the group comparison. Nevertheless, it is also the case that localizer based connectivity presents its own challenges, in terms of confounding factors from unmatched anatomy in the neighboring region of the peak activation locations. This will yield the interpretation of results challenging in a different manner, considering the finding that connectivity pattern varies significantly along the anterior-posterior direction regardless of groups. Thus, novel analysis methodologies that can combine both fMRI data and diffusion MRI data with greater sophistication will be important in future investigations. Another caveat of this study is the lack of ability to estimate significance level of the contributors to group differences in connectivity patterns, yielding somewhat arbitrary cut off points. The results of the current study were discussed with efforts to confine results to the major contributors, and thus may have limited capability of a comprehensive description of such a complex network. We hope that a statistical framework can be developed and implemented in the near future to address such needs, along with the incorporation of covariates, such as hemispheric side and acquisition site, as well as testing interactions (group x side).
In closing, in previous eras, there could only be speculation of the existence of anomalies in regions supporting reading, either in ventral occipital temporal cortex (Kinsbourne and Warrington, 1963) or in temporal parietal cortex (Warrington and Shallice, 1980), because of the limited neurobiological techniques available. With the advent of fMRI and dMRI, modern techniques are now precise enough to predict brain functions with structural information alone (Saygin et al., 2012), and fMRI studies have repeatedly found that the L-OT/F region is selectively responsive to written strings relative to other categories. Among the pioneers initiating the research of the VWFA using modern imaging techniques (Cohen et al., 2000), a decade after their initial investigation, Dehaene and Cohen recently asked (Dehaene and Cohen, 2011): “what are the precise connections of the VWFA? Can its connectivity pattern explain its specific role in written word recognition?” The findings of the current study contribute towards answering these questions, and provide a foundation for further explorations in the connectivity patterns in the VWFA, especially ones that may be less than optimal in WRD.
4. Experimental Procedure
4.1. Participants
A total of 55 children participated in this study. Before entering the study, parents of children were administered a phone screening to ensure that participants met the study’s inclusion criteria: 1) native English speakers, 2) normal hearing and vision, 3) no history of major psychiatric illness, 4) no traumatic brain injury/epilepsy, and 5) no contraindication to MRI. Each parent gave written consent while a separate written assent was obtained from each child at the beginning of the study, with procedures carried out in accordance with the university’s Institutional Review Board. Participants of this study were part of a larger study investigating the neurobiological basis of reading in children with a history of reading difficulty, Neurofibromatosis Type 1, and TD children; note that due to the specificity of our hypotheses regarding WRD, no participants with Neurofibromatosis Type 1 were selected to be in the current study. During their visit, participants were given a comprehensive battery of psychoeducational and academic achievement tests (note only selected tests are reported in the current study; see Supplemental Table 1 for performance on these tests).
Groups were defined according to a composite measure of word-level skills, which consisted of the Basic Reading composite from the Woodcock Johnson Psychoeducational Battery – III (Woodcock et al., 2001, 2003), Test of Word Reading Efficiency Composite score (Torgesen et al., 1997), and the Word Identification subtest from the Word Identification and Spelling Test (Wilson and Felton, 2004). Those in the group with WRD were required to score at less than or equal to the 19th percentile on this composite measure (mean and standard deviation: 73.41 ± 10.69; range: 1st to 19th percentile), while those in the typically developing group were required to score at or above the 34th percentile (mean and standard deviation: 102.90 ± 9.16; range: 34th to 95th percentile). Of the original sample of 55, seven children were removed from the analysis due to motion artifacts in their imaging data; two were removed due to incomplete coverage of the brain cortex. Of the eligible participants, 20 met criteria for WRD and 16 met criteria for TD.
The two groups of children did not differ significantly with regard to age, distribution of gender, or IQ. A multivariate ANOVA (MANOVA) showed that, as expected, the two groups showed statistically significant differences on all reading scores (F[1, 28] = 16.796, p < 0.001, ηp2 = 0.808), with WRD showing poorer performance. See Supplementary Table 1 for detailed demographic and behavioral profiles.
4.2. Brain imaging procedures
4.2.1. Data acquisition
The MR data were collected on Philips Achieva 3.0 T MRI scanners at the Johns Hopkins University (JHU) School of Medicine Kennedy Krieger Institute (KKI) and the Vanderbilt University Institute of Imaging Science (VUIIS). The MR imaging protocols at the two sites were the same. The diffusion-weighted (DW) images were acquired with 8-channel head coil using single shot echo planar imaging (EPI) sequence, TR/TE = 6237/75ms, flip angle = 90°, SENSE factor 2.5, field of view 212 × 212 mm2. The DW data was acquired in 96 × 96 matrices, and zero filled to 256 × 256 matrices, yielding 0.83 mm isotropic in-plane resolution. 60 axial slices were acquired with no gap, slice thickness = 2.2mm. Diffusion weighting was applied along 32 directions with the b-value of 700 s/mm2. Three non-DW images were acquired for averaging. Acquisition time was 3 min and 38 seconds per scan. A T1-weighted structural image was acquired for each participant using a magnetization-prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence (TR/TE = 8.0/3.7 ms, flip angle = 8°, SENSE factor = 2, voxel size = 1 mm3). Acquisition time was 7 min per scan.
Note that data were acquired at both the JHU-KKI and VUIIS. Therefore, to investigate any potential confounds due to scanner locations, connectivity pattern analyses using distance-based permutation tests were initially conducted between sites (JHU-KKI (N=20) and VUIIS (N=16)). Results of all analyses were not significant (JHU-KKI versus VUIIS, as well as TD-JHU-KKI versus TD-VUIIS and WRD-JHU-KKI versus WRD-VUIIS), and therefore site was removed from further analyses. See 4.3 for description of distance-based permutation analysis approach.
4.2.2. Data processing
The MR data were processed using Freesurfer (Fischl et al., 2002) and FSL (Jenkinson et al., 2012). The T1 images were used to perform brain parcellation with Freesurfer. On each side of the brain, the cortex was segmented into 34 non-overlapping regions (Desikan et al., 2006). The non-diffusion-weighted images were registered to T1 images using 12 degrees of freedom affine registration in FSL (Jenkinson et al., 2002), and the transformation was then inverted and applied to the parcellated T1 images. Head motion and eddy current artifacts were corrected by linearly registering the DW images to the non-diffusion-weighted image. Five ROIs were selected within the L-OT/F areas (Fig. 1). All ROIs have a radius of 6 mm, and lie along an anterior-posterior gradient (Brem et al., 2009). The MNI coordinates of these ROI centers were: ROI1 (−42, −80, −14), ROI2 (−42, −68, −16), ROI3 (−42, −54, −17), ROI4 (−42, −42, −18), and ROI5 (−42, −30, −20), where ROI3 is specifically thought to represent the center of the putative VWFA (Cohen et al., 2000). To localize these ROIs in each individual’s native dMRI space, the T1 image in the native space was warped to the MNI152 template (1mm isotropic resolution) using the nonlinear registration tool, FNIRT, in FSL. The warp field was then inverted. The inverted warp field (from MNI152 to native T1) and the inverted affine transformation (from native T1 to native dMRI) were applied simultaneously on the ROIs in the MNI152 space with the nearest neighbor interpolation method. The imaging data and each step of processing were visually checked to ensure the absence of motion artifacts or parcellation/registration failure. Probabilistic tractography (Behrens et al., 2003b) was performed from each of the spherical ROIs, respectively, to the whole brain cortex, which was comprised of 68 non-overlapping parcels. 10,000 samples were initiated in each seed voxel for the probabilistic fiber tracking, streamlines were excluded from the CSF region, and distance bias was corrected. To ensure no overlapping between seed and targets when performing fiber tracking, when one of them was used as seed region, this particular ROI was masked off from all cortical target parcels. For each of the ROIs, the number of streamlines found to connect to each of the 68 target cortical parcels was summed over all the voxels within the ROI. These numbers put together are furthermore referred to as the “connectivity profile”.
4.3. Connectivity pattern analysis
The focus on the connectivity pattern analysis was to examine: (1) in general, whether the five L-OT/F ROIs differed in connectivity patterns, regardless of group membership, and if differences were found, to determine what pattern feature best differentiated between the ROIs; the expected finding was progressed connectivity to more anterior regions corresponding with more anterior L-OT/F ROIs, along with a similar pattern in right OT/F ROIs and (2) whether there were differences in connectivity patterns between TD and WRD with a priori hypotheses that areas of difference would be centered on the VWFA and areas known to be anomalous in WRD (corresponding to ROIs 2, 3, and 4), and if there were differences in the connectivity patterns between groups, what pattern feature best differentiated the groups. Additionally, control analyses were performed in the homologous right hemisphere ROIs, with the expectation that we would see no group differences in the right hemisphere ROIs, therefore further supporting the specificity of group differences in the VWFA region.
To determine differences across the five ROIs and between groups, we first performed a distance-based permutation test to determine if there were any differences in connectivity patterns. It was decided a priori that if the distance-based permutation test reached significance, then linear multiple regressions of connectivity profiles between ROIs, and/or between TD versus WRD would be conducted. Such analyses would then allow for the computation of the specific cortical contributions to ROI or group difference.
4.3.1. Distance matrix and permutation test for differences in connectivity pattern
To compare between groups the overall characteristics of the connectivity patterns, number arrays per se, each consisting of 68 elements, an instinctive approach would be pairwise comparisons in a repetitive fashion, but very likely it will lead to issues arising from large number of multiple comparisons. A simple fix would be an F-test, but it is sensitive to non-normality (Box, 1953) of data being tested. To address this issue, the distance-based permutation test was developed for between-group comparisons in neuroimaging studies (Anderson and Legendre, 1999; Reiss et al., 2010; Zapala and Schork, 2006), which fits the purpose of the current study.
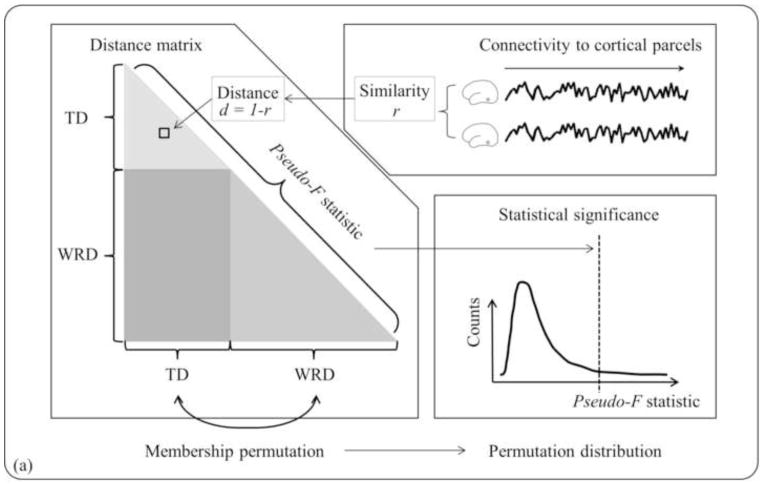
For these reasons, the distance-based permutation test was used to detect differences between specific grouping criteria of interest (heretofore referred to as “group membership”): either between TD and WRD for a specific ROI, or across the L-OT/F ROIs, as well as initially to confirm that there were no site specific confounds. For example, using the comparison between the TD and WRD groups to illustrate this approach (Fig. 4a), the first step was measuring the similarity between individuals, which was calculated as the linear correlation coefficient of the connectivity profiles between each pair of participants, rij = corr(ci, cj), (i, j = 1,2,...,N, i ≠ j), where ci is a 1 by nparc vector representing the connectivity profile of the ith participant, nparc is the number of cortical parcels, and N is the total number of participants in both the TD and WRD groups. An N by N lower triangular matrix of distance D was created, where each element, di,j = 1-ri,j, represents the dissimilarity or distance between participants, ranging from 0 for being coherently varying, to 2 for being negatively coherent, and 1 represents being completely unrelated. Then the distance matrix was transformed and centered (Shehzad et al., 2011). A pseudo-F statistic was computed to estimate how well the “group membership” (in this case, TD or WRD) explains the distances between participants. Then the “group membership” was shuffled 15,000 times to build up a permutation distribution. Significance level p was calculated as the percentage of random permutations which yielded a pseudo-F statistic greater than the real “group membership” did. The false discovery rate (FDR) method (Benjamini and Hochberg, 1995) was used to correct for multiple comparisons. The statistical significance was set as FDR<0.05 after multiple comparison correction. FDR was calculated using ‘multtest’ package in R.
Figure 4.
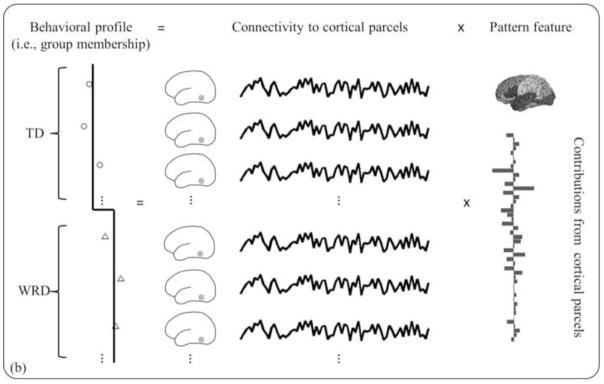
Schematic diagram of connectivity pattern analysis pipeline. (a) To test whether there is a group difference in connectivity pattern, a distance-based permutation test was performed, where pair-wise correlation coefficients were computed among participants to estimate similarity between individuals, and distance, or dissimilarity, was calculated as one minus similarity. The triangular matrix yielded a single value of pseudo-F statistic that estimates how well the “group membership” explains the distances between individuals. Then the group membership was permutated to build a pseudo-F distribution and hence to determine the statistical significance. (b) If a significant difference was found, a linear multiple regression of group membership with connectivity profiles was performed to compute cortical contributions to group difference.
As mentioned above, this same analysis approach was also used to detect differences between ROIs, as well as to confirm no site specific differences. In these cases, instead of dividing the participants into the TD and WRD groups, the “groups membership” was defined in a more generalized way, i.e., ROI1 (Ngroup1 = 36) versus ROI2 (Ngroup2 = 36), etc. Note that the dimension of the corresponding distance matrix D varies accordingly, i.e., N = Ngroup1+Ngroup2, and the rest of the test stays the same as described above.
4.3.2. Cortical contributions to group differences in connectivity pattern
To identify the connectivity pattern that best differentiated between “group membership” (TD versus WRD, or any pair of consecutive ROIs), linear multiple regressions of connectivity profiles were performed. Prior to conducting these analyses, the connectivity profiles were normalized by the total fiber counts, so that for each participant, the percentages of fiber counts originating from one particular ROI connecting to all cortical parcels sum up to 100%. The principal component analysis (PCA) was then performed on total connectivity profile C, an N by nparc matrix, and the minimum number of components accounting for >90% of total variance in dataset, nPC, was determined.
The scores of each participant corresponding to these components, X, in an N by nPC matrix, were used as predictor variables, and the group membership, Y, in an N by 1 vector, as response matrix. For example, for the TD and WRD comparisons (Fig. 4b), Y = X·βPC + ε, where Yi = 1, for 1 ≤ i ≤ NTD; Yi = 0, for NTD+1 ≤ i ≤ N, N = NTD+NWRD, NTD and NWRD represent the number of participants in TD and WRD groups respectively. The linear multiple regression coefficients βPC, an nPC by 1 vector, denotes a linear combination of nPC principal components that best differentiates between groups, which was then translated back into the connectivity profile variables, or β = PC* βPC, where β is an nparc by 1 vector, and PC is a nparc by nPC matrix comprised of the first nPC principal components. For the ROI comparison, similarly, Y = X·βPC+ ε, where Yi = 1, for 1 ≤ i ≤ NROI(k); Yi = 0, for NROI(k-1)+1 ≤ i ≤ N, N = NROI(k)+NROI(k-1), NROI(K) and NROI(k-1) represent the number of participants in the kth and (k-1)th ROI groups respectively. In this study, particularly, the TD and WRD participants were combined to identify differences between consecutive ROIs, and thus NROI(k) = NTD+NWRD for k = 1,2,...,5.
Supplementary Material
Research Highlights.
We examined left occipito-temporal/fusiform (L-OT/F) connectivity pattern.
We showed L-OT/F connectivity varies along a posterior-anterior gradient.
The visual word form area (VWFA) connectivity is related to reading ability.
Typical readers showed greater connectivity to linguistic areas.
WRD readers showed greater connectivity to visual and parahipocampal areas.
Acknowledgments
The authors are grateful to Baxter P. Rogers at Vanderbilt University for methodological discussions. This work was supported in part by the National Institute of Neurological Disorders and Stroke through the grant “Neurobiology and Treatment of Reading Disability in NF1” (NIH/NINDS R01NS049096), grant “Cognitive and Neural Processes in Reading Comprehension” (NIH/NICHD R01HD044073), grant “Predicting Late-Emerging RD: Neurobiological and Cognitive Factors” (NIH/NICHD R01HD067254), the Vanderbilt Kennedy Center for Research on Human Development (P30 HD15052), F.M. Kirby Research Center (NIH/NCRR grant P41 EB015909), the National Center for Research Resources (UL1 RR024975-01), and the National Center for Advancing Translational Sciences (2UL1 TR000445-06). This work was conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
Contributor Information
Qiuyun Fan, Email: qiuyun.fan@vanderbilt.edu.
Adam W. Anderson, Email: adam.anderson@vanderbilt.edu.
Nicole Davis, Email: nikki.davis@vanderbilt.edu.
References
- Altarelli I, Monzalvo K, Iannuzzi S, Fluss J, Billard C, Ramus F, Dehaene-Lambertz G. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: evidence for differential effects in boys and girls. J Neurosci. 2013;33:11296–11301. doi: 10.1523/JNEUROSCI.5854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, Legendre P. An empirical comparison of permutation methods for tests of partial regression coefficients in a linear model. Journal of Statistical Computation and Simulation. 1999;62:271–303. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003a;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003b;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- Box GEP. Non-normality and tests on variances. Biometrika. 1953;40:318–335. [Google Scholar]
- Braet W, Wagemans J, Op de Beeck HP. The visual word form area is organized according to orthography. Neuroimage. 2012;59:2751–2759. doi: 10.1016/j.neuroimage.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci U S A. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Brandeis D. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage. 2006;29:822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Hum Brain Mapp. 2009;30:1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123 (Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 2004;23:1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dejerine JJ. Contribution à I’étude anatomo-pathologique et clinique des différentes variétés de cécité verbale. Mémoires de la Société de Biologie. 1892;4:61–90. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Elliott JG, Grigorenko EL. The Dyslexia Debate. Cambridge University Press; 2014. [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44:962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Hedenius M, Ullman MT, Aim P, Jennische M, Persson J. Enhanced recognition memory after incidental encoding in children with developmental dyslexia. PLoS One. 2013;8:e63998. doi: 10.1371/journal.pone.0063998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Manis FR, Keating P, Seidenberg MS. Language deficits in dyslexic children: speech perception, phonology, and morphology. J Exp Child Psychol. 2000;77:30–60. doi: 10.1006/jecp.1999.2553. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M, Warrington EK. The localizing significance of limited simultaneous visual form perception. Brain. 1963;86:697–702. doi: 10.1093/brain/86.4.697. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nat Rev Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Lebihan D, Breton E. Imagerie de Diffusion In Vivo par Résonance Magnétique Nucléaire. CR Académie des Sciences de Paris. 1985;301 (Série II):1109–1112. [Google Scholar]
- LeBihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA. A definition of dyslexia. Annals of Dyslexia. 2003;53:1–14. [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Gatignol P, Duffau H. Evidence for an occipito-temporal tract underlying visual recognition in picture naming. Clin Neurol Neurosurg. 2009;111:601–605. doi: 10.1016/j.clineuro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci. 2000;267:1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nestor A, Behrmann M, Plaut DC. The Neural Basis of Visual Word Form Processing: A Multivariate Investigation. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs158. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Rueckl JG, Constable RT, Seidenberg MS, Fulbright RK, Katz L, Mencl WE. Effects of stimulus difficulty and repetition on printed word identification: an fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. J Cogn Neurosci. 2008;20:1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM, Bowen RF, Perry LM, Kevan AM, Dougherty RF, Wandell BA. Visual feature-tolerance in the reading network. Neuron. 2011;71:941–953. doi: 10.1016/j.neuron.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke K, Fernandes M, Schwindt G, O’Craven K, Grady CL. Functional specificity of the visual word form area: general activation for words and symbols but specific network activation for words. Brain Lang. 2008;104:180–189. doi: 10.1016/j.bandl.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Reiss PT, Stevens MH, Shehzad Z, Petkova E, Milham MP. On distance-based permutation tests for between-group comparisons. Biometrics. 2010;66:636–643. doi: 10.1111/j.1541-0420.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional Abnormalities in the Dyslexic Brain: A Quantitative Meta-Analysis of Neuroimaging Studies. Human Brain Mapping. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46:739–749. doi: 10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Koldewyn K, Reynolds G, Gabrieli JD, Saxe RR. Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci. 2012;15:321–327. doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Reiss PT, Adelstein J, Emerson JW, Chabernaud C, Mennes M, DiMartino A, McMahon K, Copland D, Castellanos FX, Kelly C, Milham MP. Connectome-Wide Association Studies (CWAS): A Multivariate Distance-Based Approach. Annual Meeting of the Organization for Human Brain Mapping; Quebec City, Canada. 2011. [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Cornelissen PL, Salmelin R. Dynamics of visual feature analysis and object-level processing in face versus letter-string perception. Brain. 2002;125:1125–1136. doi: 10.1093/brain/awf112. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Salmelin R. Category-specific occipitotemporal activation during face perception in dyslexic individuals: an MEG study. Neuroimage. 2003;19:1194–1204. doi: 10.1016/s1053-8119(03)00161-7. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) PRO-ED; Austin, TX: 1997. [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, Kronbichler M, Loenneker T, Klaver P, Martin E, Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012a;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012b;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL. The left occipitotemporal cortex does not show preferential activity for words. Cereb Cortex. 2012;22:2715–2732. doi: 10.1093/cercor/bhr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Wilson B, Felton R. Word Identification and Spelling Test. Super Duper Publications; Greenville, SC: 2004. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, Schrank FA. Woodcock-Johnson III (WJ III) Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, Schrank FA. Woodcock-Johnson III diagnostic supplement to the tests of cognitive abilities. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: Adjacent cortical circuits and long-range white matter connections. Brain Lang. 2012 doi: 10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapala MA, Schork NJ. Multivariate regression analysis of distance matrices for testing associations between gene expression patterns and related variables. Proc Natl Acad Sci U S A. 2006;103:19430–19435. doi: 10.1073/pnas.0609333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.